Effect of Post-Harvest Traditional Technologies on the Nutrient Content and Antioxidant Compounds of Defatted Flours from Ricinodendron heudelotti (Baill. Pierre ex Pax) Seed Kernels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Chemicals and Reagents
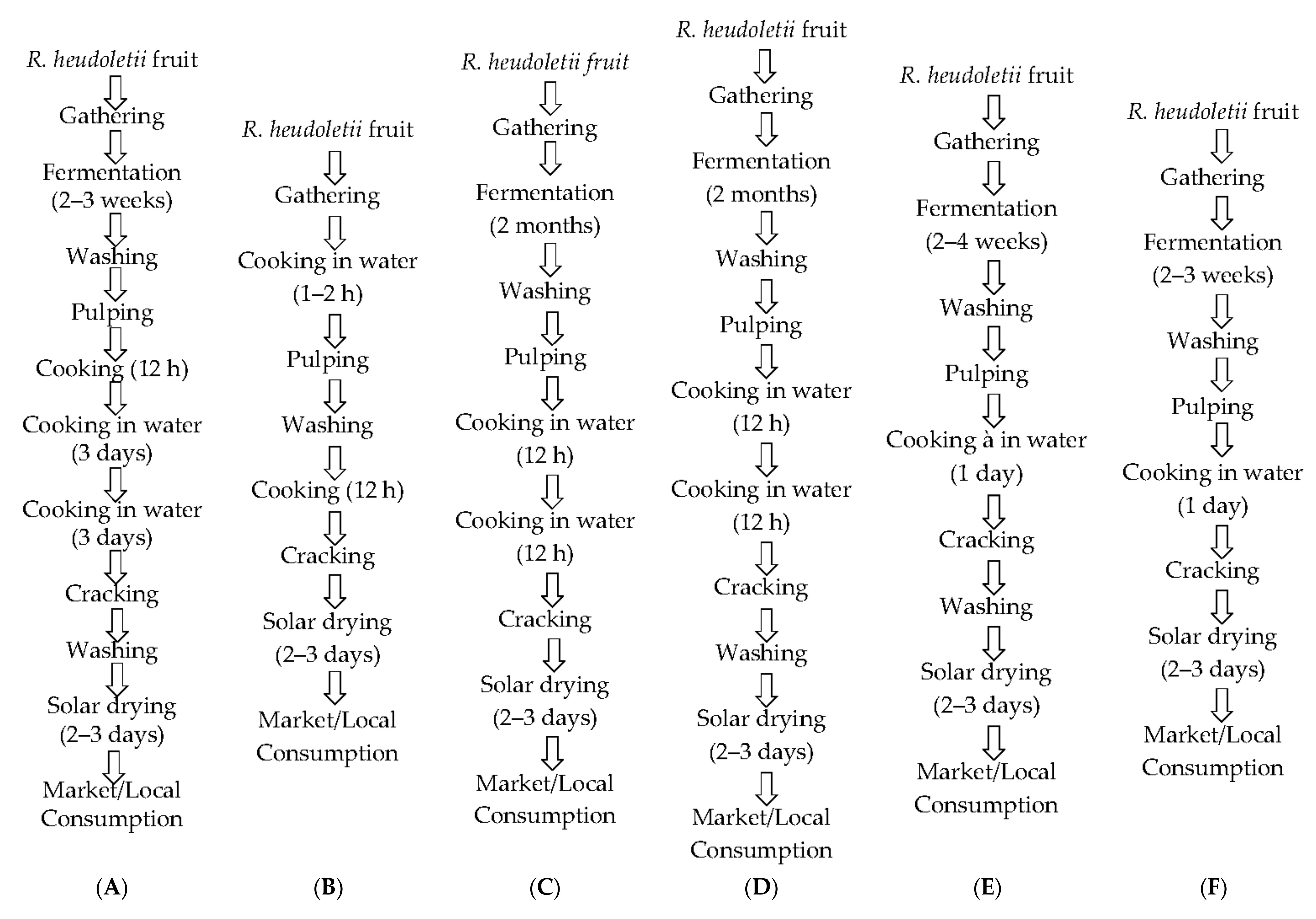
2.2. Sampling and Sample Preparation
2.3. Proximate Analysis of Defatted Flours from R. heudelotti Seeds Kernels
2.4. Mineral Composition of Defatted Flours from R. heudelotti Seeds Kernels
2.5. pH Determination of Defatted Flours from R. heudelotti Seeds Kernels
2.6. Total Titrable Acidity (TTA) of Defatted Flours from R. heudelotti Seeds Kernels
2.7. Phytochemical Analysis of Defatted Flours from R. heudelotti Seeds Kernels
2.7.1. Total Flavonoids Determination
2.7.2. Phytic Acid Content
2.7.3. Tannins
2.7.4. Oxalates
2.7.5. Total Phenolic Assay
2.8. Statistical Analyses
3. Results and Discussion
MDS Applied to Defatted Flours from R. heudelotii Sample Data
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kouakou, A.K.; Barima, Y.S.S.; Zanh, G.G.; Traoré, K.; Bogaert, J. Inventory and availability of non-timber forest products used by local residents of the classified forest of haut-Sassandra after the armed conflict period in Ivory Coast. Tropicultura 2017, 35, 121–136. [Google Scholar]
- Cosyns, H. Ricinodendron heudelotii Kernel Group Commercialization and Its Impact on Farmers’ Livelihoods in Cameroon. Ph.D. Thesis, Faculty of Bioscience Engineering, Ghent University, Ghent, Belgium, 2013. [Google Scholar]
