Detection of 1,5-anhydroglucitol as a Biomarker for Diabetes Using an Organic Field-Effect Transistor-Based Biosensor
Abstract
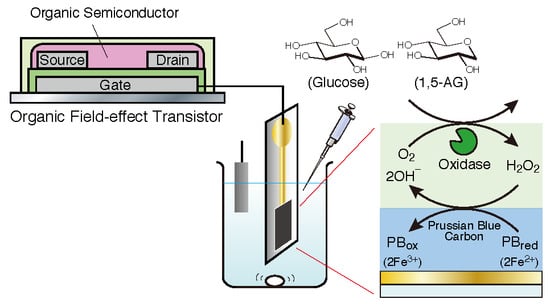
:1. Introduction
2. Materials and Methods
2.1. Materials
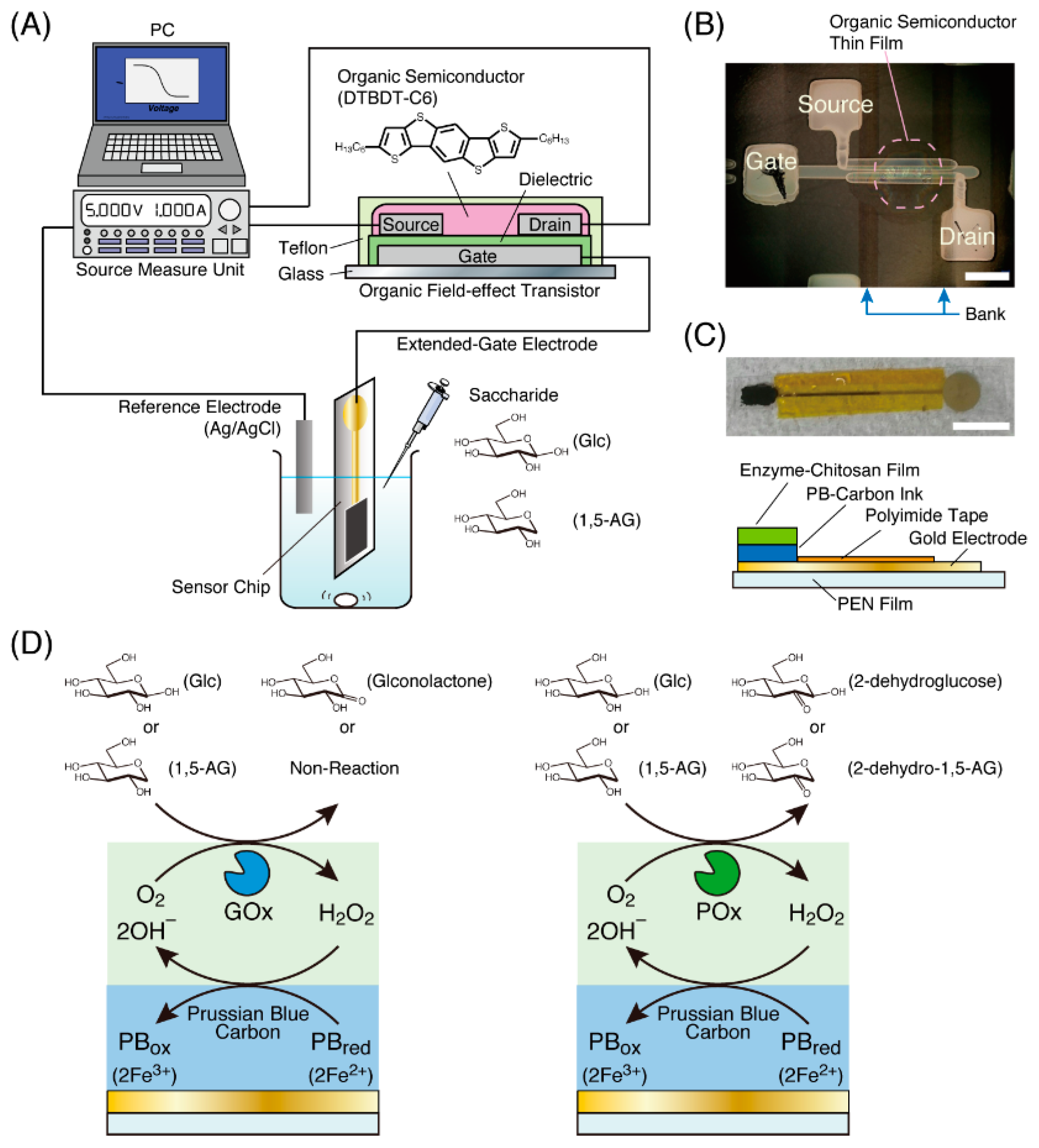
2.2. Fabrication of an OFET Device
2.3. Preparation of an Enzyme Electrode for Biomarker Detection
2.4. Measurement of 1,5-AG or Glucose Detection with an OFET-Based Biosensor
3. Results and Discussion
3.1. Characterization of the OFET Device
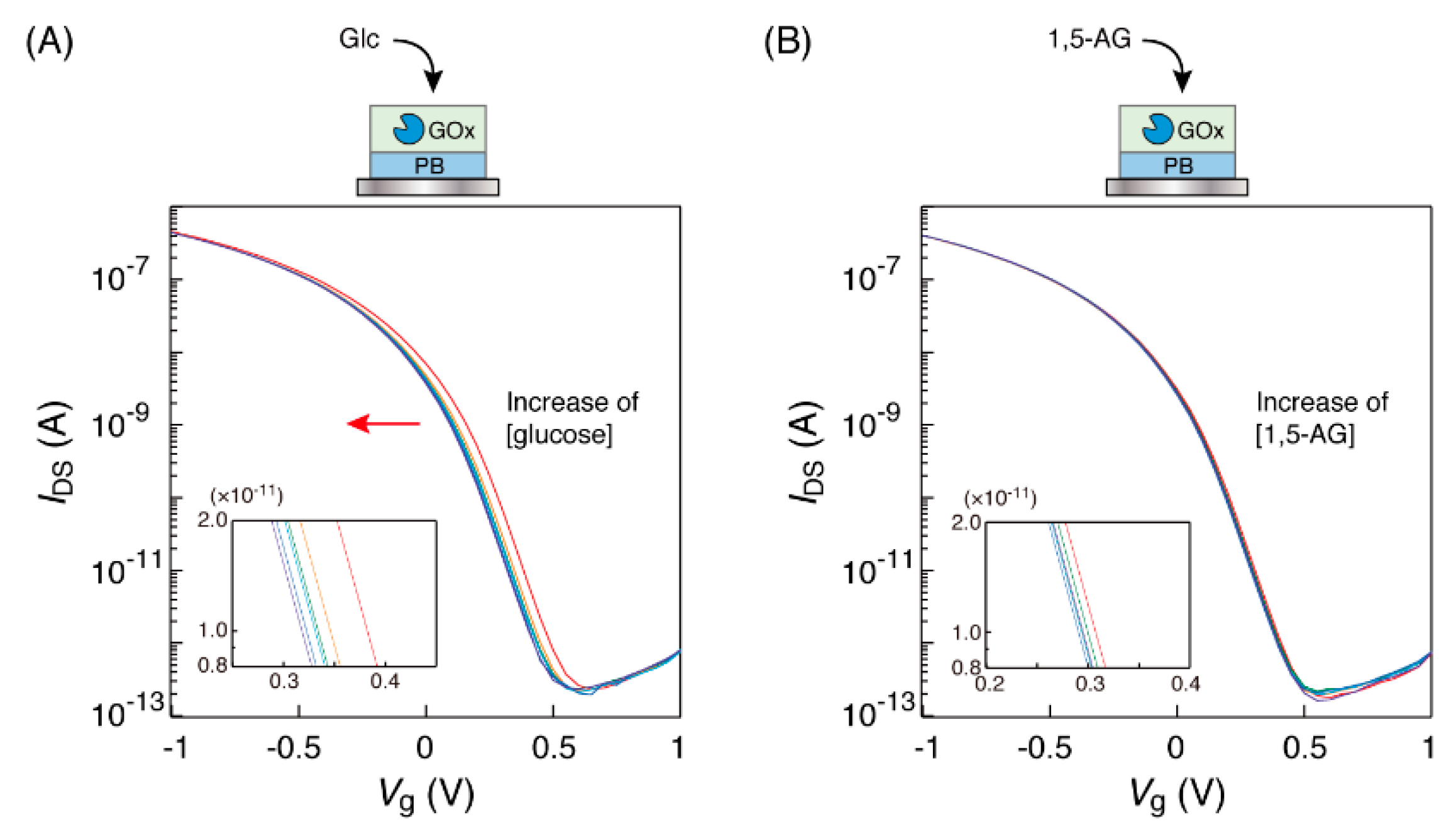
3.2. Observation of the Transfer Characteristic Curves of the OFET Equipped with a GOx-PB Electrode
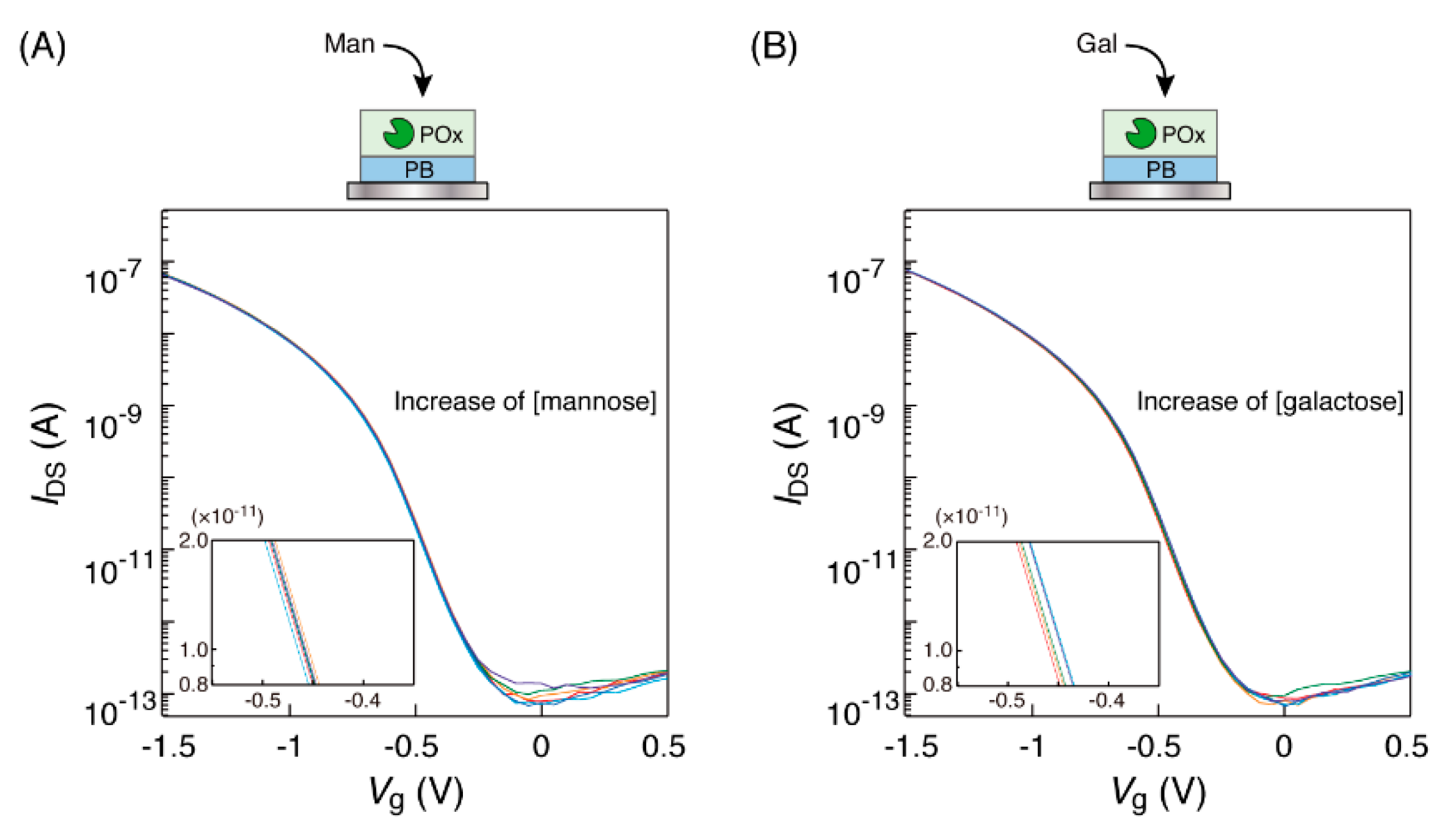
3.3. Observation of the Transfer Characteristic Curves of The OFET Equipped with A POx-PB Electrode
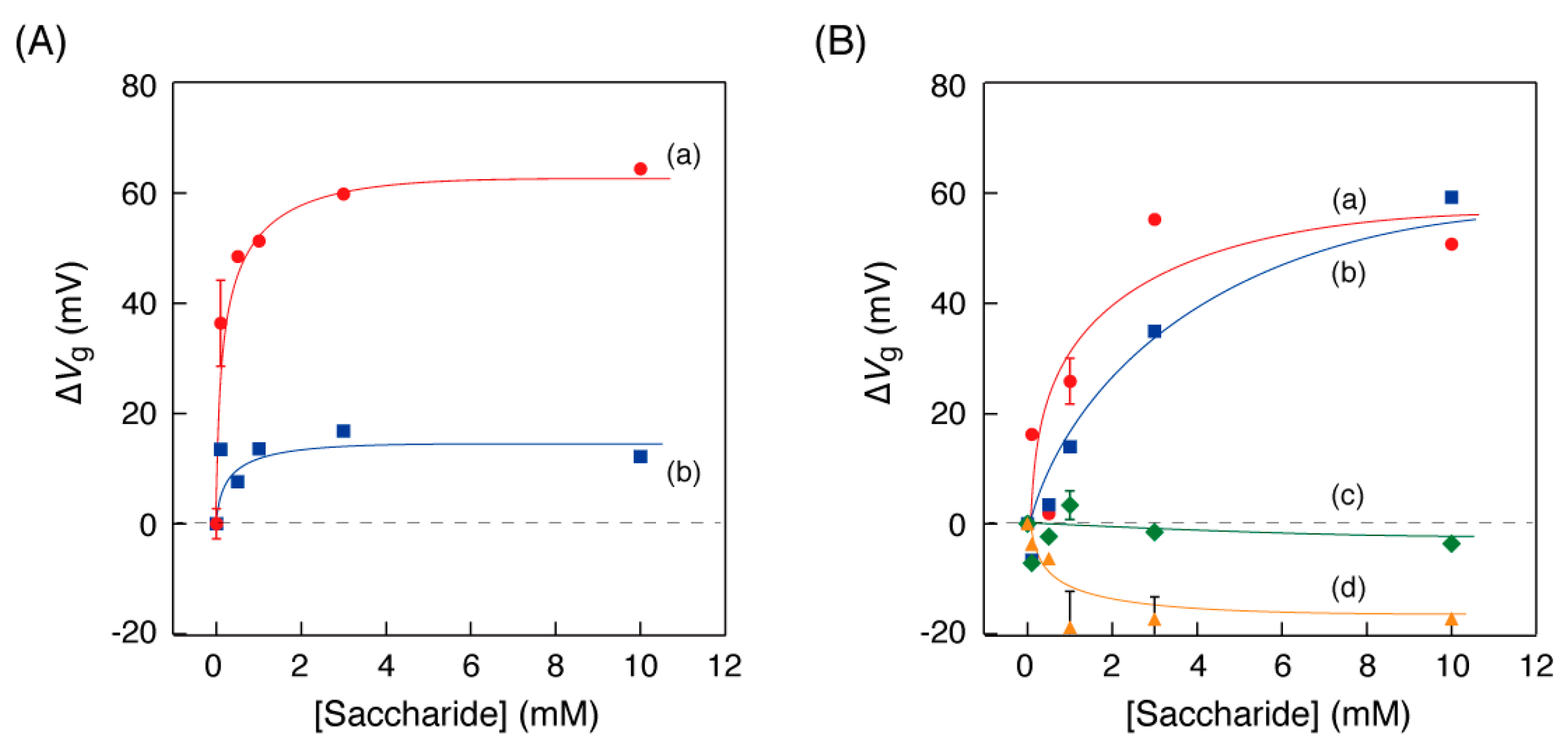
3.4. Relationship between Saccharide Concentration and Curve Shift
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Karlsson, R. SPR for molecular interaction analysis: A review of emerging application areas. J. Mol. Recognit. 2004, 17, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.P.; Kougianos, E. Biosensors: A tutorial review. IEEE Potentials 2006, 25, 35–40. [Google Scholar] [CrossRef]
