Nitrous Oxide and Methane Fluxes from Smallholder Farms: A Scoping Study in the Anjeni Watershed
Abstract
:1. Introduction
2. Methodology
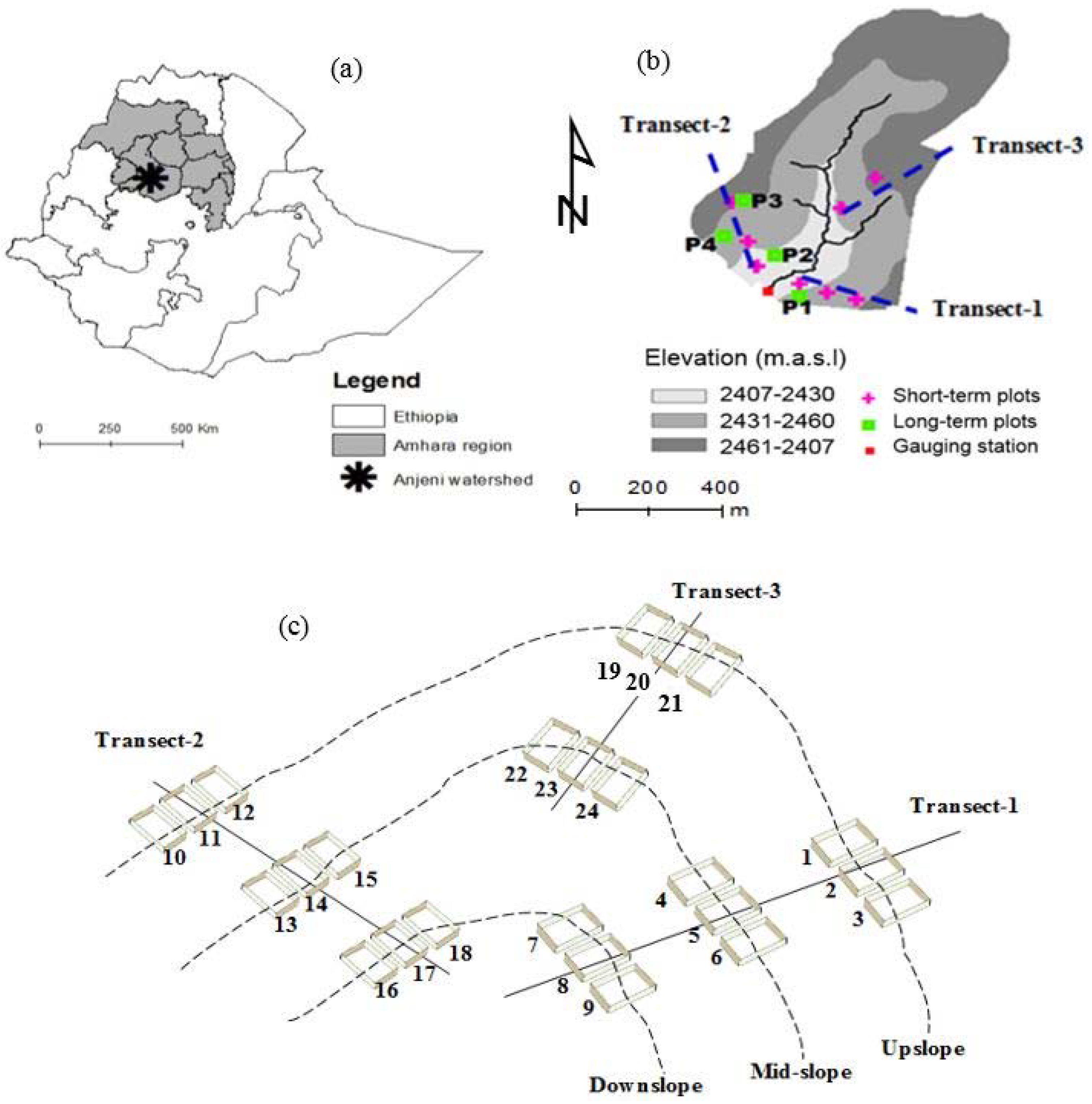
2.1. Study Site Description
2.2. Experimental Setup
2.3. Agronomic Practices on Plots
2.4. Gas Sampling and Flux Calculation
3. Results and Discussions
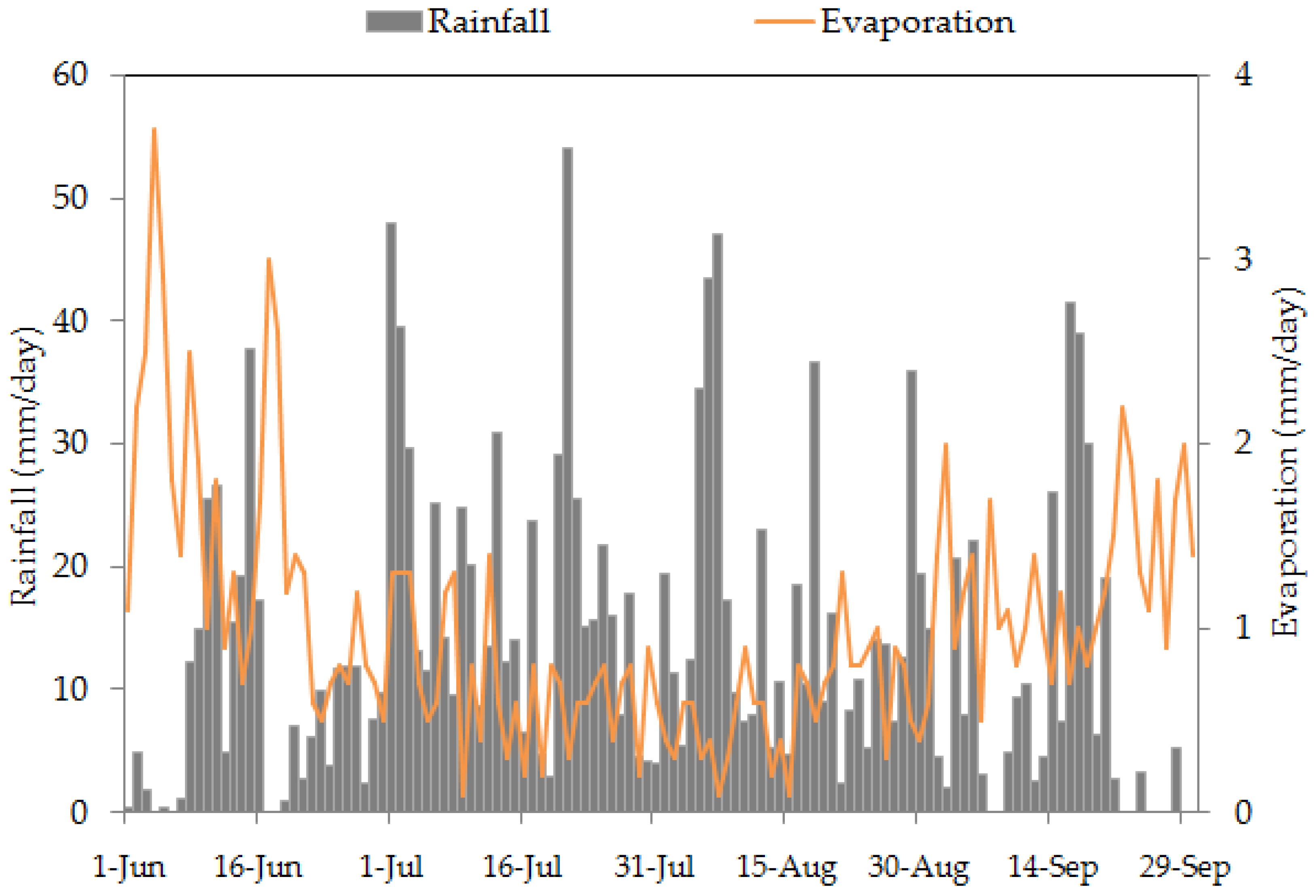
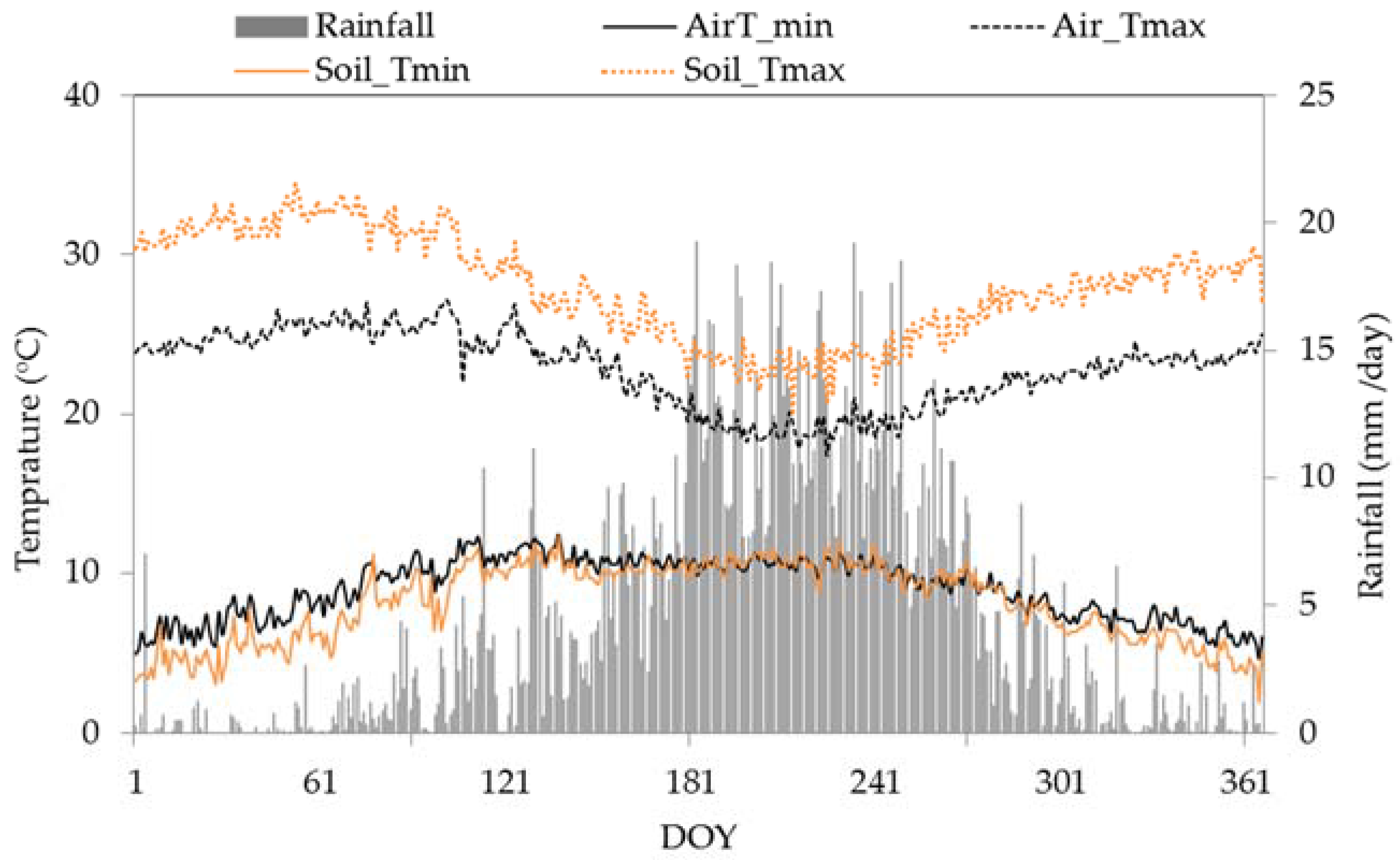
3.1. Weather Characteristics
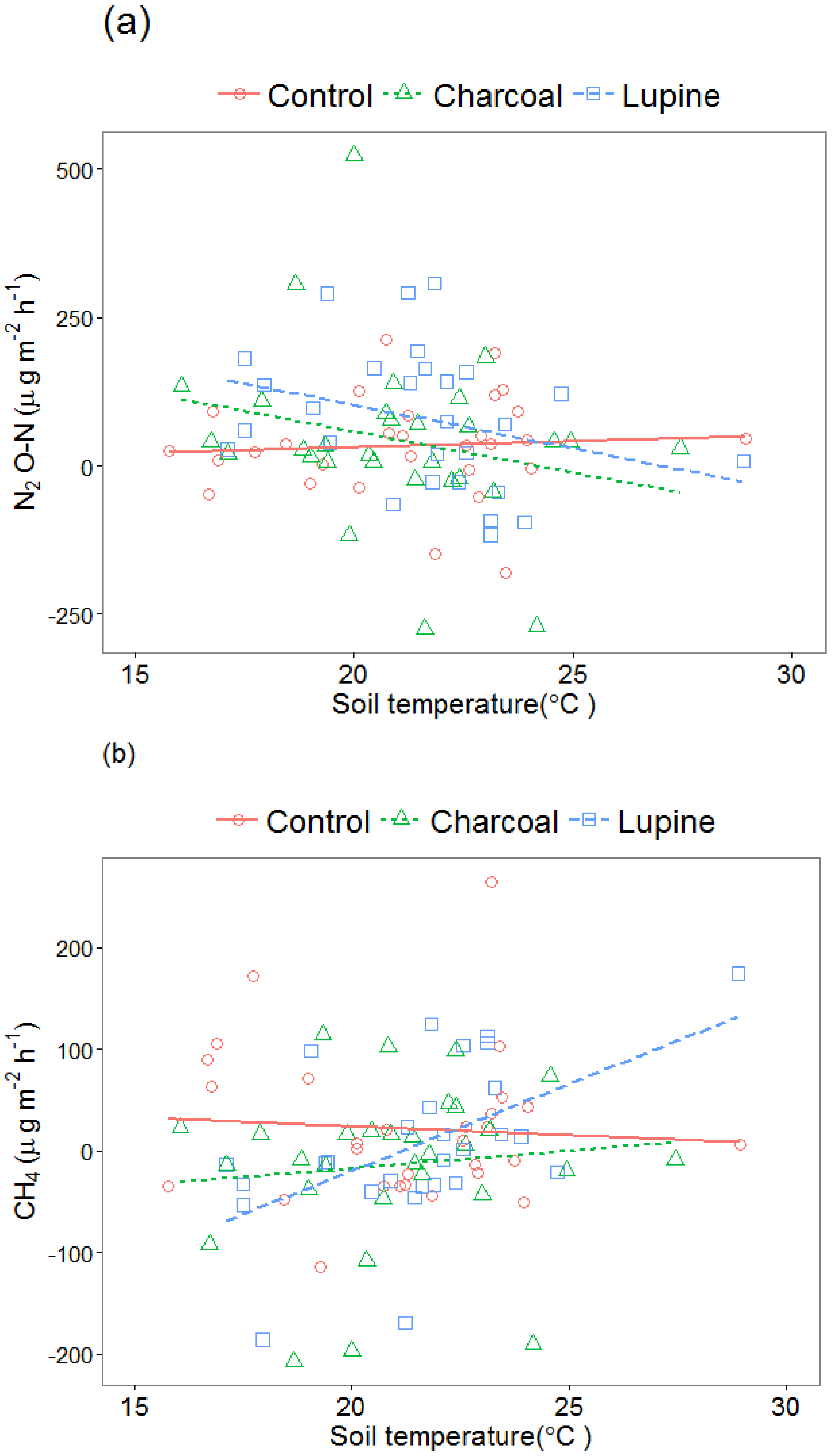
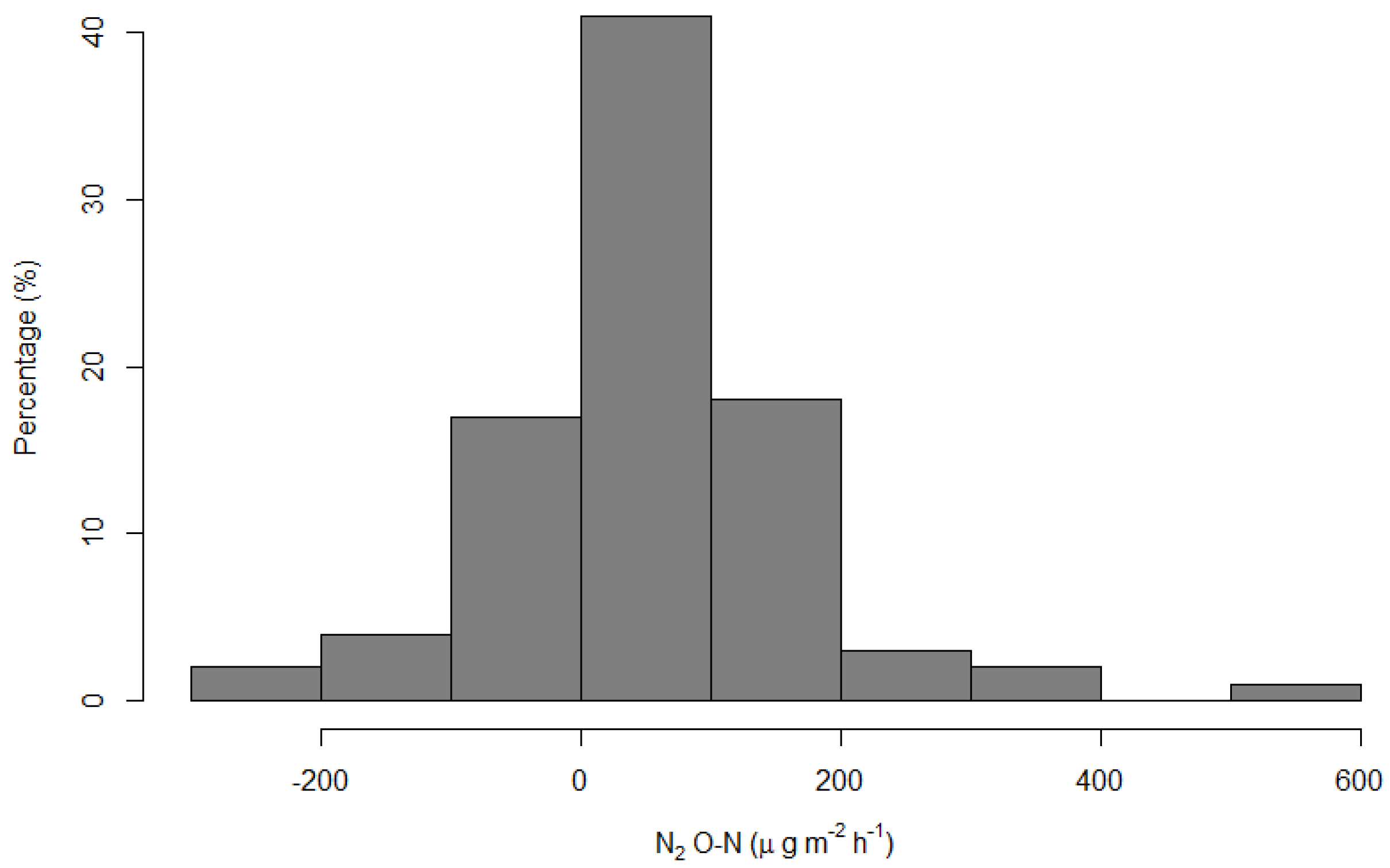
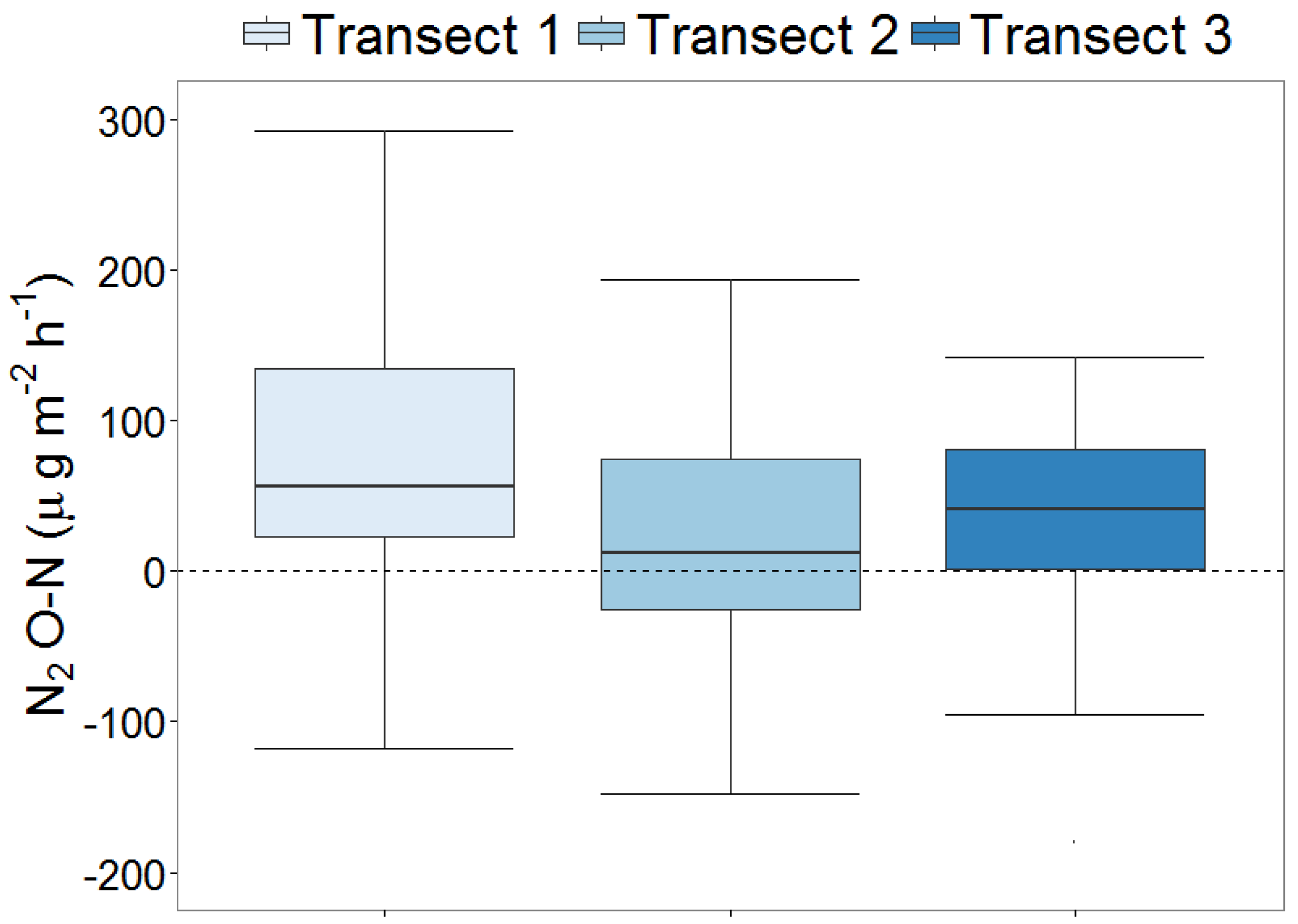
3.2. Nitrous Oxide (N2O-N) Emissions
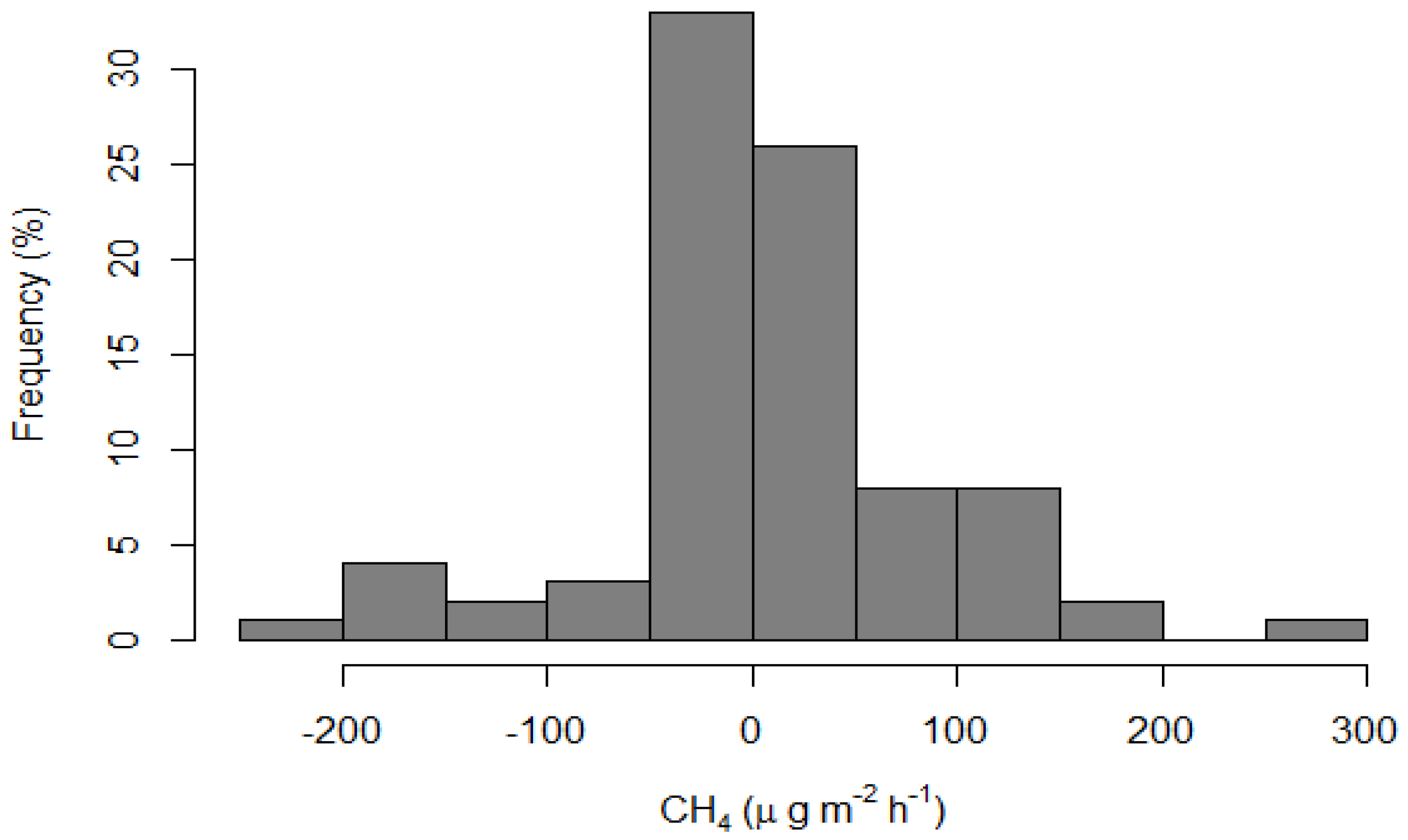
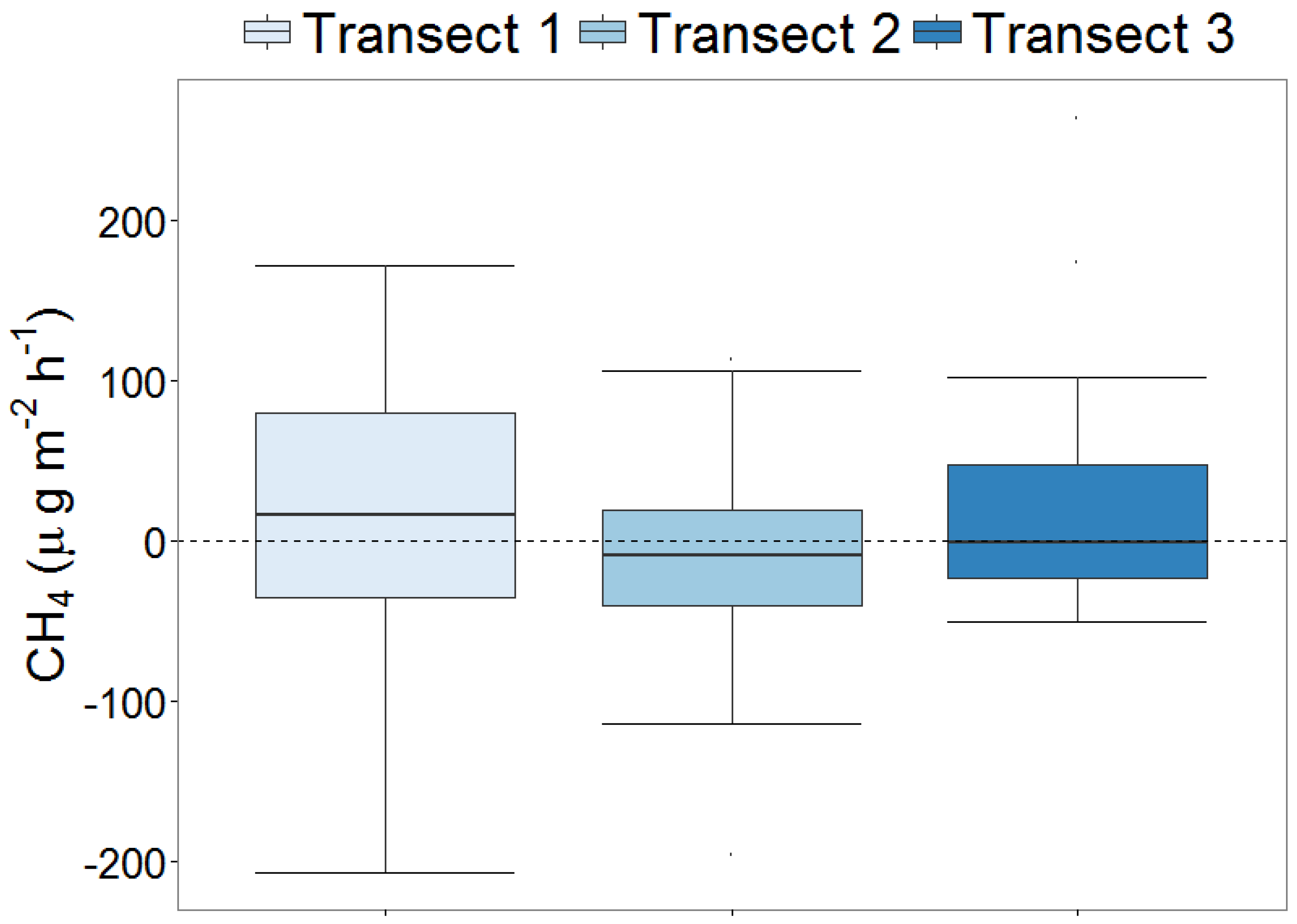
3.3. Methane (CH4) Emissions
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
Appendix B
References
- IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, B., Midgley, B.M., Eds.; IPCC: Paris, France, 2013. [Google Scholar]
- Canadell, J.G.; Raupach, M.R.; Houghton, R.A. Anthropogenic CO2 emissions in Africa. Biogeosciences 2009, 6, 463–468. [Google Scholar] [CrossRef]
- Afful-Koomson, T. The Green Climate Fund in Africa: What should be different? Clim. Dev. 2015, 7, 367–379. [Google Scholar] [CrossRef]
- Jindal, R.; Swallow, B.; Kerr, J. Forestry-based carbon sequestration projects in Africa: Potential benefits and challenges. Nat. Resour. Forum 2008, 32, 116–130. [Google Scholar] [CrossRef]
- Corbera, E.; Estrada, M.; Brown, K. Reducing greenhouse gas emissions from deforestation and forest degradation in developing countries: Revisiting the assumptions. Clim. Chang. 2010, 100, 355–388. [Google Scholar] [CrossRef]