- Léonard, J. Notulae systematicae XXXII. Observations on African species of Clutia, Ricinodendron and Sapium (Euphorbiaceae). Bull. Jardin Botan. l’État Bruxelles 1961, 31, 391–406. [Google Scholar] [CrossRef]
- Assanvo, F.E.; Gogoi, P.; Dolui, K.S.; Baruah, D.S. Synthesis, characterization, and performance characteristics of alkyd resins based on Ricinodendron heudelotii oil and their blending with epoxy resins. Ind. Crops Prod. 2015, 65, 293–302. [Google Scholar] [CrossRef]
- Saki, J.S.; Mosso, K.; Sea, B.T.; Diopoh, J.K. Determination of some essential components of Ricinodendron heudelotii (or akpi) in Cote d’Ivoire. Agron. Afr. 2005, 17, 137–142. [Google Scholar]
- Mezajoug, K.L.B.; Tchiégang, C. Physico chemical properties of defatted flours from Ricinodendron heudelotii (Bail.) and Tetracarpidium conophorum (müll. arg.) with respect to particle sizes. Glob. Adv. Res. J. Agric. Sci. 2016, 5, 94–102. [Google Scholar]
- Tchiégang, C.; Kapseu, C.; Ndjouenkeu, R.; Ngassoum, M.B. Ricinodendron heudelotii (Bail.) kernels: A novel ingredient for tropical food agro-industries. J. Food Eng. 1997, 32, 1–10. [Google Scholar] [CrossRef]
- Li, D. Proteins from land plants and potential resources for human nutrition and food security. Trends Food Sci. Technol. 2013, 32, 25–42. [Google Scholar]
- Mvomo, E.J. Training report of the basic communities in techniques of valorization of essessang / njansang seeds (Ricinodendron heudelotii). Projet GCP/RAF/441/GER, Strengthening food security in Central Africa through sustainable management of non-timber forest products, 2012; 44p. Available online: http://www.fao.org/docrep/field/009/an902f/an902f00.pdf (accessed on 23 February 2016).