- Yakovleva, M.; Bhand, S.; Danielsson, B. The enzyme thermistor—A realistic biosensor concept. A critical review. Anal. Chim. Acta 2013, 766, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, P.R.; Verma, M.; Zhao, Y.; Srivastava, S. Proteomics for Cancer Biomarker Discovery. Clin. Chem. 2002, 48, 1160–1169. [Google Scholar] [PubMed]
- Hayes, J.; Peruzzi, P.P.; Lawler, S. MicroRNAs in cancer: Biomarkers, functions and therapy. Trends Mol. Med. 2014, 20, 460–469. [Google Scholar]
- Matheson, A.; Willcox, M.D.P.; Flanagan, J.; Walsh, B.J. Urinary biomarkers involved in type 2 diabetes: A review. Diabetes Metab. Res. Rev. 2010, 26, 150–171. [Google Scholar] [CrossRef] [PubMed]
- Suhre, K.; Meisinger, C.; Döring, A.; Altmaier, E.; Belcredi, P.; Gieger, C.; Chang, D.; Milburn, M.V.; Gall, W.E.; Weinberger, K.M.; et al. Metabolic Footprint of Diabetes: A Multiplatform Metabolomics Study in an Epidemiological Setting. PLoS ONE 2010, 5, e13953. [Google Scholar] [CrossRef] [PubMed]
