A Proteomic Study of Clavibacter Michiganensis Subsp. Michiganensis Culture Supernatants
Abstract
:1. Introduction
2. Experimental Section
2.1. Strains and Growth Conditions
2.2. Isolation and Filtration of Extracellular Proteins
2.3. In-Solution Tryptic Digest
2.4. NanoLC-MS/MS Analysis
2.5. Analysis of Functional Categories and Localization of Proteins
3. Results and Discussion
3.1. Analysis of C. michiganensis Subsp. michiganensis M9 and XMM Culture Supernatants

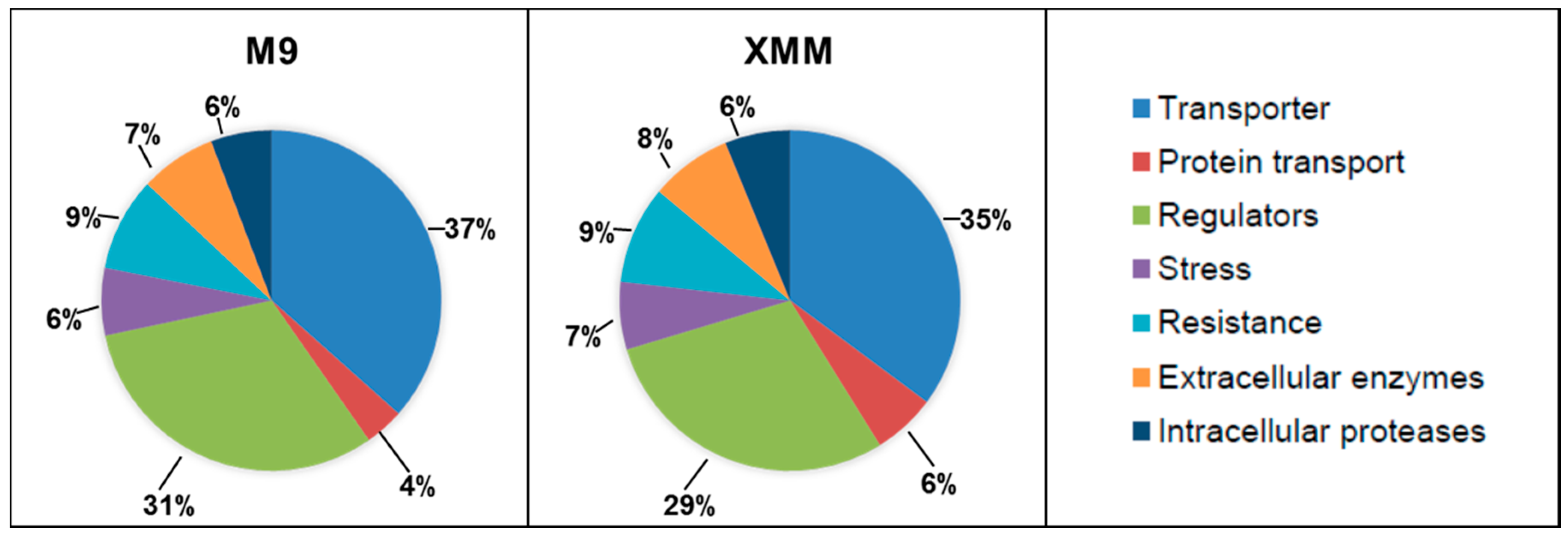
3.2. Analysis of the Putative C. michiganensis Subsp. michiganensis Secretome
| Identifier | Annotated Function | M9/XMM |
|---|---|---|
| CMM_0013 | putative sortase | +/+ |
| CMM_0017 | putative penicillin binding protein | +/+ |
| CMM_0039 | putative extracellular serine protease | +/+ |
| CMM_0041 | putative extracellular serine protease | +/+ |
| CMM_0053 | putative extracellular serine protease | +/+ |
| CMM_0059 | putative extracellular serine protease | +/+ |
| CMM_0075 | putative extracellular serine protease | +/+ |
| CMM_0090 | β-xylanase | +/+ |
| CMM_0109 | putative sugar ABC transporter substrate-binding protein | +/+ |
| CMM_0129 | putative sortase | +/− |
| CMM_0141 | putative secreted protein | +/− |
| CMM_0166 | putative iron-siderophore ABC transporter substrate-binding protein | +/+ |
| CMM_0289 | conserved secreted/exported protein | +/+ |
| CMM_0296 | putative sugar ABC transporter substrate-binding protein | +/+ |
| CMM_0345 | conserved secreted/exported protein | +/+ |
| CMM_0359 | putative sugar ABC transporter substrate-binding protein | +/+ |
| CMM_0363 | putative iron-siderophore ABC transporter substrate-binding protein | +/+ |
| CMM_0423 | putative sugar ABC transporter substrate-binding protein | +/+ |
| CMM_0430 | putative cell surface protein | +/+ |
| CMM_0431 | putative hemagglutinin/hemolysin-related protein | +/+ |
| CMM_0435 | putative iron-siderophore ABC transporter substrate-binding protein | +/+ |
| CMM_0613 | levanase | +/+ |
| CMM_0667 | secreted phosphoesterase | +/+ |
| CMM_0706 | putative penicillin binding protein | +/− |
| CMM_0792 | putative sugar ABC transporter substrate-binding protein | +/+ |
| CMM_0795 | putative extracellular nuclease/phosphatase | +/+ |
| CMM_0799 | putative peptide ABC transporter substrate-binding protein | +/+ |
| CMM_0819 | putative cell surface protein | +/+ |
| CMM_0825 | putative cell surface protein | +/+ |
| CMM_0827 | capsular polysaccharide synthesis protein | +/+ |
| CMM_0840 | putative NplC/P60 protein | +/+ |
| CMM_0866 | putative α-glucoside ABC transporter substrate-binding protein | +/+ |
| CMM_0879 | putative sugar ABC transporter substrate-binding protein | +/+ |
| CMM_0915 | putative penicillin binding protein | +/+ |
| CMM_0919 | putative penicillin binding protein | +/+ |
| CMM_0944 | putative sugar ABC transporter substrate-binding protein | +/+ |
| CMM_0975 | putative ABC transporter substrate-binding protein | +/+ |
| CMM_0976 | putative ABC transporter substrate-binding protein | +/+ |