- Womeni, H.M.; Ndjouenkeu, R.; Kapseu, C.; Tchouanguep Mbiapo, F.; Parmentier, M.; Fanni, J. Effect of Cooking and Drying of Shea Nuts (Butyrospermum parkii (G. Don) Kotschy) on the Quality of Butter. Tropicultura 2006, 24, 175–182. [Google Scholar]
- Udo, O.; Ime, A.; Akpan, A.E. Phytochemical screening of Ricinodendron heudelotii (euphorbiaceae) for insecticidal activity in the control of two storage insect pests. Int. J. Adv. Biotechnol. Res. 2017, 7, 156–162. [Google Scholar]
- Meva, E.F.; Ebongue, O.C.; Fannang, V.S.; Segnou, L.M.; Ntoumba, A.A.; Kedi, E.P.B.; Loudang, N.R.-E.; Wanlao, Y.A.; Mang, R.E.; Mpondo, M.A.E. Natural substances for the synthesis of silver nanoparticles against Escherichia coli: The Case of Megaphrynium macrostachyum (Marantaceae), Corchorus olitorus (Tiliaceae), Ricinodendron heudelotii (Euphorbiaceae), Gnetum bucholzianum (Gnetaceae), and Ipomoea batatas (Convolvulaceae). J. Nanomater. 2017, 2017, 6834726. [Google Scholar] [CrossRef]
- Nzali, G.H.; Tchiegang, C.; Sandjon, B.; Meurens, M. Comparison of some physicochemical properties of oil extracted from Ricinodendron heudelotii (Bail.) kernels by UV spectrophotometer. Int. J. Biosci. 2016, 8, 93–102. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1990. [Google Scholar]
- Joint FAO/WHO Expert Consultation. Carbohydrates in Human Nutrition; FAO Food and Nutrition Paper 66; FAO: Rome, Italy, 14–18 April 1997; Available online: http://www.fao.org/docrep/W8079E/w8079e0a.htm (accessed on 21 August 2016).
- Prosky, L.; Asp, N.G.; Schweizer, T.F.; de Vries, J.W.; Furda, I. Determination of insoluble, soluble, and total dietary fiber in foods and food products: Interlaboratory study. J. Assoc. Off. Anal. Chem. 1988, 71, 1017–1023. [Google Scholar] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for the determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bernfeld, P. Alpha and beta-amylases. In Methods in Enzymology; Colowick, S.P., Kaplan, N., Eds.; Academic Press: New York, NY, USA, 1955; Volume 1, pp. 149–158. [Google Scholar]
- Atwater, W.; Rosa, E. A new respiratory calorimeter and the conservation of energy in human body. Phys. Rev. 1899, 9, 214–251. [Google Scholar]
- American Association of Cereal Chemists (AACC). Official Method 40-70.01: Elements by atomic absorption spectrophotometry. In Approved Methods of the American Association of Cereal Chemists, 11th ed.; AACC International: St. Paul, MN, USA, 1999. [Google Scholar]
- Fiske, C.H.; Subbarow, Y. The colorimetric determination of phosphorus. J. Biol. Chem. 1925, 66, 375–400. [Google Scholar]
- Meda, A.; Laien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of total phenolic, flavonoid and proline contents in Burkina Faso honeys as well as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Mohammed, A.I.; Ponnamperuma, A.J.P.; Hafez, Y.S. New Chromophore method for phytic acid determination. Am. Assoc. Cereal Chem. 1986, 63, 475–478. [Google Scholar]
- Bainbridge, Z.; Tomlins, K.; Westby, A. Methods for Assessing Quality Characteristic of Non-Grains Starch (Part 3. Laboratory Methods); Natural Ressources Institute: Chathom, UK, 1996. [Google Scholar]
- Day, R.A.; Underwood, A.L. Quantitative Analysis, 5th ed.; Prentice Hall Publication: London, UK, 1986; pp. 1–701. [Google Scholar]
- N’Dri, D.; Mazzeo, T.; Zaupa, M.; Ferracane, R.; Fogliano, V.; Pellegrini, N. Effect of cooking on the total antioxidant capacity and phenolic profile of some whole-meal African cereals. J. Sci. Food Agric. 2013, 93, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Ogungbenle, H.N.; Oshodi, A.A.; Oladimeji, M.O. The proximate and effect of salt applications on some functional properties of quinoa (Chenopodium quinoa) flour. Pak. J. Nutr. 2009, 8, 49–52. [Google Scholar] [CrossRef]
- Yagoub, A.A.; Abdalla, A.A. Effect of domestic processing methods on chemical composition, in vitro digestibility of protein and starch and functional properties of bambara groundnut (Voandzeia subterranea) seed. Res. J. Agric. Biol. Sci. 2007, 3, 24–34. [Google Scholar]
