Testing of Candidate Icons to Identify Acetaminophen-Containing Medicines
Abstract
:1. Introduction
2. Objectives
3. Methods
3.1. Overview

3.2. Participants
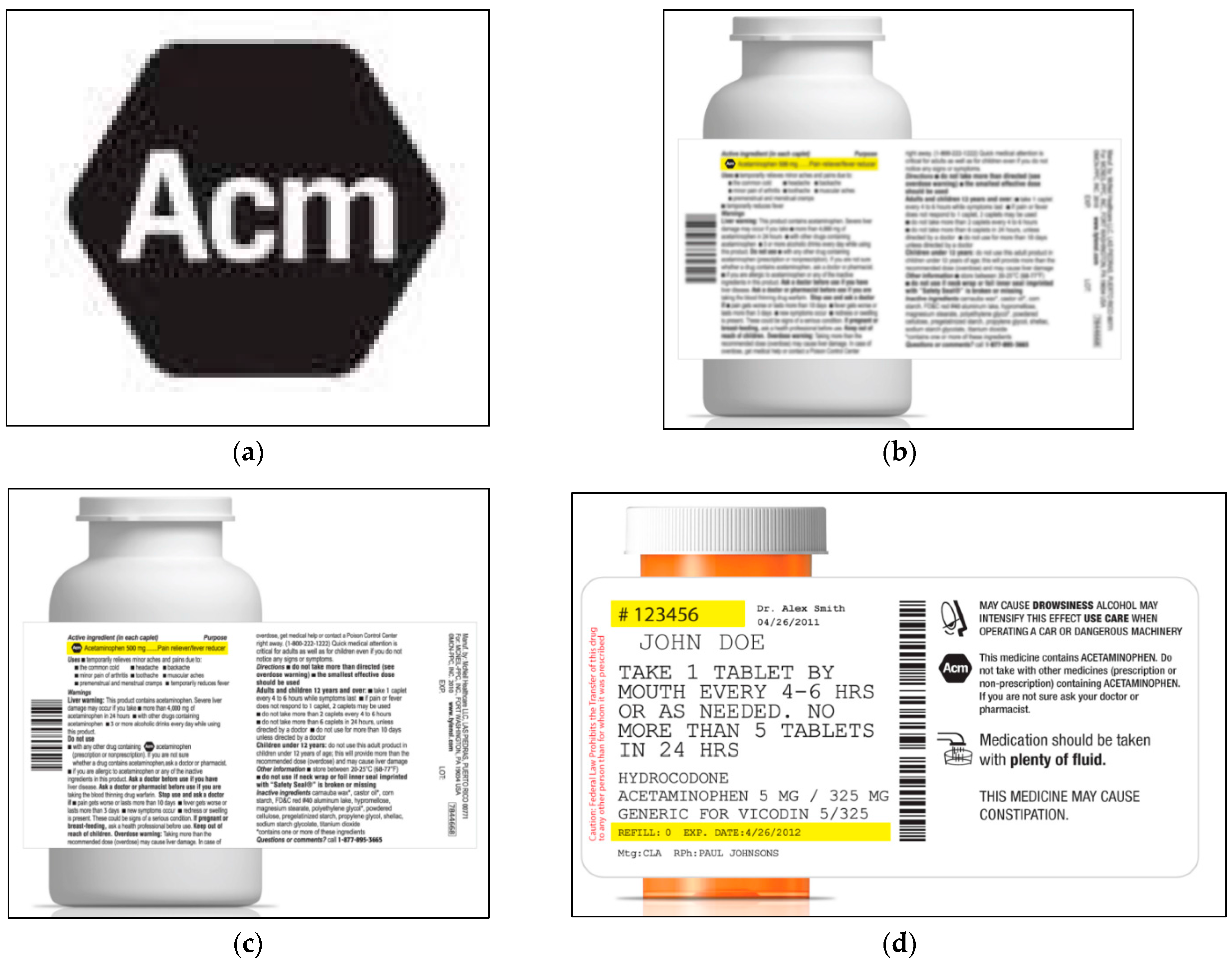
3.3. Icon Candidates
3.4. Procedures
3.5. Measurements
3.6. Analysis
3.7. Results
3.7.1. Recruitment
3.7.2. Preliminary Analyses
| Total (%) | |
|---|---|
| N = 300 | |
| Gender | |
| Female | 50.3% |
| Male | 49.7% |
| Race 1 | |
| African American or Black | 8.8% |
| Asian | 2.7% |
| Caucasian or White | 77.0% |
| Hispanic or Latino | 8.1% |
| Other or mixed | 3.4% |
| Age (years) | |
| 18–24 | 6.0% |
| 25–49 | 49.0% |
| 50–65 | 37.7% |
| 66–75 | 6.3% |
| 76+ | 1.0% |
| Employment | |
| Full-time | 52.3% |
| Homemaker | 13.0% |
| Part-time | 8.7% |
| Retired | 17.3% |
| Unemployed/seeking employment | 8.7% |
| Income (annual) 2 | |
| Under $18,000 | 10.1% |
| $18,000–$49,999 | 30.4% |
| $50,000–$74,999 | 25.0% |
| $75,000–$99,999 | 16.2% |
| $100,000–$124,999 | 10.8% |
| $125,000 or more | 7.4% |
| Education 3 | |
| Less than High School | 2.0% |
| High School graduate/GED | 39.1% |
| Associate/Bachelor’s degree | 46.2% |
| Post graduate degree | 12.7% |
| Health Literacy | |
| Limited | 14.3% |
| Adequate | 85.7% |
3.7.3. Comparisons among Icons
Open-Ended Interpretations
| Ac | Ace | Acm | APAP 1 | Abstract | p-Values 2 | |||
|---|---|---|---|---|---|---|---|---|
| N = 150 | N = 55 | N = 150 | N = 192 | N = 53 | Icons | Literacy | Interaction | |
| Open-Ended Interpretation | ||||||||
| Drug Context | ||||||||
| Acetaminophen | 30.7% a | 18.2% b | 14.7% b | 14.6% b | 9.4% b | 0.0125 | ||
| By Health Literacy | 0.10 | 0.84 | ||||||
| Limited | 23.8% | 0.0% | 9.1% | 10.7% | 12.5% | |||
| Adequate | 31.8% | 20.8% | 15.6% | 15.2% | 8.9% | |||
| Other Interpretation: | ||||||||
