A European Competence Framework for Industrial Pharmacy Practice in Biotechnology
Abstract
:- “Research and Development”,
- ‘“Upstream” and “Downstream” Processing’,
- “Product development and formulation”,
- “Aseptic processing”,
- “Analytical methodology”,
- “Product stability”, and
- “Regulation”.
1. Introduction
2. Methodology
3. Statistical Analysis
4. Results
| Industrial Employees | Academics | |||
|---|---|---|---|---|
| Number | % | Number | % | |
| Country of residence | ||||
| Austria | 1 | 0.6 | ||
| Belgium | 12 | 7.8 | 5 | 13.5 |
| Bulgaria | 2 | 1.3 | 4 | 10.8 |
| Czech Republic | 1 | 0.6 | ||
| Denmark | 5 | 3.2 | ||
| Finland | 18 | 11.7 | ||
| France | 15 | 9.7 | 1 | 2.7 |
| Germany | 8 | 5.2 | ||
| Greece | 0.0 | 2 | 5.4 | |
| Hungary | 1 | 0.6 | ||
| Ireland | 8 | 5.2 | ||
| Italy | 6 | 3.9 | 17 | 45.9 |
| Malta | 1 | 2.7 | ||
| Portugal | 14 | 9.1 | 1 | 2.7 |
| Serbia | 1 | 0.6 | ||
| Spain | 1 | 0.6 | ||
| Sweden | 3 | 1.9 | ||
| Switzerland | 20 | 13.0 | ||
| The Netherlands | 15 | 9.7 | 1 | 2.7 |
| UK | 23 | 14.9 | 5 | 13.5 |
| Total | 154 (1 did not reply) | 100 | 37 (2 did not reply) | 100 |
| Industrial Employees | Academics | |||
|---|---|---|---|---|
| Number | % | Number | % | |
| Age group (years) | ||||
| 18–30 | 13 | 8.4 | 3 | 7.7 |
| 31–40 | 29 | 18.7 | 9 | 23.1 |
| 41–50 | 46 | 29.7 | 13 | 33.3 |
| 51–60 | 54 | 34.8 | 9 | 23.1 |
| 61–70 | 11 | 7.1 | 4 | 10.3 |
| >70 | 2 | 1.3 | 1 | 2.6 |
| Total | 155 | 100 | 39 | 100 |
| Rank | Industrial Employees (n = 153) | Academics (n = 35) |
|---|---|---|
| 1 | 5.0 | 3.0 |
| 2 | 15.0 | 13.0 |
| 3 | 22.3 | 32.5 |
| 4 | 27.5 | 32.6 |
| Blanks + “I am unable to rank this premise” | 30.2 | 18.9 |
| Total | 100 | 100 |
- The clarity of the survey
- The context within which answers should be given
- The specificity to biotechnology and not to industrial pharmacy practice in general
- The educational level (foundation or specialist) at which the competence would be acquired
- The balance between the relative importance of different competences
5. Discussion
5.1. Delphi Methodology and Statistics
5.2. Profiles of Respondents
5.3. Ranking Profiles
6. Conclusions
7. Perspectives
Acknowledgement
Author Contributions
Conflicts of Interest
Appendix











| Number | Competence | Ranking | |
|---|---|---|---|
| n | 1. Research and development. | Ind. | Acad. |
| 1 | Take an active role in a multidisciplinary team to interpret the key elements of a drug development strategy and use this to design early phase clinical studies | 3.3 | 3.4 |