| CMM_1022 | putative secreted protein | +/+ |
| CMM_1031 | putative secreted protein | +/+ |
| CMM_1032 | putative secreted protein | +/+ |
| CMM_1129 | putative siderophore interacting protein | +/− |
| CMM_1243 | putative sugar ABC transporter substrate-binding protein | +/+ |
| CMM_1250 | putative secreted protein | +/+ |
| CMM_1262 | putative sugar ABC transporter substrate-binding protein | +/+ |
| CMM_1304 | conserved secreted protein | −/+ |
| CMM_1314 | putative iron ABC transporter substrate binding protein | +/+ |
| CMM_1389 | conserved secreted/exported proteins | +/+ |
| CMM_1405 | conserved secreted protein | +/+ |
| CMM_1406 | putative secreted protein | +/+ |
| CMM_1411 | putative cell surface protein | +/+ |
| CMM_1450 | putative secreted protein | +/+ |
| CMM_1478 | putative peptide ABC transporter substrate-binding protein | +/+ |
| CMM_1532 | putative proline/glycine/betaine/choline ABC transporter substrate-binding protein | +/+ |
| CMM_1557 | putative secreted protein | +/+ |
| CMM_1673 | β-xylanase | +/+ |
| CMM_1674 | β-xylanase | +/+ |
| CMM_1790 | putative anion ABC transporter substrate-binding protein | +/+ |
| CMM_1865 | putative penicillin binding protein | +/+ |
| CMM_1947 | putative extracellular serine protease | +/+ |
| CMM_1948 | putative extracellular serine protease | +/+ |
| CMM_1960 | putative peptide ABC transporter substrate-binding protein | +/+ |
| CMM_2106 | putative sugar ABC transporter substrate-binding protein | +/+ |
| CMM_2169 | putative RTX toxin | +/+ |
| CMM_2176 | conserved secreted lipoprotein | +/+ |
| CMM_2178 | putative secreted protein | +/+ |
| CMM_2180 | putative peptide ABC transporter substrate-binding protein | +/+ |
| CMM_2185 | putative peptide ABC transporter substrate-binding protein | +/+ |
| CMM_2238 | putative sugar ABC transporter substrate-binding protein | −/+ |
| CMM_2283 | putative metal ABC transporter substrate-binding protein | +/+ |
| CMM_2349 | putative iron-siderophore ABC transporter substrate-binding protein | +/+ |
| CMM_2410 | putative sugar ABC transporter substrate-binding protein | +/+ |
| CMM_2434 | putative extracellular serine protease | +/+ |
| CMM_2438 | putative L-arabinose ABC transporter substrate-binding protein | +/+ |
| CMM_2467 | putative secreted hydrolase | +/+ |
| CMM_2505 | phosphate binding protein PstS | +/+ |
| CMM_2566 | putative branched-chain amino acid ABC transporter substrate-binding protein | +/+ |
| CMM_2628 | putative polar amino acid ABC transporter substrate-binding protein | +/+ |
| CMM_2676 | putative β-lactamase | +/+ |
| CMM_2699 | putative sugar ABC transporter substrate-binding protein | +/+ |
| CMM_2733 | putative sugar ABC transporter substrate-binding protein | +/+ |
| CMM_2842 | putative sugar ABC transporter substrate-binding protein | +/+ |
| CMM_2941 | putative metal ABC transporter substrate-binding protein | +/+ |
| pCM1_0018 | putative secreted protein | +/+ |
| pCM1_0020 | CelA | +/+ |