- Jani, I.V.; Peter, T.F. How Point-of-Care Testing Could Drive Innovation in Global Health. N. Engl. J. Med. 2013, 368, 2319–2324. [Google Scholar] [CrossRef] [PubMed]
- Homola, J.; Yee, S.S.; Gauglitz, G. Surface plasmon resonance sensors: Review. Sens. Actuators B Chem. 1999, 54, 3–15. [Google Scholar] [CrossRef]
- Addas, A.; Linman, M.J.; Cheng, Q. New trends in instrumental design for surface plasmon resonance-based biosensors. Biosens. Bioelectron. 2011, 26, 1815–1824. [Google Scholar] [Green Version]
- Becker, B.; Cooper, M.A. A survey of the 2006–2009 quartz crystal microbalance biosensor literature. J. Mol. Recognit. 2011, 24, 754–787. [Google Scholar] [CrossRef] [PubMed]
- Alassi, A.; Benammar, M.; Brett, D. Quartz Crystal Microbalance Electronic Interfacing Systems: A Review. Sensors 2017, 17, 2799. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Electrochemical biosensors: Towards point-of-care cancer diagnostics. Biosens. Bioelectron. 2006, 21, 1887–1892. [Google Scholar] [CrossRef] [PubMed]
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical Biosensors - Sensor Principles and Architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furusawa, H.; Ichimura, Y.; Harada, S.; Uematsu, M.; Xue, S.; Nagamine, K.; Tokito, S. Electric Charge Detection of Sparse Organic Acid Molecules Using an Organic Field-Effect Transistor (OFET)-Based Sensor. Bull. Chem. Soc. Jpn. 2018, 91, 1020–1025. [Google Scholar] [CrossRef]
- Someya, T.; Sekitani, T.; Iba, S.; Kato, Y.; Kawaguchi, H.; Sakurai, T. A large-area, flexible pressure sensor matrix with organic field-effect transistors for artificial skin applications. Proc. Natl. Acad. Sci. USA 2004, 101, 9966–9970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minami, T.; Minamiki, T.; Hashima, Y.; Yokoyama, D.; Sekine, T.; Fukuda, K.; Kumaki, D.; Tokito, S. An extended-gate type organic field effect transistor functionalized by phenylboronic acid for saccharide detection in water. Chem. Commun. 2014, 50, 15613–15615. [Google Scholar] [CrossRef] [PubMed]
- Minami, T.; Sato, T.; Minamiki, T.; Fukuda, K.; Kumaki, D.; Tokito, S. A novel OFET-based biosensor for the selective and sensitive detection of lactate levels. Biosens. Bioelectron. 