A Modern Approach to Disinfection, as Old as the Evolution of Vertebrates
Abstract
:1. Introduction
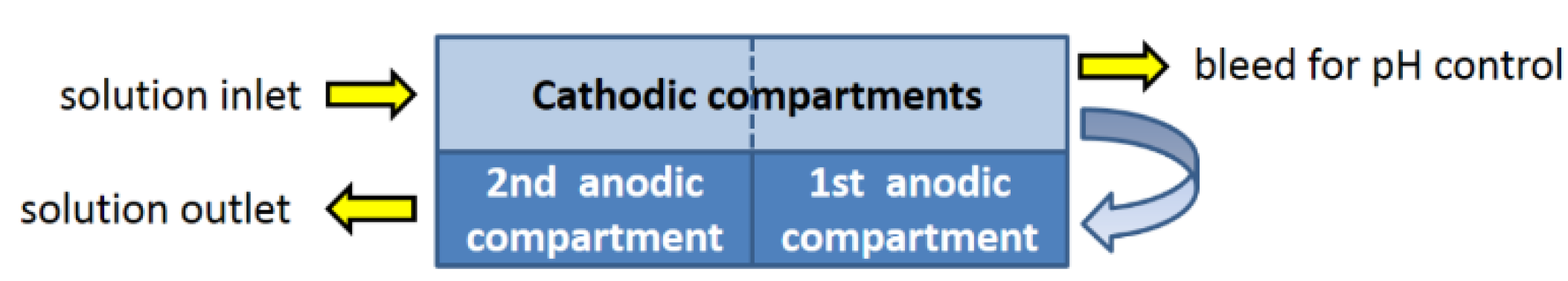
- Higher disinfectant efficacy of HOCl, with respect to ClO− (about two orders of magnitude, as demonstrated also experimentally; see e.g., [16]); accordingly, the use of HOCl makes it possible to obtain results comparable to those attainable with the use of hypochlorite, but using lower concentrations.
- HOCl apparently behaves like a source of hydroxyl radicals, rather than as a chlorine-containing oxidizing agent, thus minimizing the risk of formation of undesired byproducts [17].
- A neutral solution does not alter the pH characteristics of the treated liquid (often a potable water), and provides greater assurance concerning possible problems of corrosion for metal piping.
2. Methods
2.1. Sample Collection
2.2. Biocide Dosing
3. Results and Discussion
3.1. Decontamination of Hot Water System — Dresden University Hospital (Germany)
| Sample | Notes | 02.11 | 15.11 | 01.12 | 09.02 | 15.06 | 02.08 | *16.08 | *23.08 |
| shower in room 124 | before flushing | 2000 | 1000 | 100,000 | 10,000 | 0 | 1,000 | ||
| after flushing | 6000 | 2000 | 33,000 | 3000 | 0 | 0 | |||
| Shower in room 134 | before flushing | 1000 | 1000 | 2000 | 0 | 3000 | 0 | 0 | 0 |
| after flushing | 8000 | 11,000 | 5000 | 7000 | 11,000 | 5000 | 0 | 4 | |
| Sink in room 134 | before flushing | 12,000 | 1000 | 0 | 40 | 0 | 0 | 0 | |
| after flushing | 14,000 | 6000 | 15,000 | 3000 | 2000 | 0 | 1000 | ||
| Sink in room 61 | before flushing | 1000 | 10,000 | 3000 | 7000 | 1000 | 100 | 2,000 | |
| after flushing | 16,000 | 3000 | 6000 | 7000 | 2000 | 20 | 2,000 | ||
| Sample | Notes | *30.08 | *06.09 | *13.09 | *20.09 | *27.09 | *11.10 | *18.10 | *25.10 |
| Shower in room 124 | before flushing | 3000 | 0 | 20 | 0 | 1000 | 0 | 0 | 0 |
| after flushing | 280 | 0 | 0 | 0 | 4000 | 0 | 0 | 0 | |
| Shower in room 134 | before flushing | 1000 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| after flushing | 1000 | 0 | 0 | 0 | 1000 | 0 | 0 | 0 | |
| Sink in room 134 | before flushing | 4000 | 0 | 0 | 0 | 2000 | 0 | 0 | 0 |
| after flushing | 4000 | 0 | 0 | 0 | 1000 | 0 | 0 | 0 | |
| Sink in room 61 | before flushing | 28,000 | 14,000 | 70 | 0 | 2000 | 0 | 0 | 0 |
| after flushing | 15,000 | 1000 | 20 | 0 | 4000 | 0 | 0 | 0 |
3.2. Evaluation of Two Disinfection Systems for Legionella Eradication — Cardinal Massaia Hospital, Asti (Italy)

| Water Sample | TOC (mg/L) | Residual Free Chlorine (mg/L) * | CFU/mL | Total THMs (μg/L) | Chloroform (μg/L) |
|---|---|---|---|---|---|
| Before | 1.0 ± 0.3 | N.A. | 200 ± 30 | 0.5 | <0.1 |
| After 2 months | 1.1 ± 0.2 | 0.3 ± 0.1 | 6 ± 5 | 3.4 | <0.1 |
| After 4 months | 2.9 ± 0.6 | 0.2 ± 0.1 | 12 ± 7 | 4.6 | <0.1 |
| After 6 months | 1.7 ± 0.3 | 0.3 ± 0.1 | 2 ± 1 | 4.1 | <0.1 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Frank, S.A. Immunology and Evolution of Infectious Disease; Princeton University Press: Princeton (NJ, USA) and Oxford (UK), 2002; pp. 13–15. [Google Scholar]
- Klebanoff, S.J. Myeloperoxidase: Friend and foe. J. Leukocyte Biol. 2005, 77, 598–625. [Google Scholar] [CrossRef] [PubMed]
- Hoffstein, S.T.; Gennaro, D.E.; Manzi, R.M. Neutrophils may directly synthesize both H2O2 and O2− since surface stimuli induce their release in stimulus-specific ratios. Inflammation 1985, 9, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Bassiri, M.; Najafi, R.; Najafi, K.; Yang, J.; Khosrovi, B.; Hwong, W.; Barati, E.; Belisle, B.; Celeri, C.; et al. Hypochlorous acid as a potential wound care agent. Part I. Stabilized hypochlorous acid: A component of the inorganic armamentarium of innate immunity. J. Burns Wounds 2007, 6, 65–79. [Google Scholar]