| pCM1_0023 | putative extracellular serine protease | |
| pCM2_0025 | putative secreted protein | +/+ |
| pCM2_0028 | conserved secreted protein | +/+ |
| pCM2_0053 | putative extracellular serine protease | +/+ |
| pCM2_0054 | Pat-1 | +/+ |
3.3. Effect of Filtration on the Detectable Secretome
| Identifier | Annotated Function | Upregulated in |
|---|---|---|
| Proteins with classical signal peptide | ||
| CMM_0166 | putative Fe3+ siderophore | M9 |
| pCM2_0042 | uncharacterized protein | XMM |
| pCM2_0054 | Pat-1 | XMM |
| CMM_0052 | putative extracellular serine protease | XMM |
| Non-classically secreted proteins | ||
| CMM_1654 | uncharacterized protein | M9 |
| CMM_1841 | putative cytochrome c oxidase | M9 |
| CMM_2064 | uncharacterized protein | M9 |
| CMM_2634 | uncharacterized protein | XMM |
| CMM_1793 | elongation factor P | M9 |
| pCM2_0042 | uncharacterized protein | XMM |
| CMM_0166 | putative Fe3+ siderophore | M9 |
| CMM_0486 | uncharacterized protein | M9 |
| CMM_0517 | putative transcriptional regulator | M9 |
| CMM_0581 | uncharacterized protein | M9 |
4. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gonzalez, A.J.; Trapiello, E. Clavibacter michiganensis subsp. phaseoli subsp. nov., pathogenic in bean. Int. J. Syst. Evol. Microbiol. 2014, 64, 1752–1755. [Google Scholar] [CrossRef] [PubMed]
- Strider, D.L. Bacterial Canker of Tomato Caused by Corynebacterium Michiganense: A Literature Review and Bibliography; North Carolina Agricultural Experiment Station: Releigh, NC, USA, 1969. [Google Scholar]
- Smith, E.F. An Introduction of Bacterial Diseases of Plants; Saunders: Philadelphia, PA, USA, 1920. [Google Scholar]
- Carlton, W.M.; Braun, E.J.; Gleason, M.L. Ingress of Clavibacter michiganensis subsp. michiganensis into tomato leaves through hydathodes. Phytopathology 1998, 88, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Dreier, J.; Meletzus, D.; Eichenlaub, R. Characterization of the plasmid encoded virulence region pat-1 of phytopathogenic clavibacter michiganensis subsp. michiganensis. Mol. Plant Microbe Interact. 1997, 10, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Tancos, M.A.; Chalupowicz, L.; Barash, I.; Manulis-Sasson, S.; Smart, C.D. Tomato fruit and seed colonization by Clavibacter michiganensis subsp. michiganensis through external and internal routes. Appl. Environ. Microbiol. 2013, 79, 6948–6957. [Google Scholar] [CrossRef] [PubMed]
- Hausbeck, M.K.; Bell, J.; Medina-Mora, C.; Podolsky, R.; Fulbright, D.W. Effect of bactericides on population sizes and spread of Clavibacter michiganensis subsp. michiganensis on tomatoes in the greenhouse and on disease development and crop yield in the field. Phytopathology 2000, 90, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Fatmi, M.S.N.W. Semiselective agar medium for isolation of Clavibacter michiganense subsp. michiganense from tomato seed. Phytopathology 1988, 78, 121–126. [Google Scholar] [CrossRef]