| An active ingredient | 5.3% | 5.5% | 6.0% | 6.8% | 5.7% | |||
| Other, drug related 3 | 23.4% | 18.2% | 26.7% | 20.8% | 15.1% | |||
| Other, not drug-related | 5.3% | 3.6% | 8.0% | 4.1% | 5.7% | |||
| Don’t know | 35.3% | 54.5% | 44.7% | 53.6% | 64.2% | |||
| Full Label Context | ||||||||
| Acetaminophen 6 | 75.3% a | 76.4% a, b | 70.7% a, b | 58.9% c | 58.5% b, c | 0.005 | ||
| By Health Literacy | 0.0001 | 0.71 | ||||||
| Limited | 52.4% | 42.9% | 36.4% | 42.9% | 50.0% | |||
| Adequate | 79.1% | 81.3% | 76.6% | 61.6% | 60.0% | |||
| Other Interpretation: | ||||||||
| An active ingredient | 6.0% | 0.0% | 10.0% | 6.8% | 11.3% | |||
| Other, drug related 3 | 10.7% | 14.5% | 9.3% | 11.5% | 15.1% | |||
| Other, not drug-related | 0.7% | 0.0% | 0.0% | 2.1% | 0.0% | |||
| Don’t know | 7.3% | 9.1% | 9.3% | 20.8% | 15.1% | |||
| Open-Ended Behavioral Response | ||||||||
| Drug Context | ||||||||
| Would exercise caution 4 | 37.3% | 30.9% | 40.7% | 35.9% | 41.5% | 0.41 | ||
| By Health Literacy | 0.92 | 0.78 | ||||||
| Limited | 38.1% | 28.6% | 40.9% | 32.1% | 37.5% | |||
| Adequate | 37.2% | 31.3% | 40.6% | 36.6% | 42.2% | |||
| Dose/concomitant use 5 | 0.0% | 0.0% | 3.3% | 3.1% | 0.0% | 0.40 | ||
| By Health Literacy | 0.20 | 0.97 | ||||||
| Limited | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | |||
| Adequate | 0.0% | 0.0% | 3.9% | 3.7% | 0.0% | |||
| Full Label Context | ||||||||
| Would exercise caution 4 | 46.7% | 52.7% | 37.3% | 47.9% | 50.9% | 0.99 | ||
| By Health Literacy | 0.20 | 0.58 | ||||||
| Limited | 28.6% | 57.1% | 31.8% | 39.3% | 25.0% | |||
| Adequate | 49.6% | 52.1% | 38.3% | 49.4% | 55.6% | |||
| Dose/concomitant use 5 | 26.7% | 25.4% | 20.7% | 21.4% | 17.0% | 0.39 | ||
| By Health Literacy | 0.56 | 0.20 | ||||||
