1. Introduction
In coniferous gymnosperms (softwoods), compression wood (CW) is a reaction wood formed when growing stems are tilted from the vertical as the result of various factors such as the weight of snow or the slope of the land. CW forms on the lower side of tilted stems to correct the growth to the vertical [
1]. The wood formed on the opposite side of the stem is referred to as opposite wood (OW) and is very similar in structure and composition to normal wood (NW). Although CW formation is beneficial to the tree, CW is regarded as a defect in the timber industry. When wood is dried and rewetted, it shrinks and swells and this dimensional instability is a major obstacle in the use of wood, especially for structural purposes [
2]. CW shows particularly high longitudinal shrinkage and swelling [
1]. Because of the differences in longitudinal shrinkage and swelling between CW and OW or NW, where these wood types occur adjacent to one another in timber, severe warping can occur with a loss of value [
2]. CW also shows differences from OW and NW in tangential and radial shrinkage but the differences are much less pronounced [
1].
During drying, wood shrinkage occurs only when all the free water, which is water in the lumens of the cells or in intercellular spaces, has been removed, a point known as the fibre saturation point. Wood shrinkage and swelling depend on the amount of bound water, which is water adsorbed within the cell walls. Thus, cell walls play a pivotal role in these processes [
3,
4]. In softwoods, the cell walls are mostly of tracheids because softwoods are composed predominantly of this cell type [
5]. Tracheids are thick-walled, elongated cells that in CW are shorter and have thicker walls than in OW and NW. As in all plant cell walls, these walls consist of a fibrillar phase in the form of cellulose microfibrils set in a matrix phase. In NW and OW, the tracheid walls consist of a thin primary wall and a thick secondary wall composed of three layers (S1, S2 and S3), with the middle layer (S2) being the thickest. The angle the cellulose microfibrils make to the vertical axis of the tracheid in the S2 layer is known as the cellulose microfibril angle (MFA). In stems of the commercially important coniferous gymnosperm
Pinus radiata (radiata pine), two regions of wood have been recognized: corewood, which is formed in the first ten growth rings from the pith; and outerwood, which is formed further out [
6]. In the outerwood, the MFA in tracheid walls of CW is higher than in tracheid walls of NW or OW and is considered to be related to the high longitudinal shrinkage of CW [
7]. In corewood, the MFA in tracheid walls of NW or OW is already high (>~35°) and is the same or similar to that in tracheid walls of CW [
8,
9]. Thus, even though a high MFA may be necessary for high longitudinal shrinkage of CW, it is not sufficient and polymers in the cell-wall matrix are likely to play an important role [
10].
The cell-wall matrix of NW and OW tracheids of gymnosperms, including
P. radiata, are composed of the non-cellulosic polysaccharides heteromannans (
O-acetyl-galactoglucomannans) and smaller proportions of heteroxylans [arabino(4-
O-methylglucurono)xylans], in addition to the aromatic polymer lignin composed mostly of guaiacyl (G) units, with only trace amounts of
p-hydroxyphenyl (H) units [
8,
11]. In comparison, the cell-wall matrix of CW tracheids contains smaller proportions of heteromannans and heteroxylans but larger proportions of lignin containing significant proportions of H-units. Additionally, the cell-wall matrix of CW tracheids contains (1→4)-β-galactans (~10%) and small proportions of (1→3)-β-glucans [
8,
12,
13]. Positive statistical associations have been found between longitudinal shrinkage and the content of (1→4)-β-galactans in CW in the corewood of
P. radiata saplings [
8]. A similar association has also been found between longitudinal shrinkage and lignin content. Thus, positive associations have been found between longitudinal shrinkage of CW and two cell-wall matrix components.