2015, 74, 45–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minamiki, T.; Minami, T.; Sasaki, Y.; Wakida, S.; Kurita, R.; Niwa, O.; Tokito, S. Label-free detection of human glycoprotein (CgA) using an extended-gated organic transistor-based immunosensor. Sensors 2016, 16, 2033. [Google Scholar] [CrossRef] [PubMed]
- Yamanouchi, T.; Minoda, S.; Yabuuchi, M.; Akanuma, Y.; Akanuma, H.; Miyashita, H.; Akaoka, I. Plasma 1,5-anhydro-D-glucitol as new clinical marker of glycemic control in NIDDM patients. Diabetes 1989, 38, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Nowatzke, W.; Sarno, M.J.; Birch, N.C.; Stickle, D.F.; Eden, T.; Cole, T.G. Evaluation of an assay for serum 1,5-anhydroglucitol (GlycoMark™) and determination of reference intervals on the Hitachi 917 analyzer. Clin. Chem. Acta 2004, 350, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Fukumura, Y.; Tajima, S.; Oshitani, S.; Ushijima, Y.; Kobayashi, I.; Hara, F.; Yamamoto, S.; Yabuuchi, M. Fully enzymatic method for determining 1,5-anhydro-D-glucitol in serum. Clin. Chem. 1994, 40, 2013–2016. [Google Scholar] [PubMed]