- Keller, M.; Kaplan, W.A.; Wofsy, S.C. Emissions of N2O, CH4 and CO2 from tropical forest soils. J. Geophys. Res. Atmos. 1986, 91, 11791–11802. [Google Scholar] [CrossRef]
- Andersson, M. Soil emissions of nitrous oxide in fire-prone African savannas. J. Geophys. Res. Atmos. 2003, 108, 1769–1778. [Google Scholar] [CrossRef]
- Smith, P.; Martino, D.; Cai, Z.; Gwary, D.; Janzen, H.; Kumar, P.; McCarl, B.; Ogle, S.; O’Mara, F.; Rice, C.; et al. Greenhouse gas mitigation in agriculture. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 789–813. [Google Scholar]
- Chapuis-Lardy, L.; Metay, A.; Martinet, M.; Rabenarivo, M.; Toucet, J.; Douzet, J.M.; Razafimbelo, T.; Rabeharisoa, L.; Rakotoarisoa, J. Nitrous oxide fluxes from Malagasy agricultural soils. Geoderma 2009, 148, 421–427. [Google Scholar] [CrossRef]
- Watson, R.T.; Meira Filho, L.G.; Sanhueza, E.; Janetos, A. Greenhouse gases: Sources and sinks. Clim. Chang. 1992, 92, 25–46. [Google Scholar]
- H’enault, C.; Grossel, A.; Mary, B.; Roussel, M.; L’eonard, J. Nitrous oxide emission by agricultural soils: A review of spatial and temporal variability for mitigation. Pedosphere 2012, 22, 426–433. [Google Scholar] [CrossRef]
- Luo, G.J.; Kiese, R.; Wolf, B.; Butterbach-Bahl, K. Effects of soil temperature and moisture on methane uptake and nitrous oxide emissions across three different ecosystem types. Biogeosciences 2013, 10, 3205–3219. [Google Scholar] [CrossRef]