As described above, CW is more accurately referred to as severe CW (SCW) but it is increasingly being recognized that a continuum of CWs occur between SCW and NW or OW, with the intermediate grades, being referred to as mild CWs (MCWs) [
14,
15]. These MCWs have been much less studied than SCW. However, we recently reported two studies on two grades of MCWs, MCW1 and MCW2, as well as SCW and OW, in stem corewood of
P. radiata saplings [
12,
13]. The saplings were tilted from the vertical to induce the formation of CW but only a small tilt angle (~8–20°) was used to try to maximize MCW formation relative to SCW. The different wood types were identified based on the distribution of lignin in the tracheid cell walls in transverse sections. This was determined by the distribution of lignin autofluorescence using UV fluorescence microscopy [
16]. In OW tracheids, the lignin was mostly in the S3 layer of the secondary wall, in the compound middle lamella (middle lamella plus primary cell walls) and in the middle lamella in the cell corners. In MCW1 tracheids, some lignin was also present at the cell corners in the outer region of the S2 layer (S2L region). In MCW2 tracheids, lignin in this outer region of the S2 layer (S2L region), was present all around the cells. In SCW tracheids, greater amounts of lignin were in this S2L region and there was no lignin in the CML at any position. The cells also became more circular in transverse section in going from OW to SCW. A S3 secondary wall layer was present in OW tracheids and a very thin one was also present in MCW1 tracheids but not in MCW2 or SCW tracheids. SCW was the only wood type with tracheid walls having helical cavities in the inner region (S2i region) of the S2 layer.
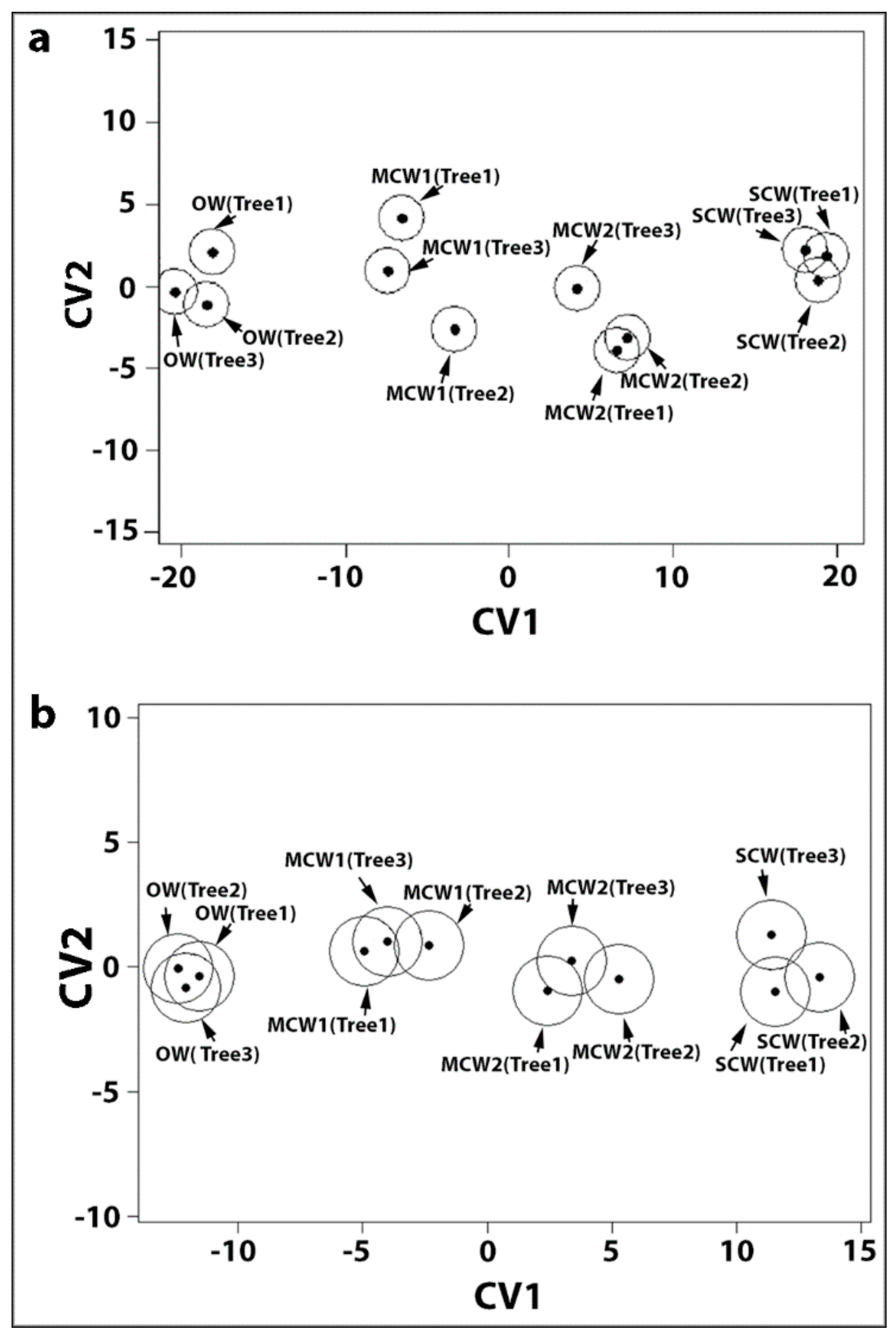
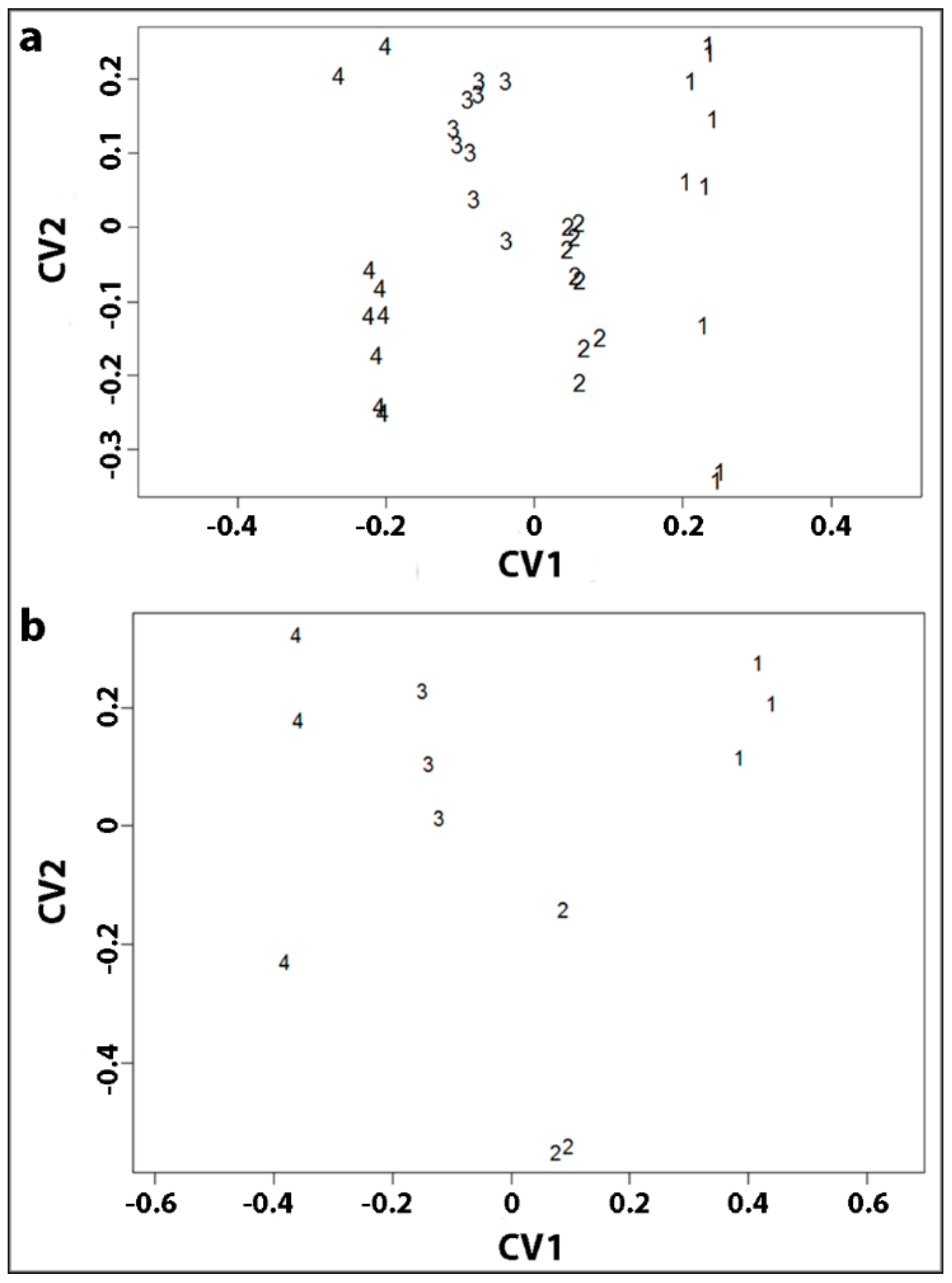
All our studies were carried out on small discs of wood, 0.5 mm in diameter that were all of a specific wood type, either OW, MCW1, MCW2 or SCW [
12,
13]. This permitted very accurately defined wood types to be used although only small amounts of wood were available for the investigations. We had earlier reported that there were no significant differences between the high cellulose MFAs in tracheid cell walls of SCW, OW and NW in stem corewood of
P. radiata saplings of similar age [
8]. We therefore focused on tracheid cell-wall matrix polymers, rather than cellulose, because of their likely importance in longitudinal shrinkage of compression corewoods. The lignin content was found to increase with CW severity, with OW, MCW1, MCW2 and SCW containing 26%, 28%, 32% and 35% lignin respectively. The H-unit content of the lignin also increased with CW severity, with OW, MCW1, MCW2 and SCW containing >1%, 7%, 11% and 14%, respectively and the H/G ratio increased in the same order [
13]. Immunofluorescence and immunogold microscopy with monoclonal antibodies was used to specifically determine the distributions of (1→4)-β-galactans and (1→3)-β-glucans in the tracheid cell walls. This showed that the (1→4)-β-galactans occurred as a band in the outer region of the S2 wall layer (S2L region) of the tracheid walls in all three grades of CW, with the band becoming wider and more intense in the order MCW1, MCW2 and SCW. The (1→4)-β-galactans thus co-located with lignin in CW tracheids. There was only extremely weak labelling of the tracheid walls in OW, probably of the primary walls. As with the (1→4)-β-galactans, the (1→3)-β-glucans also occurred as a band in the S2 wall layer of CW tracheids, which became wider and more intense with CW severity. However, the (1→3)-β-glucans were located in the inner region (S2i region) of the S2 wall layer. Differences between the wood types were also found in the neutral-monosaccharide compositions of the non-cellulosic polysaccharides. In particular, the proportion of galactose was consistent with the immunolabelling results for (1→4)-β-galactans, with the lowest in OW, the highest in SCW and intermediate proportions in MCW1 and MCW2.