| 2 | Understand the statistical principles used in preclinical and clinical research | 2.9 | 3.1 |
| 3 | Be able to critically review published studies in preclinical (including safety pharmacology) and clinical research. | 3.2 | 3.4 |
| 2. Preclinical sciences. | |||
| 4 | Have an understanding of the choice and predictive value of the non-clinical testing programme as part of the overall drug development plan for chemical and biological compounds. | 3.1 | 3.2 |
| 5 | Be able to describe the general principles of non-clinical safety testing. | 3.0 | 2.9 |
| 6 | Know how non-clinical tests are integrated into the overall drug development plan (including scheduling of toxicology tests with respect to clinical trials). | 3.1 | 3.2 |
| 7 | Be able to use animal pharmacokinetics and toxicokinetics to inform the clinical development process. | 2.6 | 2.8 |
| 8 | Describe the importance of the selection of the preclinical animal model in order to have a better and more predictive non-clinical phase. | 2.8 | 3.2 |
| 3. Biological and advanced therapy. | |||
| 9 | Describe the breadth of advanced therapy medicinal products (ATMPs) that are available and in development, including the scientific principles for the classification in to the categories of gene therapy, somatic cell therapy, tissue engineering and combined ATMPs. | 2.9 | 3.2 |
| 10 | Describe the range of products available with recombinant DNA technology. | 2.8 | 3.3 |
| 11 | Discuss the different needs between the pre-clinical and clinical trial needs of natural proteins and modified proteins | 2.8 | 3.0 |
| 12 | Describe the range of monoclonal antibodies available, and those in development, and discuss the potential long term safety issues with monoclonal antibodies. | 3.1 | 3.3 |
| 13 | Describe the global need for new and improved vaccines and the barriers to their development. | 3.0 | 3.2 |
| 14 | Define what a therapeutic vaccine is and describe how a therapeutic vaccine could influence therapy in a common disease area. | 3.0 | 3.4 |
| 15 | Describe what is a polysaccharide product and the regulatory and development challenges involved. | 2.9 | 2.7 |
| 4. Clinical pharmacology. | |||
| 16 | Take an active role in a multidisciplinary team to design clinical pharmacology studies | 2.9 | 3.3 |
| 17 | Recognise the particular ethical issues of using non patient volunteers in clinical studies | 3.0 | 3.2 |
| 18 | Understand and interpret clinical pharmacodynamic and pharmacokinetic data especially that related to safety issues | 3.2 | 3.3 |