- Molodovskaya, M.; Singurindy, O.; Richards, B.K.; Warland, J.; Johnson, M.S.; Steenhuis, T.S. Temporal variability of nitrous oxide from fertilized croplands: Hot moment analysis. Soil Sci. Soc. Am. J. 2012, 76, 1728–1740. [Google Scholar] [CrossRef]
- Kammann, C.; Ratering, S.; Eckhard, C.; Müller, C. Biochar and hydrochar effects on greenhouse gas (carbon dioxide, nitrous oxide, and methane) fluxes from soils. J. Environ. Qual. 2012, 41, 1052–1066. [Google Scholar] [CrossRef] [PubMed]
- Singla, A.; Inubushi, K. Effect of biochar on CH4 and N2O emission from soils vegetated with paddy. Paddy Water Environ. 2014, 12, 239–243. [Google Scholar] [CrossRef]
- Wang, J.; Pan, X.; Liu, Y.; Zhang, X.; Xiong, Z. Effects of biochar amendment in two soils on greenhouse gas emissions and crop production. Plant. Soil 2012, 360, 287–298. [Google Scholar] [CrossRef]
- Cayuela, M.L.; van Zwieten, L.; Singh, B.P.; Jeffery, S.; Roig, A.; Sánchez-Monedero, M.A. Biochar’s role in mitigating soil nitrous oxide emissions: A review and meta-analysis. Agric. Ecosyst. Environ. 2014, 191, 5–16. [Google Scholar] [CrossRef]
- Karhu, K.; Mattila, T.; Bergström, I.; Regina, K. Biochar addition to agricultural soil increased CH4 uptake and water holding capacity—Results from a short-term pilot field study. Agric. Ecosyst. Environ. 2011, 140, 309–313. [Google Scholar] [CrossRef]
- Zhang, A.; Bian, R.; Pan, G.; Cui, L.; Hussain, Q.; Li, L.; Zheng, J.; Zheng, J.; Zhang, X.; Han, X.; et al. Effects of biochar amendment on soil quality, crop yield and greenhouse gas emission in a Chinese rice paddy: A field study of 2 consecutive rice growing cycles. Field Crops Res. 2012, 127, 153–160. [Google Scholar] [CrossRef]