| Limited | 19.1% | 14.3% | 18.2% | 14.3% | 12.5% | |||
| Adequate | 27.9% | 27.1% | 21.1% | 22.6% | 17.8% | |||
Misinterpretations and Adjudicated Critical Confusion
Self-Reported Confusion
| Ac | Ace | Acm | APAP 1 | Abstract | p-Values 2 | |||
|---|---|---|---|---|---|---|---|---|
| N = 150 | N = 55 | N = 150 | N = 192 | N = 53 | Icons | Literacy | Interaction | |
| Confusing (Y/N) | 22.7% a | 21.8% a | 26.7% a | 50.0% b | 32.1% a, b | <0.0001 | ||
| By Health Literacy | 0.05 | 0.84 | ||||||
| Limited | 9.5% | 14.3% | 31.8% | 35.7% | 0.0% | |||
| Adequate | 24.8% | 22.9% | 25.8% | 52.4% | 37.8% | |||
| Correctly Identified Ingredient | 90.0% | 92.7% | 88.7% | 87.0% | 84.9% | 0.27 | ||
| By Health Literacy | <0.0001 | 0.08 | ||||||
| Limited | 71.4% | 85.7% | 63.6% | 71.4% | 50.0% | |||
| Adequate | 93.0% | 93.8% | 93.0% | 89.6% | 91.1% | |||
| Communication Effectiveness (1–7) | ||||||||
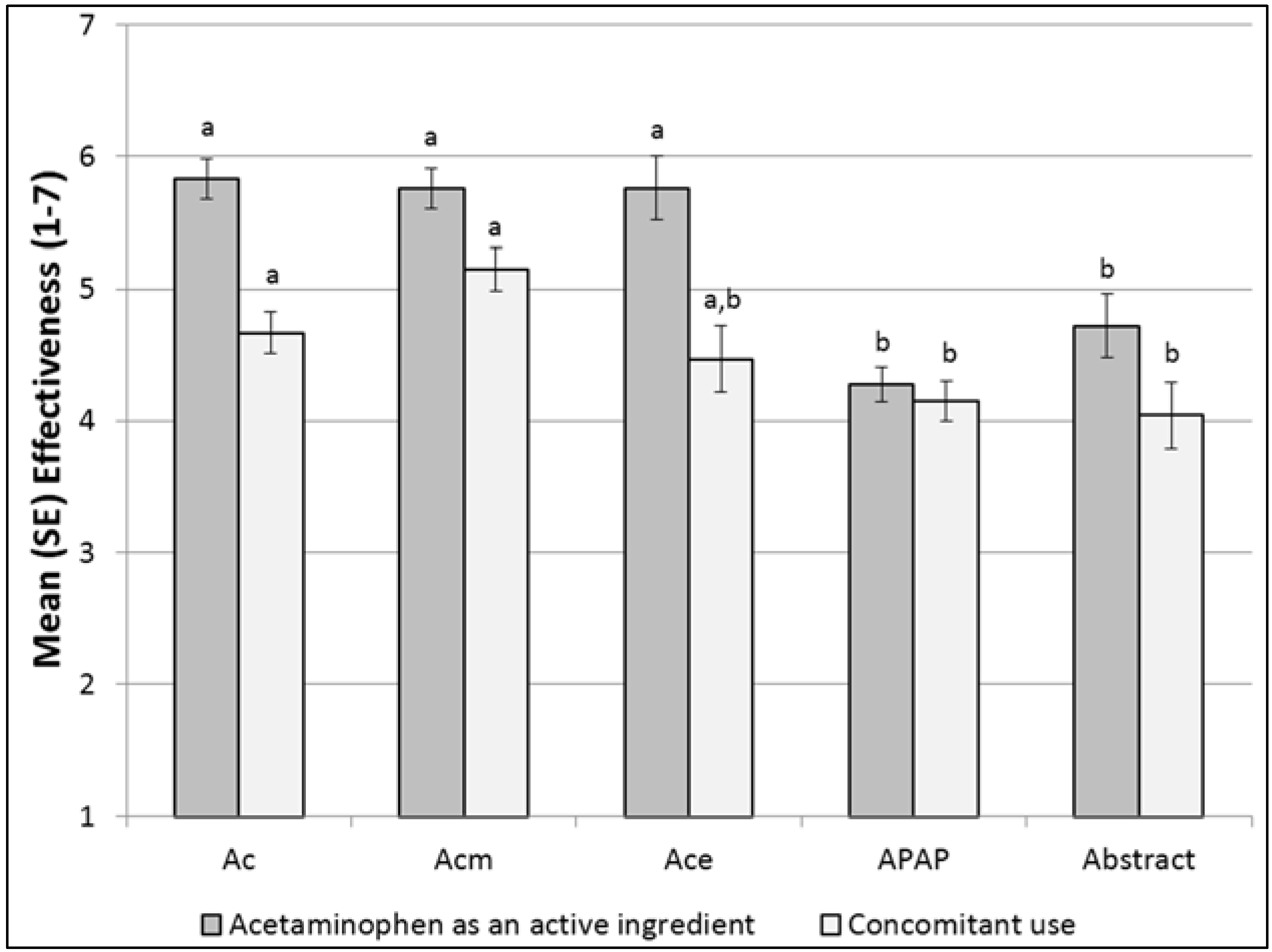
| Acetaminophen as an ingredient | 5.83 a (0.15) | 5.76 a (0.24) | 5.76 a (0.15) | 4.28 b (0.13) | 4.72 b (0.24) | <0.0001 | ||
| By Health Literacy | 0.35 | 0.03 | ||||||
| Limited | 5.67 (0.40) | 5.57 (0.67) | 5.59 (0.39) | 4.79 (0.35) | 6.25 (0.63) | 0.15 | ||
| Adequate | 5.86 a (0.16) | 5.79 a (0.26) | 5.79 a (0.16) | 4.19 b (0.14) | 4.44 b (0.26) | <0.0001 | ||
| Concomitant Use | 4.67 a (0.16) | 4.47 a, b (0.25) | 5.15 a (0.16) | 4.15 b (0.14) | 4.04 b (0.25) | <0.0001 | ||
| By Health Literacy | 0.003 | 0.88 | ||||||
| Limited | 5.24 (0.43) | 5.29 (0.68) | 5.73 (0.42) | 4.93 (0.38) | 4.88 (0.64) | |||
| Adequate | 4.57 (0.17) | 4.35 (0.26) | 5.05 (0.17) | 4.02 (0.15) | 3.89 (0.27) | |||
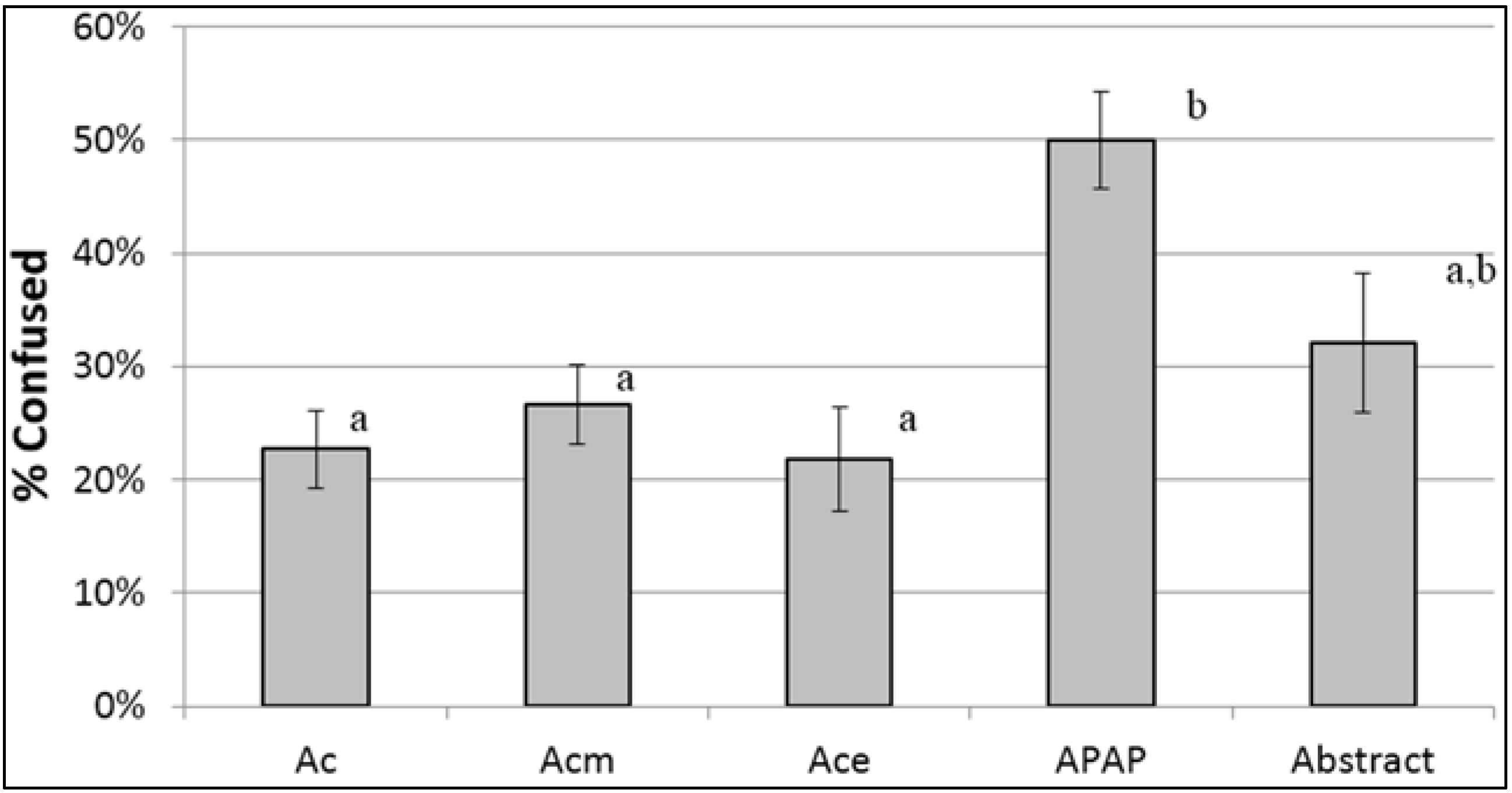
Identification of Acetaminophen as an Ingredient
Communication Effectiveness
3.7.4. One vs. Two OTC Icons, Rx vs. OTC