| 19 | Discuss how data from a clinical pharmacology study can inform the future development of a medicine | 3.3 | 3.3 |
| 5. Clinical development. | |||
| 20 | Use pre-clinical pharmacology and safety data to prepare a clinical trial plan | 2.9 | 3.3 |
| 21 | Write a protocol for a study including the choice of design, the end points, whether to use a placebo and the inclusion and exclusion criteria | 2.9 | 3.3 |
| 22 | Interpret the elements of GCP that apply to the design and execution of clinical trials | 3.2 | 3.1 |
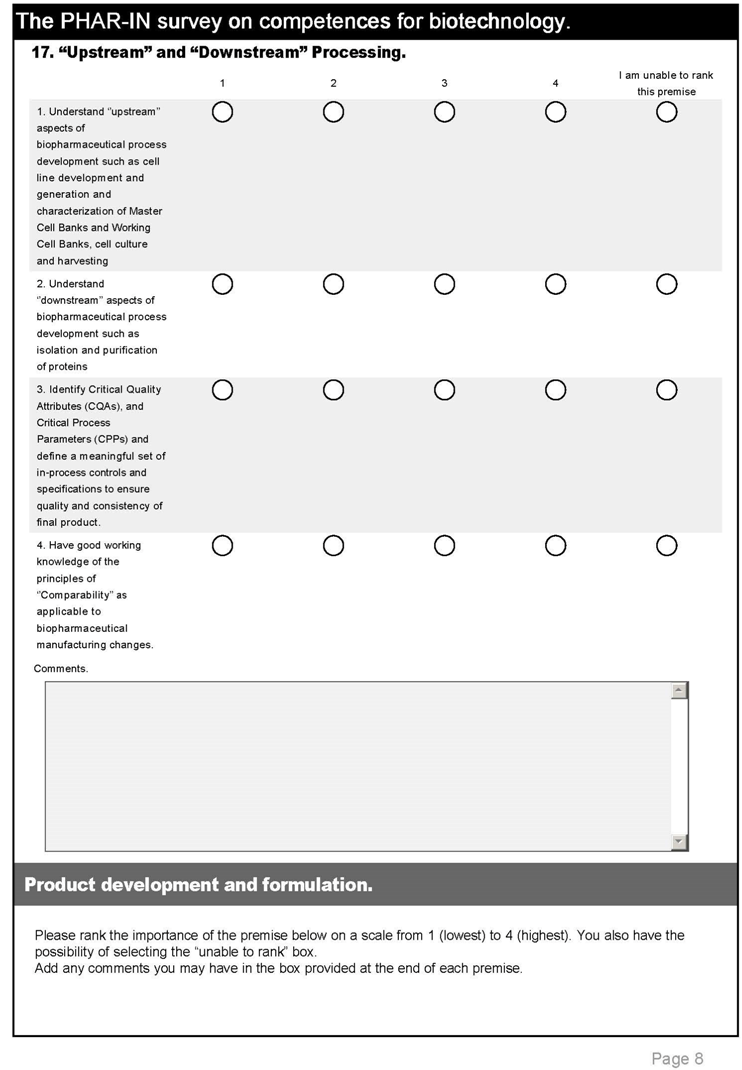
| 6. “Upstream” and “Downstream” Processing. | |||
| 23 | Understand ‘’upstream’’ aspects of biopharmaceutical process development such as cell line development and generation and characterization of Master Cell Banks and Working Cell Banks, cell culture and harvesting | 3.2 | 3.1 |
| 24 | Understand ‘’downstream’’ aspects of biopharmaceutical process development such as isolation and purification of proteins | 3.2 | 3.2 |
| 25 | Identify Critical Quality Attributes (CQAs), and Critical Process Parameters (CPPs) and define a meaningful set of in-process controls and specifications to ensure quality and consistency of final product. | 3.3 | 3.2 |
| 26 | Have good working knowledge of the principles of “Comparability” as applicable to biopharmaceutical manufacturing changes. | 3.3 | 3.1 |
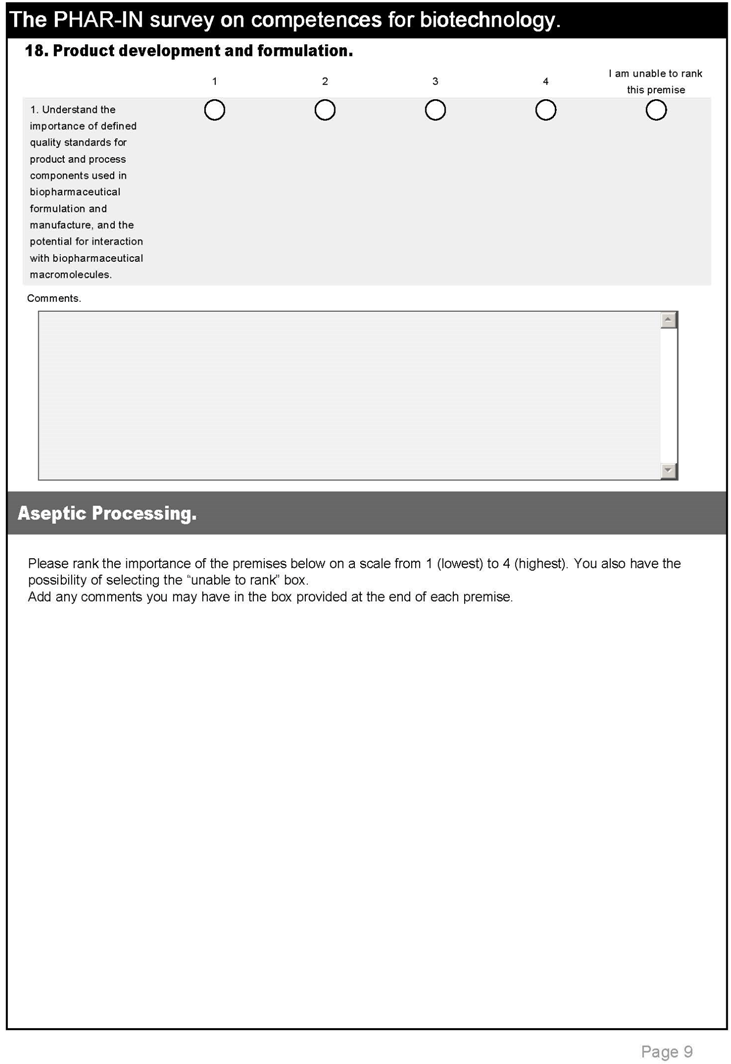
| 7. Product development and formulation. | |||
| 27 | Understand the importance of defined quality standards for product and process components used in biopharmaceutical formulation and manufacture, and the potential for interaction with biopharmaceutical macromolecules. | 3.3 | 3.5 |
| 8. Aseptic processing. | |||
| 28 | Understand microbiological principles as they apply to sterility assurance in biopharmaceutical manufacturing. | 3.3 | 3.2 |
| 29 | Understand unit operations in aseptic processing and design of facilities and utilities in sterile manufacturing suite. | 3.2 | 3.1 |
| 30 | Understand concepts of Good Manufacturing Practice (GMP) and Good Distribution Practice (GDP) as applicable to the aseptic production, control, storage and handling of biopharmaceuticals. | 3.4 | 3.3 |
| 9. Analytical methodology. | |||
| 31 | Understand the principles, instrumentation and application of analytical methods (especially bioassay) used to characterize biopharmaceutical raw materials, intermediates and finished products. | 3.1 | 3.4 |
| 10. Product stability. | |||