- Zhang, A.; Cui, L.; Pan, G.; Li, L.; Hussain, Q.; Zhang, X.; Zheng, J.; Crowley, D. Effect of biochar amendment on yield and methane and nitrous oxide emissions from a rice paddy from Tai Lake plain, China. Agric. Ecosyst. Environ. 2010, 139, 469–475. [Google Scholar] [CrossRef]
- Bremner, J.M.; Blackmer, A.M. Nitrous oxide: Emission from soils during nitrification of fertilizer nitrogen. Science 1978, 199, 295–296. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.A.; Ball, T.; Conen, F.; Dobbie, K.E.; Massheder, J.; Rey, A. Exchange of greenhouse gases between soil and atmosphere: Interactions of soil physical factors and biological processes. Eur. J. Soil Sci. 2003, 54, 779–791. [Google Scholar] [CrossRef]
- Armstrong, R.D.; Kuskopf, B.J.; Millar, G.; Whitbread, A.M.; Standley, J. Changes in soil chemical and physical properties following legumes and opportunity cropping on a cracking clay soil. Anim. Prod. Sci. 1999, 39, 445–456. [Google Scholar] [CrossRef]
- Dakora, F.D.; Keya, S.O. Contribution of legume nitrogen fixation to sustainable agriculture in Sub-Saharan Africa. Soil Biol. Biochem. 1997, 29, 809–817. [Google Scholar] [CrossRef]
- Yang, L.; Cai, Z. The effect of growing soybean (Glycine max. L.) on N2O emission from soil. Soil Biol. Biochem. 2005, 37, 1205–1209. [Google Scholar] [CrossRef]
- Herweg, K.; Ludi, E. The performance of selected soil and water conservation measures—Case studies from Ethiopia and Eritrea. Catena 1999, 36, 99–114. [Google Scholar] [CrossRef]