- Ragab, D.M.; Babiker, E.E.; El Tinay, A.H. Fractionation, solubility and functional properties of cowpea (Vigna unguiculata) proteins as affected by pH and/or salt concentration. Food Chem. 2004, 84, 207–212. [Google Scholar] [CrossRef]
- Tchiegang, C.; Mezajoug, K.L.; Tenin, D.; Ndjouenkeu, R. Physicochemical and functional properties of defatted cakes from two Euphorbiaceae from Cameroon: Ricinodendron heudelotii (Bail) and Tetracarpidium conophorum (Müll. Arg.). J. Food Technol. 2006, 4, 96–100. [Google Scholar]
- Adeoti, O.A.; Alabi, A.O.; Adedokun, S.O.; Jimoh, K.O.; Elutilo, O.O.; Azeez, L.A. Influence of Processing Methods on the Nutrient, Anti-Nutrient, Mineral Compositions and Functional Properties of Akee Apple (Blighia Sapida Konig) Seed and Aril Flour. J. Hum. Nutr. Food Sci. 2017, 5, 1101. [Google Scholar]
- Fabbrin, D.T.A.; Crosby, G.A. A review of the impact of preparation and cooking on the nutritional quality of vegetables and legumes. Int. J. Gastron. Food Sci. 2016, 3, 2–11. [Google Scholar] [CrossRef]
- Mezajoug, K.L.B.; Arab-Tehrany, E.; Tchiégang, C.; Linder, M. Compositional and nutritional studies of two defatted flours obtained from Ricinodendron heudelotii (Bail.) and Tetracarpidium conophorum (Müll. Arg.). Food 2011, 5, 52–57. [Google Scholar]
- D’Evoli, L.; Lucarini, M.; Gabrielli, P.; Aguzzi, A.; Lombardi-Boccia, G. Nutritional Value of Italian Pistachios from Bronte (Pistacia vera, L.), Their Nutrients, Bioactive Compounds and Antioxidant Activity. Food Nutr. Sci. 2015, 6, 1267–1276. [Google Scholar] [CrossRef]
- Ibeji, C.C. Effect of different processing methods on the nutritional composition of three different cultivars of Dioscorea bulbifera. Bachelor’s Thesis, Food Science and Technology, Michael Okpara University of Agriculture, Umudike, Nigeria, 2011; p. 29. [Google Scholar]
- Fombang, E.N.; Agamou, J.A.A.; Mbofung, C.M.F. Incorporation of Ricinodendron heudolotti meal into blends of wheat and precooked taro flour for production of nutrient dense biscuits. Indian J. Nutr. 2016, 3, 131. [Google Scholar]
- Mezajoug, K.B.L. Nutritional and Functional Properties, Concentrate and Isolate Proteins of Ricinodendron Heudelotii Oilseed (Bail). Pierre ex Pax and Tetracarpidium conophorum (Müll. Arg). Ph.D. Thesis, Ecole Nationale Supérieure des Sciences Agro—Industrielles, Laboratoire de Biochimie et Technologie Alimentaire, Nancy, France, 2010; 226p. [Google Scholar]
- Chapeland-Leclerc, F.; Papon, N.; Noël, T.; Villard, J. Molds and food risks (Mycotoxicoses). Rev. Franç. Lab. 2000, 373, 61–66. [Google Scholar]
- Aletor, V.A.; Aladetimi, O.O. Compositional evaluation of some cowpea varieties and some under-utilized edible legumes in Nigeria. Die Nahrung 1989, 33, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Andualem, B.; Gessesse, A. Proximate composition, mineral content and antinutritional factors of Brebra (Millettia ferruginea) seed flour as well as physicochemical characterization of its seed oil. SpringerPlus 2014, 3, 298. [Google Scholar] [CrossRef] [PubMed]
- Bezzala, A. Argan Tree Introduction Test in Amdoukal Area and Evaluation of Some Parameters of Resistance to Drought. Master’s Thesis, University El Hadj Lakhdar, Batna, Algeria, 2005; 106p. [Google Scholar]
- Pomeranz; Clifton, D. Properties of defatted soybean, peanut field pea and pecan flours. J. Food Sci. 1981, 42, 1440–1450. [Google Scholar]
- Mayombo, P.A.; Baldwin, P.; Wathelet, J.; Marlier, M.; Istasse, L. Incorporation of rapeseed meal extracted by pressure in a diet for growing fattening bulls. I. Intake, digestibility and fermentation in the rumen. I. Ingestion, digestibilite et fermentation dans le rumen. Ann. Zootech. INRA/EDP Sci. 1997, 46, 57–70. [Google Scholar] [CrossRef]
- Treche, S.; Tchiloemra-Poba, G.; Gallon, J.M. Variation Factors in the Quality of Cassava Jours Produced at the Village Level in the Congo; Agbor Egbe, T., Brauman, A., Griffon, D., Trèche, S., Eds.; Colloques et Séminaires; ORSTOM: Congo, 1995; pp. 450–460. ISBN 2-7099-1279-1. Available online: https://core.ac.uk/download/pdf/39854995.pdf (accessed on 2 August 2016).