- Hampton, M.B.; Kettle, A.J.; Winterbourn, C.C. Inside the neutrophil phagosome: Oxidants, myeloperoxidase, and bacterial killing. Blood 1998, 92, 3007–3017. [Google Scholar] [PubMed]
- Klebanoff, S.J. Mieloperoxidase. Proc. Assoc. Am. Physicians 1999, 111, 383–389. [Google Scholar] [PubMed]
- Winterbourn, C.C. Biological reactivity and biomarkers of the neutrophil oxidant, hypochlorous acid. Toxicology 2002, 181–182, 223–227. [Google Scholar]
- Len, S.V.; Hung, Y.C.; Erickson, M.; Kim, C. Ultraviolet spectrophotometric characterization and bactericidal properties of electrolyzed oxidizing water as influenced by amperage and pH. J. Food Prot. 2000, 63, 1534–1537. [Google Scholar] [PubMed]
- Abadias, M.; Usall, J.; Oliveira, M.; Alegre, I.; Viñas, I. Efficacy of neutral electrolyzed water (NEW) for reducing microbial contamination on minimally processed vegetables. Int. J. Food Microbiol. 2008, 123, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Xiong, K.; Liu, H.-J.; Liu, R.; Li, L.-T. Differences in fungicidal efficiency against Aspergillus flavus for neutralized and acidic electrolyzed oxidizing waters. Int. J. Food Microbiol. 2010, 137, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Neodo, S.; Rosestolato, D.; Ferro, S.; de Battisti, A. On the electrolysis of dilute chloride solutions: Influence of the electrode material on Faradaic efficiency for active chlorine, chlorate and perchlorate. Electrochim. Acta 2012, 80, 282–291. [Google Scholar] [CrossRef]
- Bohnstedt, R.; Surbeck, U.; Bartsch, R. Membrane electrolytic reactors system with four chambers. European Patent n. 1969159 B1, 13 May 2009. [Google Scholar]
- Regolamento concernente i materiali e gli oggetti che possono essere utilizzati negli impianti fissi di captazione, trattamento, adduzione e distribuzione delle acque destinate al consumo umano. D. Min. 174, 17.07.2004, G.U. n. 166 (In Italian).
- Bakhir, V.M. Regulating physical and chemical properties of technological aqueous solutions by unipolar electrochemical exposure and experience of its practical application. Ph.D. Thesis, Kazan Institute of Chemical Technologies, Kazan, Tatarstan (Russia), 1985. [Google Scholar]
- Prilutsky, V.I.; Bakhir, V.M. Electrochemically Actuating Water: Anomalous Characteristics, Mechanism of Biological Action; Nauka: Moscow, Russia, 1997; p. 232. [Google Scholar]
- Faust, S.D.; Aly, O.M. Chemistry of Water Treatment, 2nd ed.; Lewis Publishers: New York, NY, USA, 1998; pp. 509–517. [Google Scholar]
- Tamburini, E.; Bernardi, T.; Castaldelli, G.; Tumiatti, G.; Ferro, S. Green electrochemical approach for delignification of wheat straw in second-generation bioethanol production. Energy Environ. Sci. 2011, 4, 551–557. [Google Scholar] [CrossRef]
- Quadrelli, S.; Ferro, S. Electrochemical Reactor. Intern. Pat. Appl. n. WO 2010/055108 A1, 2010. [Google Scholar]
- Lee, J.S.; Quan, N.D.; Hwang, J.M.; Lee, S.D.; Kim, H.; Lee, H.; Kim, H.S. Polymer electrolyte membranes for fuel cells. J. Ind. Eng. Chem. 2006, 12, 175–183. [Google Scholar] [CrossRef]
- Thorn, R.M.S.; Lee, S.W.H.; Robinson, G.M.; Greenman, J.; Reynolds, D.M. Electrochemically activated solutions: Evidence for antimicrobial efficacy and applications in healthcare environments. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Liste der Aufbereitungsstoffe und Desinfektionsverfahren, Trinkwasserverordnung 2008, gemäβ § 11—Teil I c. Available online: http://www.lra-mue.de/shared/data/pdf/liste_paragraf_11_stand_juni_2008.pdf (accessed on 17 December 2014). (In German)
- Italian guidelines for prevention and control of Legionellosis. 05.05.2000, G.U. n. 103.