- Zeleke, G. Landscape Dynamics and Soil Erosion Process Modelling in the North-Western Ethiopian Highlands; African Studies Series A 16; Geographica Bernensia: Berne, Switzerland, 2000. [Google Scholar]
- Bayabil, H.K.; Tebebu, T.Y.; Stoof, C.R.; Steenhuis, T.S. Effects of a deep-rooted crop and soil amended with charcoal on spatial and temporal runoff patterns in a degrading tropical highland watershed. Hydrol. Earth Syst. Sci. 2016, 20, 875–885. [Google Scholar] [CrossRef]
- Bayabil, H.K.; Tebebu, T.Y.; Stoof, C.R.; Steenhuis, T.S. Spatial and temporal runoff processes in the degraded Ethiopian Highlands: The Anjeni Watershed. Hydrol. Earth Syst. Sci. Discuss. 2015, 19, 1–25. [Google Scholar] [CrossRef]
- Bayabil, H.K.; Stoof, C.R.; Lehmann, J.C.; Yitaferu, B.; Steenhuis, T.S. Assessing the potential of biochar and charcoal to improve soil hydraulic properties in the humid Ethiopian Highlands: The Anjeni watershed. Geoderma 2015, 243–244, 115–123. [Google Scholar] [CrossRef]
- Mason, C. Spring-Thaw Nitrous Oxide Emissions from Reed Canary Grass on Wet Marginal Soil in New York State. Master’s Thesis, Cornell University, Ithaca, NY, USA, 2014. [Google Scholar]
- Molodovskaya, M.; Warland, J.; Richards, B.K.; Öberg, G.; Steenhuis, T.S. Nitrous oxide from heterogeneous agricultural Landscapes: Source contribution analysis by eddy covariance and Chambers. Soil Sci. Soc. Am. J. 2011, 75, 1829–1838. [Google Scholar] [CrossRef]
- Gogoi, B.; Baruah, K.K. Nitrous oxide emissions from fields with different wheat and rice varieties. Pedosphere 2012, 22, 112–121. [Google Scholar] [CrossRef]