Open-Ended Interpretations
| One-Placement OTC | Two-Placement OTC | Rx | OTC Placement: One Icon vs. Two Icons (p-Values) | Product Class: Rx vs. OTC (p-Values) | |||
|---|---|---|---|---|---|---|---|
| N = 200 | N = 200 | N = 200 | |||||
| Placement | Interaction 2 | Class | Interaction 2 | ||||
| Open-Ended Interpretation | |||||||
| Drug Context | |||||||
| Acetaminophen | 22.0% | 22.0% | 11.5% | 0.89 | 0.005 | ||
| By Health Literacy 2 | 0.39 | 0.77 | |||||
| Limited | 20.0% | 13.9% | 0.0% | ||||
| Adequate | 22.4% | 23.8% | 12.8% | ||||
| Other Interpretations: | |||||||
| An active ingredient | 9.0% | 6.0% | 3.0% | ||||
| Other, drug related | 18.5% | 17.5% | 30.5% | ||||
| Other, not drug related | 4.5% | 7.0% | 5.0% | ||||
| Don’t know | 46.0% | 47.5% | 50.0% | ||||
| Full Context | |||||||
| Acetaminophen | 61.0% | 69.0% | 72.5% | 0.14 | 0.14 | ||
| By Health Literacy 2 | 0.16 | 0.82 | |||||
| Limited | 46.7% | 38.9% | 50.0% | ||||
| Adequate | 63.5% | 75.6% | 75.0% | ||||
| Other Interpretations: | |||||||
| An active ingredient | 13.5% | 5.0% | 3.0% | ||||
| Other, drug related | 11.0% | 9.0% | 14.0% | ||||
| Other | 1.0% | 0.0% | 1.5% | ||||
| Don’t know | 13.0% | 17.0% | 9.0% | ||||
| Open-Ended Behavioral Response | |||||||
| Drug Context | |||||||
| Would exercise caution | 36.0% | 34.0% | 42.5% | 0.77 | 0.14 | ||
| By Health Literacy 2 | 0.047 | 0.52 | |||||
| Limited | 53.3% | 22.2% | 35.0% | 0.04 | |||
| Adequate | 32.9% | 36.6% | 43.3% | 0.59 | |||
| Dose/concomitant use 1 | 0.5% | 4.0% | 1.0% | 0.08 | 0.13 | ||
| By Health Literacy 2 | 0.42 | 0.83 | |||||
| Limited | 0.0% | 0.0% | 0.0% | ||||
| Adequate | 0.6% | 4.9% | 1.1% | ||||
| Full Context | |||||||
| Would exercise caution | 30.0% | 38.0% | 69.0% | 0.17 | <0.0001 | ||