- Sabo, H.; Sadou, H.S.; Mahamane, L.C-L. Global chemical composition of seeds and physicochemical characteristics of Luffa aegyptiaca and Luffa cylindrica oils from Niger. J. Soc. Ouest-Afr. Chim. 2005, 20, 119–133. [Google Scholar]
- Al-wahsh, A.I.; Horner, T.H.; Palmer, G.R.; Reddy, B.M.; Massey, K.L. Oxalate and Phytate of Soy Foods. J. Agric. Food Chem. 2005, 53, 5670–5674. [Google Scholar] [CrossRef] [PubMed]
- Sauvant, D.; Perez, J.M.; Tran, G. INRA-AFZ Tables of Composition and Nutritional Value of Feed Materials for Livestock, 2nd ed.; INRA Editions Versailles: Paris, France, 2004; 306p, ISBN 2738011586. [Google Scholar]
- Lestradet, H.; Machinot, S. Daily calcium intake. Cah. Nutr. Diet. 1990, 25, 135–137. [Google Scholar]
- FAO/WHO. Requirement of Vitamin A, Iron, Folate and Vitamin B12; Report of a Joint Expert Consultation; FAO/WHO: Rome, Italy, 1988. [Google Scholar]
- Vasco, C.; Ruales, J.; Kamal-Eldin, A. Total phenolic compounds and antioxidant capacities of major fruits from Ecuador. Food Chem. 2008, 111, 816–823. [Google Scholar] [CrossRef]
- Mehinagic, E.; Bourles, E.; Jourjon, F. Nutritional quality of fruits: Impact of processing on phenolic compounds. Rev. Suisse Vitic. Arboric. Horticult. 2011, 43, 364–368. [Google Scholar]
- Odinga, T.; Worlu-Wodu, Q.E.; Deekae, S. Bioprospective Screening of Ricinodendron Heudelotii Seeds. J. Anal. Pharm. Res. 2016, 3, 84. [Google Scholar] [CrossRef]
- Bolanho, B.C.; Beléia, A.P. Bioactive compounds and antioxidant potential of soy products. Alim. Nutr. Araraquara 2011, 22, 539–546. [Google Scholar]
- Sowndhararajan, K.; Siddhuraju, P.; Manian, S. Antioxidant and free radical scavenging capacity of the underutilized legume, Vigna vexillata (L.) A. rich. J. Food Compos. Anal. 2011, 24, 160–165. [Google Scholar] [CrossRef]
- Gill, L.S. Ethnomedical Uses of Plants in Nigeria; University of Benin: Benin City, Nigeria, 1992; pp. 1–268. [Google Scholar]
- Zhang, M.; Hettiarachchy, S.N.; Horax, R.; Kannan, A.; Praisoody, M.D.A.; Muhundan, A.; Mallangi, C.R. Phytochemicals, antioxidant and antimicrobial activity of Hibiscus sabdariffa, Centella asiatica, Moringa oleifera and Murraya koenigii leaves. J. Med. Plants Res. 2011, 5, 6672–6680. [Google Scholar]