- Shiwaku, R.; Matsui, H.; Nagamine, K.; Uematsu, M.; Mano, T.; Maruyama, Y.; Nomura, A.; Tsuchiya, K.; Hayasaka, K.; Takeda, Y.; et al. A Printed Organic Circuit System for Wearable Amperometric Electrochemical Sensors. Sci. Rep. 2018, 8, 6368. [Google Scholar] [CrossRef] [PubMed] [Green Version]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furusawa, H.; Ichimura, Y.; Nagamine, K.; Shiwaku, R.; Matsui, H.; Tokito, S. Detection of 1,5-anhydroglucitol as a Biomarker for Diabetes Using an Organic Field-Effect Transistor-Based Biosensor. Technologies 2018, 6, 77. https://doi.org/10.3390/technologies6030077
Furusawa H, Ichimura Y, Nagamine K, Shiwaku R, Matsui H, Tokito S. Detection of 1,5-anhydroglucitol as a Biomarker for Diabetes Using an Organic Field-Effect Transistor-Based Biosensor. Technologies. 2018; 6(3):77. https://doi.org/10.3390/technologies6030077
Chicago/Turabian StyleFurusawa, Hiroyuki, Yusuke Ichimura, Kuniaki Nagamine, Rei Shiwaku, Hiroyuki Matsui, and Shizuo Tokito. 2018. "Detection of 1,5-anhydroglucitol as a Biomarker for Diabetes Using an Organic Field-Effect Transistor-Based Biosensor" Technologies 6, no. 3: 77. https://doi.org/10.3390/technologies6030077