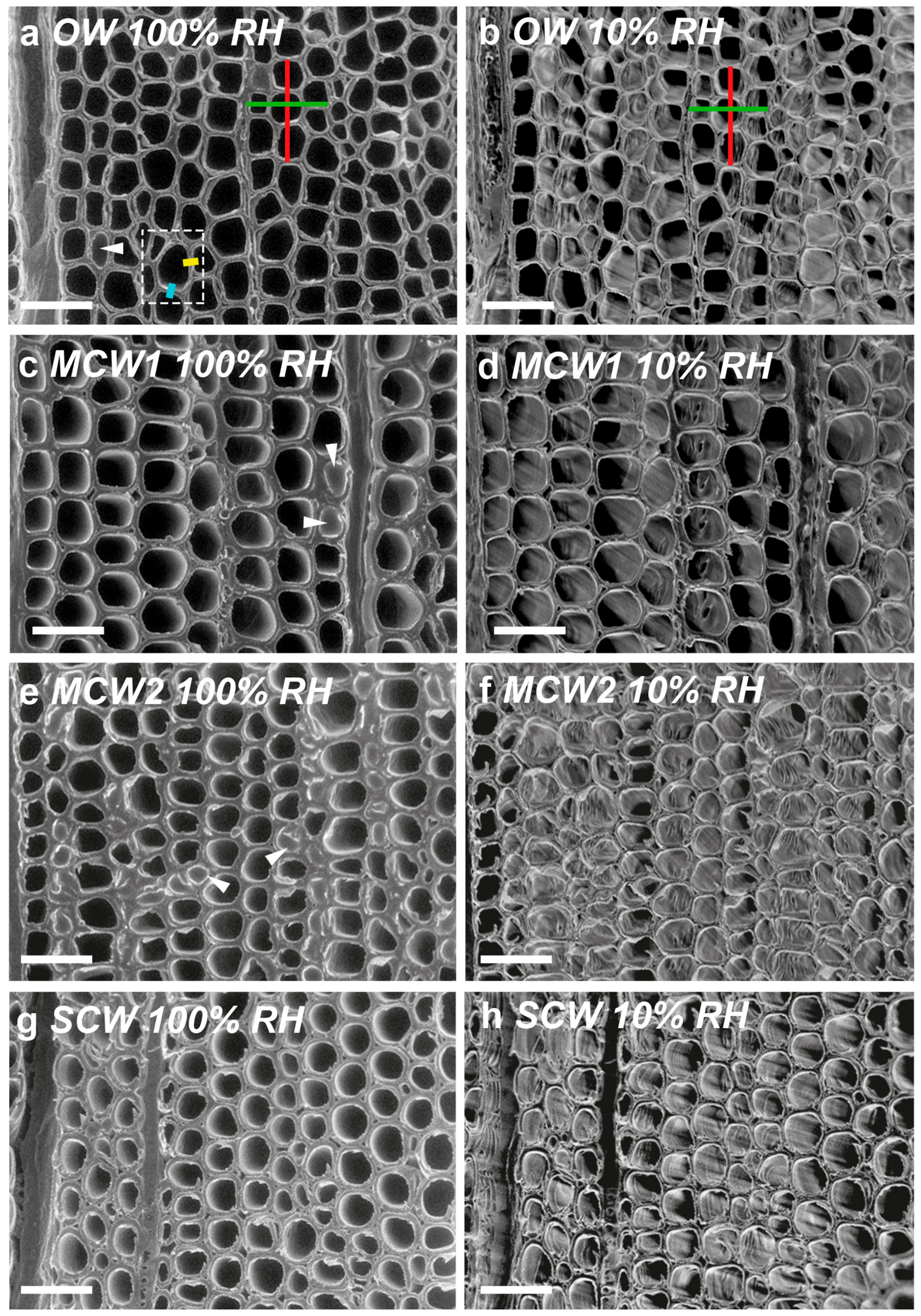
In the present study, we used similar, small discs cut from the same four corewood types and determined the longitudinal shrinkage and swelling of the tracheids, as well as their radial and tangential shrinkage, by variable-pressure scanning electron microscopy (VP-SEM), sometimes referred to as environmental scanning electron microscopy (ESEM). VP-SEM is an excellent technique for this purpose because the samples can be imaged without pre-treatment. The samples can be hydrated (adsorption) and dehydrated (desorption) by altering the water vapour pressure and hence the relative humidity in the chamber [
17]. The technique has been used to study tracheid shrinkage in earlywood and latewood of Norway spruce (
Picea abies) but CW has not been examined [
18]. We hypothesized that, based on the composition of the cell-wall matrix polymers, the longitudinal shrinkage and swelling of the tracheids in the MCW1 and MCW2 would be intermediate between those in OW and SCW.