- N’Dri, Y.D.; Kouakou, K.N.; Erba, D.; Scazzina, F.; Pellegrini, N.; Casiraghi, C.M. Nutritive Evaluation of the Bambara Groundnut Ci12 Landrace (Vigna subterranea (L.) Verdc. (Fabaceae)) Produced in Côte d’Ivoire. Int. J. Mol. Sci. 2015, 16, 21428–21441. [Google Scholar]
- Fekadu, H.; Beyene, F.; Desse, G. Effect of traditional processing methods on nutritional composition and anti-nutritional factors of anchote (Coccinia Abyssinica (lam.) Cogn) tubers grown in Western Ethiopia. J. Food Process. Technol. 2013, 4, 249. [Google Scholar] [CrossRef]
- Suárez, M.H.; Hernández, A.I.M.; Galdón, B.R.; Rodríguez, L.H.; Cabrera, C.E.M.; Mesa, D.R.; Rodríguez Rodríguez, E.M.; Romero, C.D. Application of multidimensional scaling technique to differentiate sweet potato (Ipomoea batatas (L.) Lam) cultivars according to their chemical composition. J. Food Compos. Anal. 2016, 46, 43–49. [Google Scholar] [CrossRef]


| Defatted Kernel Flour Samples | ||||||
|---|---|---|---|---|---|---|
| Composition | Akpi-Agb1 | Akpi-Agb2 | Akpi-Div | Akpi-Lak | Akpi-Bon | Akpi-Vav |
| Dry matter (%) | 94.3 ± 0.0 a | 92.7 ± 0.0 b | 95.6 ± 0.0 c | 94.0 ± 0.0 a,b | 93.7 ± 0.0 d | 95.0 ± 0.0 e |
| TS (%) | 7.8 ± 0.8 a | 9.8 ± 0.1 a | 12.9 ± 0.1 a | 17.1 ± 0.2 a | 13.6 ± 0.1 a | 7.3 ± 0.6 a |
| RS (%) | 0.2 ± 0.0 a | 0.3 ± 0.0 a | 0.3 ± 0.0 a | 0.2 ± 0.0 a | 0.2 ± 0.0 a | 0.2 ± 0.0 a |
| Ash (%) | 12.5 ± 0.1 a,b,c | 12.8 ± 0.1 b,c | 13.1 ± 0.0 c | 12.0 ± 0.1 a | 12.2 ± 0.0 a,b | 11.2 ± 0.1 d |
| Protein (%) | 57.0 ± 0.0 a | 49.4 ± 0.1 b | 48.8 ± 0.1 b | 61.3 ± 0.1 c | 56.6 ± 0.1 a | 47.0 ± 0.0 d |
| TCHO (%) | 24.7 ± 0.1 a | 31.1 ± 0.8 b | 33.8 ± 0.1 c | 20.5 ± 0.0 d | 24.9 ± 0.1 a | 37.8 ± 0.1 e |
| Crude fibre (%) | 10.0 ± 0.1 a | 13.7 ± 0.0 b | 12.5 ± 0.0 c | 11.1 ± 0.0 d | 14.4 ± 0.0 e | 9.5 ± 0.0 f |
| pH | 6.2 ± 0.0 a | 6.1 ± 0.0 b | 6.7 ± 0.0 c | 6.5 ± 0.0 d | 7.1 ± 0.0 e | 6.8 ± 0.0 f |
| TTA (meq/100 g) | 4.3 ± 0.0 a | 4.5 ± 0.0 b | 2.3 ± 0.0 c | 3.1 ± 0.0 d | 1.9 ± 0.0 e | 2.4 ± 0.0 c |
| Energy (kcal/100g) | 326.9 ± 0.4 a | 322.0 ± 0.2 b | 330.4 ± 0.1 c | 327.4 ± 0.3 a | 325.9 ± 0.2 a | 339.2 ± 0.2 d |
| Mineral Elements | |||||||
|---|---|---|---|---|---|---|---|
| Defatted Kernel Flour | Mg (%) | P (%) | Ca (%) | K (%) | Fe (%) | Zn (%) | Cl (%) |
| Akpi-Agb1 | 19.0 ± 0.0 a,b | 2.0 ± 0.0 a | 0.6 ± 0.0 a | 0.2 ± 0.0 a,b | 3.0 ± 0.0 b | n.d. | n.d. |
| Akpi-Agb2 | 30.0 ± 0.0 b | 1.0 ± 0.0 a | 0.5 ± 0.0 b | 0.3 ± 0.0 c | 2.0 ± 0.0 a | n.d. | n.d. |