| By Health Literacy 2 | 0.94 | 0.51 | |||||
| Limited | 16.7% | 25.0% | 80.0% | ||||
| Adequate | 32.4% | 40.9% | 67.8% | ||||
| Dose/concomitant use 1 | 5.5% | 14.5% | 47.5% | 0.03 | <0.0001 | ||
| By Health Literacy 2 | 0.91 | 0.17 | |||||
| Limited | 0.0% | 8.3% | 55.0% | ||||
| Adequate | 6.5% | 15.9% | 46.7% | ||||
Self-Reported Confusion
| One-Placement OTC | Two-Placement OTC | Rx | OTC Placement: one Icon vs. two Icons (p-Values) | Placement: Rx vs. OTC (p-Values) | |||
|---|---|---|---|---|---|---|---|
| N = 200 | N = 200 | N = 200 | |||||
| Placement | Interaction 1 | Class | Interaction 1 | ||||
| Confusing (Y/N) | 34.5% | 32.5% | 32.5% | 0.52 | 0.89 | ||
| By Health Literacy 1 | 0.10 | 0.66 | |||||
| Limited | 33.3% | 13.9% | 25.0% | ||||
| Adequate | 34.7% | 36.6% | 33.3% | ||||
| Correctly Identified Ingredient | 90.0% | 86.0% | 84.5% | 0.42 | 0.63 | ||
| By Health Literacy 1 | 0.85 | 0.006 | |||||
| Limited | 63.3% | 61.1% | 80.0% | 0.04 | |||
| Adequate | 94.7% | 91.5% | 85.0% | 0.23 | |||
| Communication Effectiveness (1–7) | |||||||
| Acetaminophen as an ingredient | 5.37 (0.16) | 5.13 (0.16) | 5.14 (0.16) | 0.45 | 0.49 | ||
| By Health Literacy 1 | 0.025 | 0.26 | |||||
| Limited | 6.33 (0.40) | 4.97 (0.36) | 4.80 (0.49) | 0.02 | |||
| Adequate | 5.19 (0.17) | 5.16 (0.18) | 5.18 (0.17) | 0.82 | |||
| Concomitant Use | 4.14 (0.19) | 4.37 (0.19) | 5.14 (0.19) | 0.36 | 0.0001 | ||
| By Health Literacy 1 | 0.49 | 0.22 | |||||
| Limited | 5.30 (0.47) | 5.08 (0.43) | 5.40 (0.58) | ||||
| Adequate | 3.94 (0.20) | 4.21 (0.21) | 5.11 (0.20) | ||||
Identification of Ingredient
Communication Effectiveness

4. Discussion
4.1. Variation among Icons
4.2. Adding a Second Icon to OTC Labels
4.3. Icons on Rx vs. OTC Labels
4.4. Effects of Health Literacy
4.5. Limitations