3. Discussion
As we hypothesized, the present study showed that the longitudinal shrinkage of the tracheids of the two grades of MCW, MCW1 and MCW2, was intermediate between that of OW and SCW, with the shrinkage of MCW1 being less than MCW2. The percentage longitudinal shrinkage of OW and SCW tracheids was similar to that reported by others for radiata pine in which the shrinkage of small strips (e.g., 3 mm radial × 10 mm tangential × 100 mm longitudinal) of wood was measured using micrometre callipers [
20,
21,
22]. The shrinkage was also similar to the longitudinal swelling of OW, NW and SCW reported by Brennan et al. [
8] also using similar strips of radiata pine corewood. Interestingly, one of these studies [
22] included wood strips that contained MCW, although the size of the strips would preclude examining all the tracheids to check if they were indeed MCW. Nevertheless, the MCW strips also showed longitudinal shrinkage intermediate between SCW and NW.
Our longitudinal shrinkage results are consistent with the tracheid walls of the MCWs MCW1 and MCW2 containing intermediate proportions of (1→4)-β-galactans determined both by immunomicroscopy, which showed they were present in the S2L region of the tracheid walls and by determining the neutral monosaccharide compositions of the cell-wall matrix polysaccharides [
12]. Statistically, there was a positive association between longitudinal shrinkage and the proportions of galactosyl residues as a percentage of the total neutral monosaccharides released by acid hydrolysis conditions that did not hydrolyse cellulose. Similar associations have previously been reported between longitudinal swelling and the proportions of galactose in acid hydrolysates of OW, NW and SCW of corewood of radiata pine [
8] and between longitudinal shrinkage and the proportions of galactose in acid hydrolysates of logs of 24-year-old, plantation-grown loblolly pine (
Pinus taeda) [
23]. (1→4)-β-Galactans are known to have a high capacity for binding water and swell on adsorbing water (hydration) [
24,
25,
26]. These polysaccharides may thus play a functional role in the longitudinal shrinkage and swelling of CWs.
A statistically positive association was also found in the present study between longitudinal shrinkage and lignin contents. A similar association has also been reported between lignin content and longitudinal swelling of corewood SCW [
8]. However, lignin is a hydrophobic molecule and does not have a high capacity for binding water [
27]. Lignin therefore probably does not play a functional role in the longitudinal shrinkage and swelling of CWs. Nevertheless, the formation and location of lignin and (1→4)-β-galactans are closely related and it is possible that this close relationship results in the statistical association. Our earlier studies showed that with increasing CW severity, the band of (1→4)-β-galactans in the S2L region of the tracheid walls increased in width and, in the same region, lignin autofluorescence also increased [
12,
13]. The (1→4)-β-galactans are therefore co-located with lignin and together increase in concentration with CW severity. The two components are probably covalently linked as partial delignification of radiata pine SCW resulted in increased amounts of (1→4)-β-galactans that could be extracted using 6 M sodium hydroxide [
28]. This finding is consistent with the isolation of a complex containing (1→4)-β-galactans and lignin from Japanese red pine (
Pinus densiflora) [
29]. The synthesis of the (1→4)-β-galactans probably slightly precedes lignification in the S2L region, with the covalent cross links between the two components probably being formed by reactions involving quinone methide intermediates during lignin synthesis [
27].
In addition to (1→4)-β-galactans, small proportions of (1→3)-β-glucans were found in all grades of CW and increase with increasing CW severity [
12]. They were detected by immunomicroscopy and also by staining with pure, synthetic aniline blue fluorochrome, which specifically binds to (1→3)-β-glucans and were found to be located in the inner region of the S2 layer (S2i region) [
12]. Like (1→4)-β-galactans, these polysaccharides have a high capacity for binding water and are known to swell on adsorbing water (hydration) [
30,
31,
32]. Consequently, they may also contribute to the longitudinal shrinkage and swelling of CW. Our immunogold microscopy study [
12] showed that, in SCW, these polysaccharides were located in the helical cavities in the inner region of the S2 layer. A previous immunogold microscopy study [
33] also reported the same location of (1→3)-β-glucans in the tracheid walls in SCW of radiata pine and of Sitka spruce (
Picea sitchensis) and Norway spruce (
Picea abies). Interestingly, in the two MCWs of radiata pine, the (1→3)-β-glucans were also located in the S2i region of the tracheid walls, despite there being no cavities [
12]. The presence of the glucans in the helical cavities of the SCW walls led to the suggestion they may act as sealants for these cavities [
33] but the absence of cavities in the MCW walls suggests this is not their only function.