| 32 | Understand the potential impact of environmental factors (such as temperature, light, oxidation) on biopharmaceutical proteins and consequences for product quality, safety and efficacy. | 3.4 | 3.4 |
| 11. Regulation. | |||
| 33 | Understand the regulatory framework applicable to the development, manufacture, quality assurance and testing of biopharmaceutical products | 3.5 | 3.3 |
| 34 | Use research skills to find regulatory documents used for the preparation of a Clinical Trial Application. | 2.8 | 2.9 |
| 35 | Use knowledge of specific legislation for biopharmaceuticals to review preclinical and clinical parts of a Marketing Authorisation dossier. | 2.8 | 3.0 |
| 36 | Make decisions based on regulatory and commercial information about what text should be included in a Summary of Product Characteristics and Patient Information for a biopharmaceutical. | 3.0 | 3.0 |
| 37 | Know how National Agencies conduct GXP inspections and how to prepare for them. | 3.0 | 3.0 |
| 38 | Have an appreciation of post-licensing responsibilities for drug safety and how to construct a risk management plan. | 3.0 | 3.0 |
| 39 | Understand the life cycle management of biopharmaceuticals | 3.2 | 3.2 |
| 40 | Understand the current regulatory requirements for biosimilars | 3.2 | 3.1 |
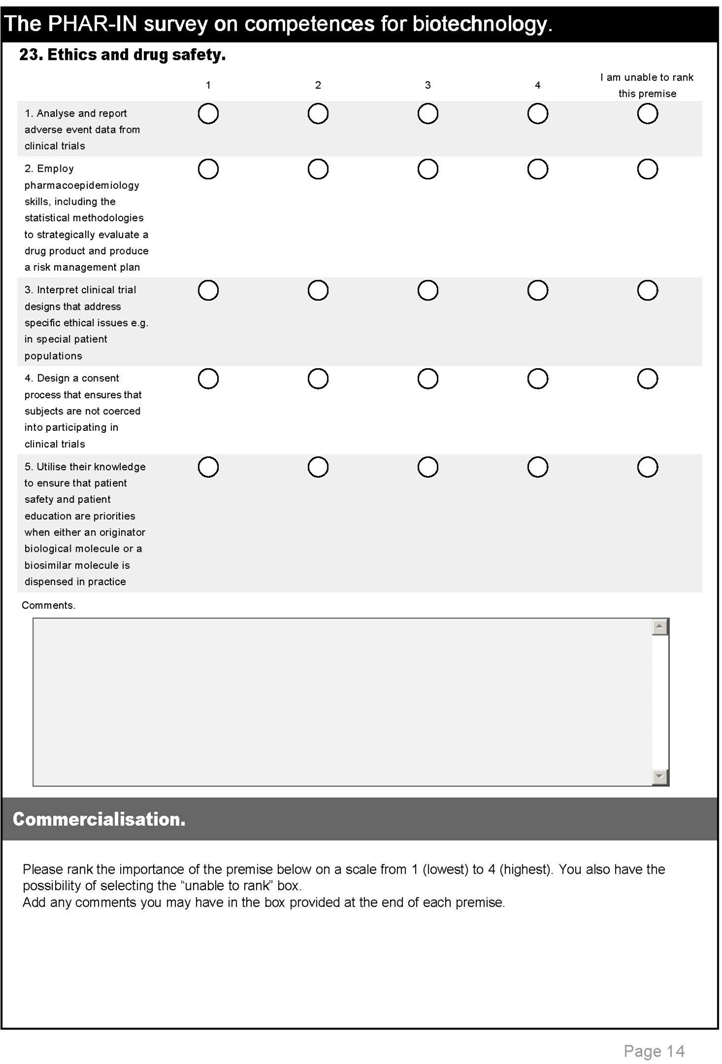
| 12. Ethics and drug safety. | |||
| 41 | Analyse and report adverse event data from clinical trials | 2.9 | 3.2 |
| 42 | Employ pharmaco-epidemiology skills, including the statistical methodologies to strategically evaluate a drug product and produce a risk management plan | 2.5 | 3.0 |
| 43 | Interpret clinical trial designs that address specific ethical issues e.g., in special patient populations | 2.6 | 3.1 |
| 44 | Design a consent process that ensures that subjects are not coerced into participating in clinical trials | 2.5 | 3.1 |
| 45 | Utilise their knowledge to ensure that patient safety and patient education are priorities when either an originator biological molecule or a biosimilar molecule is dispensed in practice | 2.9 | 3.2 |
| 13. Commercialisation. | |||
| 46 | Understand the significance of biomarkers as an integral part of the development process and economic evaluation of biopharmaceuticals | 3.0 | 3.2 |
References
- Competences for industrial pharmacy practice in biotechnology (PHAR-IN). PHAR-IN is funded by the European Commission via its Education, Audio-visual and Culture Agency; the EU reference is 538252-LLP-1-2013-1-BE-ERASMUS-EKA. Available online: http://www.phar-in.eu/ (accessed on 23 July 2015).