5. Conclusions
6. Practice Implications
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kaufman, D.W.; Kelly, J.P.; Rosenberg, L.; Anderson, T.E.; Mitchell, A.A. Recent patterns of medication use in the ambulatory adult population of the united states: The slone survey. JAMA 2002, 287, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Careful: Acetaminophen in Pain Relief Medicines Can Cause Liver Damage. 2011. Available online: http://www.Webcitation.Org/6fw0a6vuc (accessed on 19 May 2015). [Google Scholar]
- Food and Drug Administration. Briefing Information for the 29–30 June 2009 Joint Meeting of the Drug Safety and Risk Management Advisory Committee with the Anesthetic and Life Support Drugs Advisory Committee and the Nonprescription Drugs Advisory Committee. 2009. Available online: http://www.webcitation.org/6fvzgqcar (accessed on 19 May 2015). [Google Scholar]
- Krenzelok, E.P. The fda acetaminophen advisory committee meeting—What is the future of acetaminophen in the united states? The perspective of a committee member. Clin. Toxicol. 2009, 47, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, D.W.; Kelly, J.P.; Rohay, J.M.; Malone, M.K.; Weinstein, R.B.; Shiffman, S. Prevalence and correlates of exceeding the labeled maximum dose of acetaminophen among adults in a U.S.-based internet survey. Pharmacoepidemiol. Drug Saf. 2012, 21, 1280–1288. [Google Scholar] [CrossRef] [PubMed]
- Shiffman, S.; Rohay, J.M.; Battista, D.R.; Kelly, J.P.; Malone, M.K.; Weinstein, R.B.; Kaufman, D.W. Patterns of acetaminophen medication use associated with exceeding the recommended maximum daily dose. Pharmacoepidemiol. Drug Saf. 2015, 24, 915–921. [Google Scholar] [CrossRef] [PubMed]