- Wang, Z.; Hao, X.; Shan, D.; Han, G.; Zhao, M.; Willms, W.D.; Wang, Z.; Han, X. Influence of increasing temperature and nitrogen input on greenhouse gas emissions from a desert steppe soil in Inner Mongolia. Soil Sci. Plant Nutr. 2011, 57, 508–518. [Google Scholar] [CrossRef]
- Sainju, U.M.; Stevens, W.B.; Caesar-TonThat, T.; Liebig, M.A. Soil greenhouse gas emissions affected by irrigation, tillage, crop rotation, and nitrogen fertilization. J. Environ. Qual. 2012, 41, 1774–1786. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Tang, J.; Zhang, R.; Wu, Q.; Gong, M. Effects of biochar application on soil methane emission at different soil moisture levels. Biol. Fertil. Soils 2013, 49, 119–128. [Google Scholar] [CrossRef]
- Sanz-Cobena, A.; García-Marco, S.; Quemada, M.; Gabriel, J.L.; Almendros, P.; Vallejo, A. Do cover crops enhance N2O, CO2 or CH4 emissions from soil in Mediterranean arable systems? Sci. Total Environ. 2014, 466–467, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Whalen, S.; Reeburgh, W. Moisture and temperature sensitivity of CH4 oxidation in boreal soils. Soil Biol. Biochem. 1996, 28, 1271–1281. [Google Scholar] [CrossRef]

| Transect | Position | Plots | Elevation | Slope | Sand | Silt | Clay | OM | Pb | D |
|---|---|---|---|---|---|---|---|---|---|---|
| (m a.s.l.) | (%) | (%) | (g·cm−3) | (m) | ||||||
| One | Upslope | 1, 2, 3 | 2438 | 3.0 | 24.8 | 35.4 | 39.8 | 2.2 | 1.1 | 1.15 |
| Mid-slope | 4, 5, 6 | 2431 | 2.5 | 31.7 | 28.0 | 40.3 | 2.1 | 1.1 | 1.22 | |
| Down slope | 7, 8, 9 | 2411 | 1.5 | 23.6 | 36.7 | 39.6 | 2.2 | 1.1 | >1.3 | |
| Two | Upslope | 10, 11, 12 | 2461 | 2.5 | 23.8 | 32.2 | 44.0 | 2.1 | 1.1 | 0.84 |