- European Industrial Pharmacists’ Group (EIPG). Available online: http://www.eipg.eu/ (accessed on 23 July 2015).
- European Federation of Pharmaceutical Industries and Associations, (EFPIA). Available online: http://www.efpia.eu/ (accessed on 23 July 2015).
- European Association of Faculties of Pharmacy (EAFP). Available online: http://www.eafponline.eu/ (accessed on 23 July 2015).
- European Federation for Pharmaceutical Sciences (EUFEPS). Available online: http://www.eufeps.org/ (accessed on 23 July 2015).
- Atkinson, J.; Nicholson, J.; Rombaut, B. Survey of Pharmaceutical Education in Europe Pharmine—Report on the integration of the industry component in pharmacy education and training. European Industrial Pharmacy 2012, 13, 17–20. Available online: http://eipg.eu/wp-content/uploads/2013/11/eip13-jun12.pdf#page=17 (accessed on 23 July 2015). [Google Scholar]
- International Pharmaceutical Federation (FIP). Global pharmacy workforce report. 2012. Available online: http://www.fip.org/humanresources (accessed on 23 July 2015).
- Rusu, A.; Kuokkanen, K.; Heier, A. Current trends in the pharmaceutical industry—A case study approach. Eur. J. Pharm. Sci. 2011, 44, 437–440. Available online: http://www.sciencedirect.com/science/article/pii/S0928098711002314 (accessed on 23 July 2015). [Google Scholar] [CrossRef] [PubMed]
- Atkinson, J.; Rombaut, B. The PHARMINE paradigm—Matching the supply of pharmacy education and training to demands. Eur. Ind. Pharmacy 2010, 6, 4–7. Available online: http://eipg.eu/wp-content/uploads/2013/11/eip6-jun10.pdf#page=4 (accessed on 23 July 2015). [Google Scholar]
- Bjerrum, O.J. Excellence in education and training advances competitiveness of the pharmaceutical industry in Europe. Eur. J. Pharmaceutical Sci. 2011, 44, 174–175. Available online: http://www.sciencedirect.com/science/article/pii/S0928098711002296 (accessed on 23 July 2015). [Google Scholar] [CrossRef] [PubMed]
- Stupans, I.; McAllister, S.; Clifford, R.; Hughes, J.; Krasse, I.; March, G.; Owen, S.; Woulf, J. Nationwide collaborative development of learning outcomes and exemplar standards for Australian pharmacy programmes. Int. J. Pharmacy Pract. 2014, 1–9. Available online: http://onlinelibrary.wiley.com/advanced/search/results (accessed on 23 July 2015). [Google Scholar] [CrossRef] [PubMed]
- Wu-Pong, S.; Gobburu, J.; O’Barr, S.; Shah, K.; Huber, J.; Weiner, D. The Future of the Pharmaceutical Sciences and Graduate Education: Recommendations from the AACP Graduate Education Special Interest Group. Am. J. Pharm. Educ. 2013, 77, 1–8. Available online: http://www.ncbi.nlm.nih.gov/pmc/journals/383/ (accessed on 23 July 2015). [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.C.; Sandford, B.A. The Delphi Technique: Making Sense of Consensus. Pract. Assess. Res. Eval. 2007, 12, 1. Available online: http://pareonline.net/getvn.asp?v=12&n = 10 (accessed on 23 July 2015). [Google Scholar]
- The TUNING network. Competences: Nursing practice and clinical decision making. Available online: http://www.unideusto.org/tuningeu/competences/specific/nursing.html (accessed on 23 July 2015).