- McNeil Consumer Healthcare. Frequently Asked Questions about New Dosing for Extra Strength Tylenol® Products. 2011. Available online: http://www.webcitation.org/6mp1ffyga (accessed on 19 May 2015).
- Wolf, M.S.; Davis, T.C.; Bass, P.F.; Curtis, L.M.; Lindquist, L.A.; Webb, J.A.; Bocchini, M.V.; Bailey, S.C.; Parker, R.M. Improving prescription drug warnings to promote patient comprehension. Arch. Intern. Med. 2010, 170, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Suydam, L. Chpa Overview and Approach: Consumer Healthcare Products Association (Chpa) Presentation for the 29 June 2009 Joint Meeting of the Drug Safety and Risk Management Advisory Committee with the Anesthetic and Life Support Drugs Advisory Committee and the Nonprescription Drugs Advisory Committee. Available online: http://www.webcitation.org/6ggfykzxl (accessed on 30 April 2013).
- Houts, P.S.; Doak, C.C.; Doak, L.G.; Loscalzo, M.J. The role of pictures in improving health communication: A review of research on attention, comprehension, recall, and adherence. Patient Educ. Couns. 2006, 61, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Montagne, M. Pharmaceutical pictograms: A model for development and testing for comprehension and utility. Res. Soc. Adm. Pharm. 2013, 9, 609–620. [Google Scholar] [CrossRef] [PubMed]
- American National Standards Institute. Criteria for Safety Symbols (American National Standards Institute z535.3-Revised); National Electrical Manufacturers Association: Arlington, VA, USA, 2007. [Google Scholar]
- Chan, A.H.; Chan, K.W. Effects of prospective-user factors and sign design features on guessability of pharmaceutical pictograms. Patient Educ. Couns. 2013, 90, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Federal Register. Over-the-Counter Human Drugs: Labeling Requirements; Federal Register: Washington, DC, USA, 1999; pp. 13254–13303.