The hysteresis we found for the sorption curves for the four different corewood types has previously been reported for softwoods [
4]. The bound water in tracheid walls that is responsible for the dimensional changes, is thought to be mostly hydrogen bonded to the hydroxyl groups of the non-cellulosic polysaccharides. The hysteresis being explained by it being more difficult for water to penetrate tracheid walls, with low water contents and hydrogen bond to hydroxyl groups of the non-cellulosic polysaccharides (adsorption) than for these hydrogen bonds to be broken and the water leave the walls (desorption). However, the complete recovery of the starting dimensions when the samples were “rehydrated” in the VP-SEM is consistent with the finding that all wood cell-wall hydroxyl groups were accessible to water vapour after air drying [
34]. The degree of hysteresis was greater for CW and increased with CW severity. This may be related to the differences in the cell-wall matrix compositions of the different corewood types. Alternatively, it may be related to the differences in the tracheid wall thickness, which also increased with increasing CW severity. The degree of hysteresis in transverse shrinkage (tangential and radial) has previously been related to tracheid wall thickness in Norway spruce (
Picea abies) [
35].
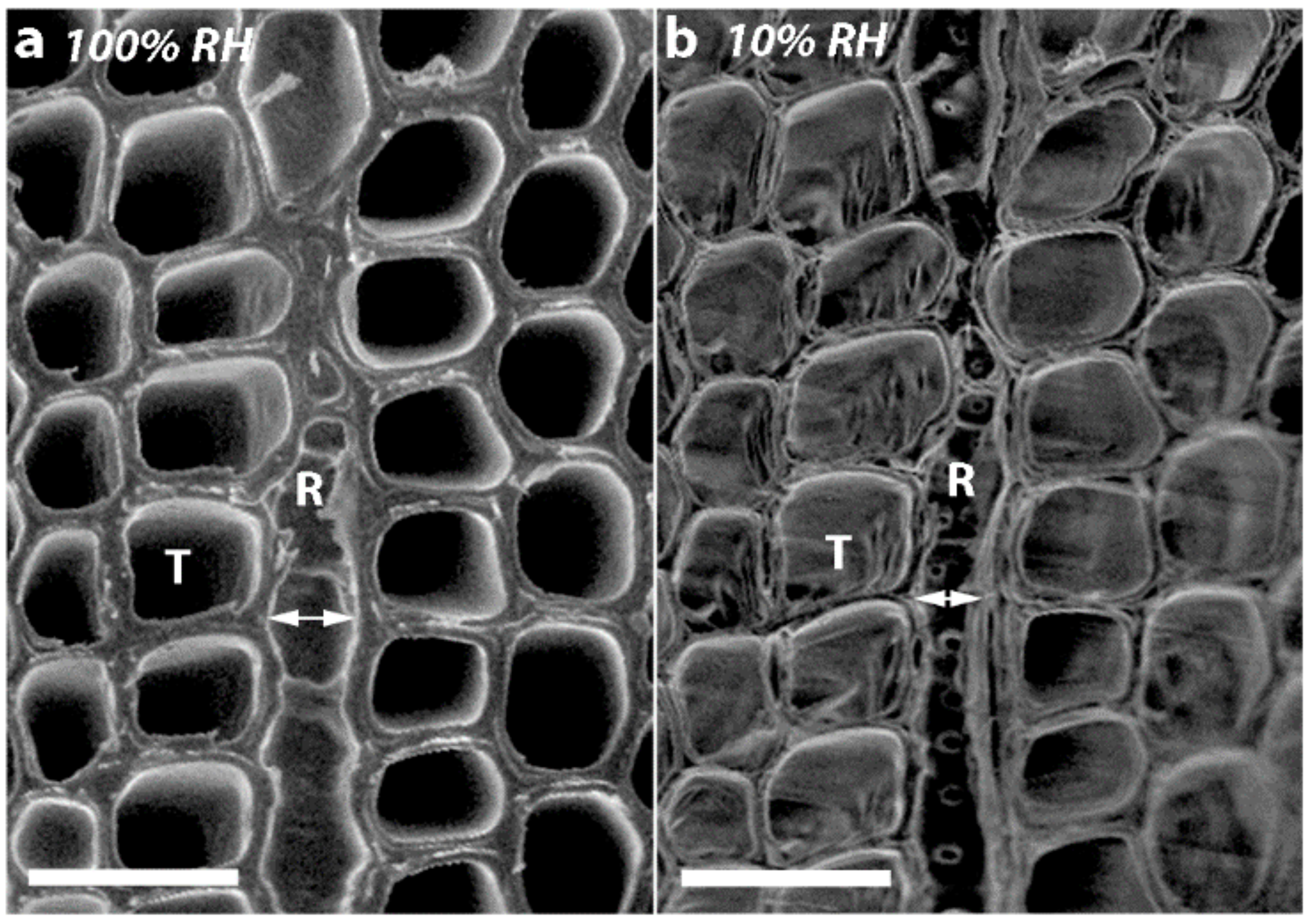
As all the tracheids in softwoods are joined through their MLs, it is not surprising that if there is shrinkage in the longitudinal direction, there will also be dimensional changes in the tangential and radial directions. Indeed, in all the corewood types, the tracheids also showed tangential and radial shrinkage, with the tangential shrinkage being about twice that of the radial shrinkage. Similar higher values for tangential than for radial shrinkage have been reported for NW of softwood species, including radiata pine [