| Akpi-Div | 20.0 ± 0.0 a,b | 0.4 ± 0.0 b | 0.4 ± 0.0 c,d | 0.1 ± 0.0 a | 2.1 ± 0.0 a | n.d. | n.d. |
| Akpi-Lak | 12.0 ± 0.0 a | 0.3 ± 0.0 b | 0.3 ± 0.0 c | 0.2 ± 0.0 a,b | 2.0 ± 0.0 a | n.d. | n.d. |
| Akpi-Bon | 30.0 ± 0.0 a,b | 0.4 ± 0.0 c | 0.4 ± 0.0 d | 0.4 ± 0.0 d | 2.1 ± 0.0 a | n.d. | n.d. |
| Akpi-Vav | 40.0 ± 0.0 c | 1.0 ± 0.0 a | 0.6 ± 0.0 a,b | 0.2 ± 0.0 a,b | 2.3 ± 0.0 a | n.d. | n.d. |
| Bioactive Compounds | |||||
|---|---|---|---|---|---|
| Defatted Kernel Flour | Polyphenols (mg/100g) | Phytates (mg/100g) | Flavonoids (mg/100g) | Tannins (mg/100g) | Oxalate (mg/100g) |
| Akpi-Agb1 | 403.9 ± 0.6 a | 62.3 ± 0.1 a | 1.8 ± 0.0 a,b | 16.4 ± 0.0 a | 972.9 ± 0.0 a |
| Akpi-Agb2 | 346.4 ± 0.1 a,b | 52.3 ± 0.3 a | 2.7 ± 0.0 a,b | 23.9 ± 0.0 b | 972.3 ± 0.0 a |
| Akpi-Div | 347.4 ± 1.2 a,b | 52.2 ± 0.1 a | 3.1 ± 0.0 b | 21.8 ± 0.0 a,b | 973.6 ± 0.0 a |
| Akpi-Lak | 285.2 ± 0.1 b | 61.9 ± 0.1 a | 1.0 ± 0.0 a | 41.8 ± 0.1 c | 881.1 ± 0.0 a |
| Akpi-Bon | 216.6 ± 0.2 c | 63.3 ± 0.0 a | 1.1 ± 0.0 a | 18.5 ± 0.1 a,b | 908.1 ± 0.0 a |
| Akpi-Vav | 348.4 ± 0.0 a,b | 71.4 ± 0.1 a | 4.6 ± 0.0 c | 22.0 ± 0.0 a,b | 714.7 ± 0.0 a |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coulibaly, M.; Kouamé, C.A.; N’dri, D.Y.; Kouassi, N.K.; Pereko, K.K.A.; Amani, G.N. Effect of Post-Harvest Traditional Technologies on the Nutrient Content and Antioxidant Compounds of Defatted Flours from Ricinodendron heudelotti (Baill. Pierre ex Pax) Seed Kernels. Technologies 2018, 6, 37. https://doi.org/10.3390/technologies6020037
Coulibaly M, Kouamé CA, N’dri DY, Kouassi NK, Pereko KKA, Amani GN. Effect of Post-Harvest Traditional Technologies on the Nutrient Content and Antioxidant Compounds of Defatted Flours from Ricinodendron heudelotti (Baill. Pierre ex Pax) Seed Kernels. Technologies. 2018; 6(2):37. https://doi.org/10.3390/technologies6020037
Chicago/Turabian StyleCoulibaly, Mamadou, Camille Adam Kouamé, Denis Yao N’dri, Nestor Kouakou Kouassi, Kingsley Kwadwo Asare Pereko, and Georges N’guessan Amani. 2018. "Effect of Post-Harvest Traditional Technologies on the Nutrient Content and Antioxidant Compounds of Defatted Flours from Ricinodendron heudelotti (Baill. Pierre ex Pax) Seed Kernels" Technologies 6, no. 2: 37. https://doi.org/10.3390/technologies6020037