- Gitaitis, R.D.; Beaver, R.W.; Voloudakis, A.E. Detection of Clavibacter michiganensis subsp. michiganensis in symptomless tomato transplants. Plant Dis. 1991, 74, 834–838. [Google Scholar] [CrossRef]
- Jahr, H.; Dreier, J.; Meletzus, D.; Bahro, R.; Eichenlaub, R. The endo-β-1,4-glucanase CelA of Clavibacter michiganensis subsp. michiganensis is a pathogenicity determinant required for induction of bacterial wilt of tomato. Mol. Plant Microbe Interact. 2000, 13, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Kaup, O.; Gräfen, I.; Zellermann, E.M.; Eichenlaub, R.; Gartemann, K.H. Identification of a tomatinase in the tomato-pathogenic actinomycete Clavibacter michiganensis subsp. michiganensis NCPPB382. Mol. Plant Microbe Interact. 2005, 18, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Gartemann, K.H.; Abt, B.; Bekel, T.; Burger, A.; Engemann, J.; Flügel, M.; Gaigalat, L.; Goesmann, A.; Gräfen, I.; Kalinowski, J.; et al. The genome sequence of the tomato-pathogenic actinomycete Clavibacter michiganensis subsp. michiganensis NCPPB382 reveals a large island involved in pathogenicity. J. Bacteriol. 2008, 190, 2138–2149. [Google Scholar] [CrossRef] [PubMed]
- Stork, I.; Gartemann, K.H.; Burger, A.; Eichenlaub, R. A family of serine proteases of Clavibacter michiganensis subsp. michiganensis: ChpC plays a role in colonization of the host plant tomato. Mol. Plant Pathol. 2008, 9, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Eichenlaub, R.; Gartemann, K.H. The Clavibacter michiganensis subspecies: Molecular investigation of gram-positive bacterial plant pathogens. Annu. Rev. Phytopathol. 2011, 49, 445–464. [Google Scholar] [CrossRef] [PubMed]
- Savidor, A.; Chalupowicz, L.; Teper, D.; Gartemann, K.H.; Eichenlaub, R.; Manulis-Sasson, S.; Barash, I.; Sessa, G. Clavibacter michiganensis subsp. michiganensis Vatr1 and Vatr2 transcriptional regulators are required for virulence in tomato. Mol. Plant Microbe Interact. 2014, 27, 1035–1047. [Google Scholar] [CrossRef] [PubMed]
- Bentley, S.D.; Corton, C.; Brown, S.E.; Barron, A.; Clark, L.; Doggett, J.; Harris, B.; Ormond, D.; Quail, M.A.; May, G.; et al. Genome of the actinomycete plant pathogen Clavibacter michiganensis subsp. sepedonicus suggests recent niche adaptation. J. Bacteriol. 2008, 190, 2150–2160. [Google Scholar] [CrossRef] [PubMed]
- Monteiro-Vitorello, C.B.; Camargo, L.E.; van Sluys, M.A.; Kitajima, J.P.; Truffi, D.; do Amaral, A.M.; Harakava, R.; de Oliveira, J.C.; Wood, D.; de Oliveira, M.C.; et al. The genome sequence of the gram-positive sugarcane pathogen Leifsonia xyli subsp. xyli. Mol. Plant Microbe Interact. 2004, 17, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Flügel, M.; Becker, A.; Gartemann, K.H.; Eichenlaub, R. Analysis of the interaction of Clavibacter michiganensis subsp. michiganensis with its host plant tomato by genome-wide expression profiling. J. Biotechnol. 2012, 160, 42–54. [Google Scholar]