- Food and Drug Administration. Pdufa Pilot Project: Proprietary Name Review. 2008. Available online: http://www.webcitation.org/6mopifbqx (accessed on 19 May 2015). [Google Scholar]
- Weiss, B.D.; Mays, M.Z.; Martz, W.; Castro, K.M.; DeWalt, D.A.; Pignone, M.P.; Mockbee, J.; Hale, F.A. Quick assessment of literacy in primary care: The newest vital sign. Ann. Fam. Med. 2005, 3, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Zeger, S.L.; Liang, K.Y. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 1986, 42, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.Y.; Zeger, S.L. Longitudinal data analysis using generalized linear models. Biometrika 1986, 73, 13–22. [Google Scholar] [CrossRef]
- Lipsitz, S.R.; Laird, N.M.; Harrington, D.P. Generalized estimating equations for correlated binary data: Using the odds ratio as a measure of association. Biometrika 1991, 78, 153–160. [Google Scholar] [CrossRef]
- National Council for Prescription Drug Programs. Ncpdp Recommendations for Improved Prescription Container Labels for Medicines Containing Acetaminophen, Version 1.1. 2013. Available online: http://www.Webcitation.Org/6yeb19byf (accessed on 19 May 2015).
- Kaufman, D.W.; Kelly, J.P.; Battista, D.R.; Malone, M.K.; Weinstein, R.B.; Shiffman, S. The relation of health literacy to exceeding the labeled maximum daily dose of acetaminophen. Am. J. Prev. Med. 2015. [Google Scholar] [CrossRef] [PubMed]
- Coomber, K.; Martino, F.; Barbour, I.R.; Mayshak, R.; Miller, P.G. Do consumers “get the facts”? A survey of alcohol warning label recognition in australia. BMC Public Health 2015, 15, 816. [Google Scholar] [CrossRef] [PubMed]
- Struik, M.H.; Koster, F.; Schuit, A.J.; Nugteren, R.; Veldwijk, J.; Lambooij, M.S. The preferences of users of electronic medical records in hospitals: Quantifying the relative importance of barriers and facilitators of an innovation. Implement. Sci. 2014, 9, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unni, E.; Shiyanbola, O.O. Clustering medication adherence behavior based on beliefs in medicines and illness perceptions in patients taking asthma maintenance medications. Curr. Med. Res. Opin. 2015, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hays, R.D.; Liu, H.; Kapteyn, A. Use of internet panels to conduct surveys. Behav. Res. Methods 2015, 47, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Shiffman, S.; Cotton, H.; Jessurun, C.; Sembower, M.A.; Pype, S.; Phillips, J. Pharmacist and physician interpretation of abbreviations for acetaminophen intended for use in a consumer icon. Pharmacy 2015, 3, 169–181. [Google Scholar] [CrossRef]
- McNeil Consumer Healthcare. Get Relief Responsibly. 2012. Available online: http://www.webcitation.org/6kosochr0 (accessed on 19 May 2015).
- Acetaminophen Awareness Coalition. Know Your Dose. 2012. Available online: http://www.webcitation.org/6koszinr2 (accessed on 19 May 2015).
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiffman, S.; Cotton, H.; Jessurun, C.; Sembower, M.A.; Pype, S.; Phillips, J. Testing of Candidate Icons to Identify Acetaminophen-Containing Medicines. Pharmacy 2016, 4, 10. https://doi.org/10.3390/pharmacy4010010
Shiffman S, Cotton H, Jessurun C, Sembower MA, Pype S, Phillips J. Testing of Candidate Icons to Identify Acetaminophen-Containing Medicines. Pharmacy. 2016; 4(1):10. https://doi.org/10.3390/pharmacy4010010
Chicago/Turabian StyleShiffman, Saul, Helene Cotton, Christina Jessurun, Mark A. Sembower, Steve Pype, and Jerry Phillips. 2016. "Testing of Candidate Icons to Identify Acetaminophen-Containing Medicines" Pharmacy 4, no. 1: 10. https://doi.org/10.3390/pharmacy4010010