| Mid-slope | 13, 14, 15 | 2426 | 2.0 | 17.8 | 39.0 | 43.2 | 2.4 | 1.2 | 1.09 | |
| Down slope | 16, 17, 18 | 2415 | 1.0 | 24.7 | 36.3 | 39.0 | 2.4 | 1.3 | >1.3 | |
| Three | Upslope | 19, 20, 21 | 2455 | 3.0 | 21.0 | 37.7 | 41.4 | 1.3 | 1.4 | 0.33 |
| Mid-slope | 22, 23, 24 | 2438 | 2.0 | 30.6 | 37.4 | 32.0 | 1.4 | 1.3 | 0.72 | |
| Transect | Elevation | Control | Charcoal | Lupine | |||
|---|---|---|---|---|---|---|---|
| Range | Mean (±SE) | Range | Mean (±SE) | Range | Mean (±SE) | ||
| (μg·m−2·h−1) | |||||||
| Transect 1 | Upslope | −48.9–51.5 | 9.3 ± 21.3 | 20.4–139.8 | 101.1 ± 27.7 | −27.4–135.6 | 48.9 ± 34 |
| Mid-slope | 35.5–212 | 93.7 ± 41.5 | −117.5–41.3 | −20.7 ± 34.7 | 74.3–180.8 | 127.6 ± 25 | |
| Downslope | −29.5–188.8 | 77.7 ± 49.4 | −270.9–305.1 | 33.2 ± 118.1 | 290.5–307.1 | 296.5 ± 5.3 | |
| Transect 2 | Upslope | −6.3–126.7 | 51.9 ± 34 | −43.1–6.7 | −13.7 ± 12.2 | −91.9–165.2 | 15.5 ± 77.2 |
| Mid-slope | −53.3–36.1 | −17.8 ± 27.4 | 26.5–183.3 | 99.4 ± 45.6 | −116.5–193.2 | 32.8 ± 89.6 | |
| Downslope | −148.4–56 | −30.4 ± 61.1 | 6.2–522.3 | 182.1 ± 170.1 | 38.5–163.7 | 90.8 ± 37.6 | |
| Transect 3 | Upslope | −179.9–84.6 | 0.1 ± 60.6 | −26.2–114.5 | 48.4 ± 30.4 | −95.1–139.1 | 18 ± 47.9 |
| Mid-slope | −6–118.4 | 43.2 ± 27.1 | −275–70.4 | −30.9 ± 81.7 | −65–141.8 | 38.4 ± 54.1 | |
| Average | 32 ±15.2 | 43.8 ± 26.3 | 79.9 ± 22.2 | ||||
| Control | Charcoal | Lupine | |||||
|---|---|---|---|---|---|---|---|
| Transect | Elevation | Range | Mean ± SE | Range | Mean ± SE | Range | Mean ± SE |