- Savidor, A.; Teper, D.; Gartemann, K.H.; Eichenlaub, R.; Chalupowicz, L.; Manulis-Sasson, S.; Barash, I.; Tews, H.; Mayer, K.; Giannone, R.J.; et al. The Clavibacter michiganensis subsp. michiganensis-tomato interactome reveals the perception of pathogen by the host and suggests mechanisms of infection. J. Proteome Res. 2012, 11, 736–750. [Google Scholar] [CrossRef] [PubMed]
- Hiery, E.; Adam, S.; Reid, S.; Hofmann, J.; Sonnewald, S.; Burkovski, A. Genome-wide transcriptome analysis of clavibacter michiganensis subsp. michiganensis grown in xylem mimicking medium. J. Biotechnol. 2013, 168, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Chalupowicz, L.; Cohen-Kandli, M.; Dror, O.; Eichenlaub, R.; Gartemann, K.H.; Sessa, G.; Barash, I.; Manulis-Sasson, S. Sequential expression of bacterial virulence and plant defense genes during infection of tomato with Clavibacter michiganensis subsp. michiganensis. Phytopathology 2010, 100, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Hansmeier, N.; Chao, T.C.; Pühler, A.; Tauch, A.; Kalinowski, J. The cytosolic, cell surface and extracellular proteomes of the biotechnologically important soil bacterium Corynebacterium efficiens YS-314 in comparison to those of Corynebacterium glutamicum ATCC 13032. Proteomics 2006, 6, 233–250. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Proteomes–Clavibacter michiganensis subsp. michiganensis (strain NCPPB 382). Available online: http://www.uniprot.org/proteomes/UP000001564 (accessed on 15 April 2015).
- Amin, B.; Maurer, A.; Voelter, W.; Melms, A.; Kalbacher, H. New poteintial serum biomarkers in multiple sclerosis identified by proteomic strategies. Curr. Med. Chem. 2014, 21, 1544–1556. [Google Scholar] [CrossRef] [PubMed]
- Maurer, A.; Zeyher, C.; Amin, B.; Kalbacher, H. A periodate-cleavable linker for functional proteomics under slightly acidic conditions: Application for the analysis of intracellular aspartic proteases. J. Proteome Res. 2012, 12, 199–207. [Google Scholar] [CrossRef] [PubMed]
- SignalP 4.1 Server. Available online: http://www.cbs.dtu.dk/services/SignalP/ (accessed on 27 October 2015).
- SecretomeP 2.0 Server. Available online: http://www.cbs.dtu.dk/services/SecretomeP/ (accessed on 27 October 2015).
- Comprehensive Microbial Resource. Available online: http://cmr.jcvi.org/tigr-scripts/CMR/CmrHomePage.cgi (accessed on 17 July 2015).
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hiery, E.; Poetsch, A.; Moosbauer, T.; Amin, B.; Hofmann, J.; Burkovski, A. A Proteomic Study of Clavibacter Michiganensis Subsp. Michiganensis Culture Supernatants. Proteomes 2015, 3, 411-423. https://doi.org/10.3390/proteomes3040411
Hiery E, Poetsch A, Moosbauer T, Amin B, Hofmann J, Burkovski A. A Proteomic Study of Clavibacter Michiganensis Subsp. Michiganensis Culture Supernatants. Proteomes. 2015; 3(4):411-423. https://doi.org/10.3390/proteomes3040411
Chicago/Turabian StyleHiery, Eva, Ansgar Poetsch, Tanja Moosbauer, Bushra Amin, Jörg Hofmann, and Andreas Burkovski. 2015. "A Proteomic Study of Clavibacter Michiganensis Subsp. Michiganensis Culture Supernatants" Proteomes 3, no. 4: 411-423. https://doi.org/10.3390/proteomes3040411