- The TUNING network. Competences: Medical doctors. Available online: http://www.unideusto.org/tuningeu/subject-areas/medicine.html (accessed on 23 July 2015).
- Scott, D.G.; Washer, B.A.; Wright, M.D. A Delphi study to identify recommended biotechnology competences for first-year/initially certified technology education teachers. J. Technol. Educ. 2006, 17, 43–55. Available online: http://scholar.lib.vt.edu/ejournals/JTE/v17n2/pdf/scott.pdf (accessed on 23 July 2015). [Google Scholar]
- Wells, J.G. Establishing a taxonometric structure for the study of biotechnology in secondary school technology education. J. Technol. Educ. 1994, 6, 1–7. Available online: http://scholar.lib.vt.edu/ejournals/JTE/v6n1/pdf/wells.pdf (accessed on 23 July 2015). [Google Scholar]
- Innovative Medicines Initiative (IMI). Available online: http://www.imi.europa.eu/ (accessed on 23 July 2015).
- Surveymonkey®. The PHAR-IN survey is given in the annex. Available online: https://www.surveymonkey.com/ (accessed on 23 July 2015).
- Likert scales. Available online: http://www.socialresearchmethods.net/kb/scallik.php (accessed on 23 July 2015).
- Leik, R.K. A measure of ordinal consensus. Pac. Sociol. Rev. 1966, 9, 85–90. Available online: http://www.jstor.org/stable/1388242 (accessed on 23 July 2015). [Google Scholar] [CrossRef]
- Graphpad®. Available online: http://www.graphpad.com/ (accessed on 23 July 2015).
- De Villiers, M.R.; de Villiers, P.J.T.; Kent, A.P. The Delphi technique in health sciences education research. Med. Teacher 2005, 27, 639–643. Available online: http://informahealthcare.com/doi/pdf/10.1080/13611260500069947 (accessed on 23 July 2015). [Google Scholar] [CrossRef] [PubMed]
- Goodman, C.M. The Delphi technique: A critique. J. Adv. Nurs. 1987, 12, 729–734. Available online: http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2648.1987.tb01376.x/abstract (accessed on 23 July 2015). [Google Scholar] [CrossRef] [PubMed]
- Marz, R.; Dekker, F.W.; van Schravendijk, C.; O’Flynn, S.; Ross, M.T. Tuning research competences for Bologna three cycles in medicine: Report of a MEDINE2 European consensus survey. Perspect. Med. Educ. 2013, 2, 1–15. Available online: http://www.ncbi.nlm.nih.gov/pmc/issues/222987/ (accessed on 23 July 2015). [Google Scholar] [CrossRef] [PubMed]
- CoDEG: Competency development and evaluation group. The general level framework. Available online: http://www.codeg.org/frameworks/general-level-practice/ (accessed on 23 July 2015).
- Joint FIP/WHO guidelines on good pharmacy practice: Standards for quality of pharmacy services. Available online: http://www.fip.org/good_pharmacy_practice (accessed on 23 July 2015).
- APEL: Accreditation of Prior Experiential Learning. Available online: http://www.city.ac.uk/”courses/cpd/accreditation-of-prior-experiential-learning-apel-claim (accessed on 23 July 2015).
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atkinson, J.; Crowley, P.; De Paepe, K.; Gennery, B.; Koster, A.; Martini, L.; Moffat, V.; Nicholson, J.; Pauwels, G.; Ronsisvalle, G.; et al. A European Competence Framework for Industrial Pharmacy Practice in Biotechnology. Pharmacy 2015, 3, 101-128. https://doi.org/10.3390/pharmacy3030101
Atkinson J, Crowley P, De Paepe K, Gennery B, Koster A, Martini L, Moffat V, Nicholson J, Pauwels G, Ronsisvalle G, et al. A European Competence Framework for Industrial Pharmacy Practice in Biotechnology. Pharmacy. 2015; 3(3):101-128. https://doi.org/10.3390/pharmacy3030101
Chicago/Turabian StyleAtkinson, Jeffrey, Pat Crowley, Kristien De Paepe, Brian Gennery, Andries Koster, Luigi Martini, Vivien Moffat, Jane Nicholson, Gunther Pauwels, Giuseppe Ronsisvalle, and et al. 2015. "A European Competence Framework for Industrial Pharmacy Practice in Biotechnology" Pharmacy 3, no. 3: 101-128. https://doi.org/10.3390/pharmacy3030101