2]. Interestingly, when the transverse surfaces of the discs were examined, the shrinkage in thickness of the radial walls, which is shrinkage in the tangential direction, was about twice that of the shrinkage in thickness of the tangential walls, which is shrinkage in the radial direction. Thus, the relative shrinkage of the tangential and radial walls appears to play a major part in determining the anisotropy of transverse shrinkage. What actually determines these shrinkage differences between the radial and tangential walls is less clear. One factor may be the presence of bordered pits in the radial but not in the tangential walls. The cellulose microfibrils in the radial walls deviate around pits, probably affecting the wall shrinkage [
36]. This is consistent with the finding in our study that the diameters of the bordered pits remain almost unchanged on drying in all the corewood types. A factor that may also cause less radial than tangential shrinkage of tracheids is the arrangement of the tracheids. As was also shown in the present study, they are regularly ordered in the radial direction but less so in the tangential direction. In hardwoods, the rays have also been found to restrain shrinkage in the radial direction [
2,
37]. However, in the softwood
Pinus sylvestris, cross sections of wood with and without rays showed no difference in shrinkage, suggesting they are not a limiting factor in softwood shrinkage [
38].
In the present study, we found that in addition to the tracheids of SCW having a much higher (~300% higher) longitudinal shrinkage than the tracheids in OW, the tangential and radial shrinkage of the tracheids was less by ~37% and ~32%, respectively. Similar, lower tangential and radial shrinkage values for SCW than NW (or OW) have been reported for a number of softwood species [
1]. Our results extend this information by showing that the tangential and radial shrinkage of the tracheids of the two mild CWs was intermediate between that of OW and SCW. In a similar way to the tangential and radial shrinkage of OW, the relative shrinkage of the tangential and radial walls appears to play a major part in determining the anisotropy of transverse shrinkage of the CWs. The lower shrinkage of the tangential and radial walls of the CWs, relative to that of OW, may result from the much greater longitudinal shrinkage driven by the presence of the (1→4)-β-galactans and possibly (1→3)-β-glucans.