- International Organization for Standardization (ISO). Water Quality: Detection and Enumeration of Legionella. ISO 11731–2: 2008.
- Descours, G.; Cassier, P.; Forey, F.; Ginevra, C.; Etienne, J.; Lina, G.; Jarraud, S. Evaluation of BMPA, MWY, GVPC and BCYE media for the isolation of Legionella species from respiratory samples. J. Microbiol. Methods 2014, 98, 119–121. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization (ISO). Colorimetric method using N,N-diethyl-1,4-phenylenediamine, for routine control purposes. In Water Quality: Determination of Free Chlorine and Total Chlorine; ISO 7393–2: 2002; Switzerland.
- Verfahren zur Desinfektion von Trinkwasser mit Chlor und Hypochloriten, Arbeitsblatt W 229. 2008; DVGW technical rule. Available online: http://www.beuth.de/de/technische-regel/dvgw-w-229/110879513 (accessed on 17 December 2014). (In German)
- Attuazione della direttiva 98/83/CE relativa alla qualità delle acque destinate al consumo umano. D. Lgs. n. 31, 03.03.2001, G.U. n. 52.
- Migliarina, F.; Administrative services business, ASL AT, Asti, Italy. Personal Communication, 2014.
- American Public Health Association (APHA); American Water Works Association (AWWA); Water Environment Federation (WEF). Standard Methods for the Examination of Water and Wastewater, 21st ed. 2005. Available online: http://www.mwa.co.th/download/file_upload/SMW W_1000-3000.pdf (accessed on 17 December 2014).
- APAT; IRSA-CNR. Metodi analitici per le Acque; Manuali e Linee Guida, APAT: Roma, 2003; p. 29. [Google Scholar]
- Martinez-Huitle, C.A.; Ferro, S. Electrochemical oxidation of organic pollutants for the wastewater treatment: Direct and indirect processes. Chem. Soc. Rev. 2006, 35, 1324–1340. [Google Scholar] [CrossRef] [PubMed]
- Ghernaout, D.; Naceur, M.W.; Aouabed, A. On the dependence of chlorine by-products generated species formation of the electrode material and applied charge during electrochemical water treatment. Desalination 2011, 270, 9–22. [Google Scholar] [CrossRef]
- Bonfatti, F.; Ferro, S.; Lavezzo, F.; Malacarne, M.; Lodi, G.; de Battisti, A. Electrochemical incineration of glucose as a model organic substrate. Part 1: Role of the electrode material. J. Electrochem. Soc. 1999, 146, 2175–2179. [Google Scholar] [CrossRef]
- Martinez-Huitle, C.A.; de Battisti, A.; Ferro, S.; Reyna, S.; Cerro-Lopez, M.; Quiroz, M.A. Removal of the pesticide Methamidophos from aqueous solutions by electrooxidation using Pb/PbO2, Ti/SnO2 and Si/BDD electrodes. Environ. Sci. Technol. 2008, 42, 6929–6935. [Google Scholar] [CrossRef] [PubMed]
- Ferro, S.; Martinez-Huitle, C.A.; de Battisti, A. Electroxidation of oxalic acid at different electrode materials. J. Appl. Electrochem. 2010, 40, 1779–1787. [Google Scholar] [CrossRef]
- Furuta, T.; Tanaka, H.; Nishiki, Y.; Pupunat, L.; Haenni, W.; Rychen, P. Legionella inactivation with diamond electrodes. Diamond Relat. Mater. 2004, 13, 2016–2019. [Google Scholar] [CrossRef]
- Chen, D.-Q.; Huang, S.-S.; Lu, Y.-J. Efficient transformation of Legionella pneumophila by high-voltage electroporation. Microbiol. Res. 2006, 161, 246–251. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Migliarina, F.; Ferro, S. A Modern Approach to Disinfection, as Old as the Evolution of Vertebrates. Healthcare 2014, 2, 516-526. https://doi.org/10.3390/healthcare2040516
Migliarina F, Ferro S. A Modern Approach to Disinfection, as Old as the Evolution of Vertebrates. Healthcare. 2014; 2(4):516-526. https://doi.org/10.3390/healthcare2040516
Chicago/Turabian StyleMigliarina, Franco, and Sergio Ferro. 2014. "A Modern Approach to Disinfection, as Old as the Evolution of Vertebrates" Healthcare 2, no. 4: 516-526. https://doi.org/10.3390/healthcare2040516