| Transect 1 | Upslope | −35.1–105.4 | 34.6 ± 36.5 | −13.8–22.5 | 10.6 ± 8.3 | −185.8–42.7 | −52.4 ± 48.6 |
| Mid-slope | −47.9–62.5 | −2.4 ± 25 | −92.6–98 | −4 ± 40.7 | −33.1–103.5 | 46.2 ± 33.1 | |
| Downslope | 36.2–172.1 | 95.7 ± 28.9 | −206.7–114.6 | −69.3 ± 78 | −169–124.7 | −18.6 ± 84.9 | |
| Transect 2 | Upslope | −9.8–43.8 | 15 ± 11.8 | −15.4–20.1 | 3.5 ± 8.1 | −40.1–106.5 | 11.7 ± 47.5 |
| Mid-slope | −13.7–24.1 | 5.9 ± 10.9 | −47.1–−9.3 | −33.1 ± 12 | −45.7–113 | 22.9 ± 47 | |
| Downslope | −114.2–20.3 | −45.8 ± 38.9 | −196.1–19.5 | −94.7 ± 62.6 | −34.7–16.2 | −9.9 ± 14.7 | |
| Transect 3 | Upslope | −35.4–52.3 | −2.6 ± 20.6 | −9.3–102.8 | 45.7 ± 22.9 | −33.2–174.5 | 44.7 ± 45 |
| Mid-slope | −50.9–264.4 | 53.9 ± 71.9 | −23.1–73.4 | 4.8 ± 23 | −29.5–61.9 | 0.8 ± 20.8 | |
| Average | 21.9 ± 13.5 | −13.9 ± 14.5 | 6.3 ± 15.1 | ||||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bayabil, H.K.; Stoof, C.R.; Mason, C.; Richards, B.K.; Steenhuis, T.S. Nitrous Oxide and Methane Fluxes from Smallholder Farms: A Scoping Study in the Anjeni Watershed. Climate 2016, 4, 62. https://doi.org/10.3390/cli4040062
Bayabil HK, Stoof CR, Mason C, Richards BK, Steenhuis TS. Nitrous Oxide and Methane Fluxes from Smallholder Farms: A Scoping Study in the Anjeni Watershed. Climate. 2016; 4(4):62. https://doi.org/10.3390/cli4040062
Chicago/Turabian StyleBayabil, Haimanote K., Cathelijne R. Stoof, Cedric Mason, Brian K. Richards, and Tammo S. Steenhuis. 2016. "Nitrous Oxide and Methane Fluxes from Smallholder Farms: A Scoping Study in the Anjeni Watershed" Climate 4, no. 4: 62. https://doi.org/10.3390/cli4040062






