Short-Term Response of Sasa Dwarf Bamboo to a Change of Soil Nitrogen Fertility in a Forest Ecosystem in Northern Hokkaido, Japan
Abstract
:1. Introduction
2. Results
2.1. The Response of Sasa and Soil to a Change of Soil N Fertility
2.2. Estimation of the N Budget
3. Discussion
3.1. Effect of Soil N Fertility Changes on the Leaf N, Biomass and Litter Decomposition
3.2. The Response of the Sasa and Soil System to Increased Soil N Fertility
4. Experimental Section
4.1. Site Description
4.2. N Addition Experiment
4.3. Aboveground Biomass
4.4. Litterfall
4.5. Litter Decomposition Experiment
4.6. Soil and Belowground Biomass
4.7. Inorganic N Leaching
4.8. Chemical Analysis
4.9. Statistical Analysis
4.10. Estimation of the N Budget
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Aber, J.D.; Nadelhoffer, K.J.; Steudler, P.; Melillo, J.M. Nitrogen saturation in northern forest ecosystems. Bioscience 1989, 39, 378–386. [Google Scholar] [CrossRef]
- Gundersen, P.; Emmett, B.A.; Kj’naas, O.J.; Koopmans, C.J.; Tietema, A. Impact of nitrogen deposition on nitrogen cycling in forests: A synthesis of NITREX data. For. Ecol. Manage. 1998, 101, 37–55. [Google Scholar] [CrossRef]
- Galloway, J.N.; Aber, J.D.; Erisman, J.W.; Seitzinger, S.P.; Howarth, R.W.; Cowling, E.B.; Cosby, B.J. The nitrogen cascade. BioScience 2003, 53, 341–356. [Google Scholar] [CrossRef]
- Galloway, J.N.; Dentener, F.J.; Capone, D.G.; Boyer, E.W.; Howarth, R.W.; Seitzinger, S.P.; Asner, G.P.; Cleveland, C.C.; Green, P.A.; Holland, E.A.; et al. Nitrogen cycles: Past, present, and future. Biogeochemistry 2004, 70, 153–226. [Google Scholar] [CrossRef]
- Fenn, M.E.; Lambert, K.F.; Blett, T.F.; Burns, D.A.; Pardo, L.H.; Lovett, G.M.; Haeuber, R.A.; Evers, D.C.; Driscoll, C.T.; Jeffries, D.S. Setting Limits: Using Air Pollution Thresholds to Protect and Restore U.S. Ecosystems; Issues in Ecology, Report No. 14; Ecological Society of America: Washington, DC, USA, 2011. [Google Scholar]
- Shibata, H.; Branquinho, C.; McDowell, W.H.; Mitchell, M.J.; Monteith, D.T.; Tang, J.; Arvola, L.; Cruz, C.; Cusack, D.F.; Halada, L.; et al. Consequence of altered nitrogen cycles in the coupled human and ecological system under changing climate: The need for long-term and site-based research. Ambio 2015, 44, 178–193. [Google Scholar] [CrossRef] [PubMed]
- Galloway, J.N.; Cowling, E.B. Reactive nitrogen and the world: 200 years of change. Ambio 2002, 31, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Gundersen, P.; Vogt, R.D.; Koba, K.; Chen, F.; Chen, X.Y.; Yoh, M. Atmospheric deposition and leaching of nitrogen in Chinese forest ecosystems. J. For. Res. 2011, 16, 341–350. [Google Scholar] [CrossRef]
- Driscoll, C.T.; Whitall, D.; Aber, J.D.; Boyer, E.; Castro, M.; Cronan, C.; Goodale, C.L.; Groffman, P.; Hopkinson, C.; Lambert, K.; et al. Nitrogen pollution in the northeastern United States: Sources, effects, and management options. Bioscience 2003, 53, 357–374. [Google Scholar] [CrossRef]
- Thomas, R.Q.; Canham, C.D.; Weathers, K.C.; Goodale, C.L. Increased tree carbon storage in response to nitrogen deposition in the US. Nat. Geosci. 2010, 3, 13–17. [Google Scholar] [CrossRef]
- Magill, A.H.; Downs, M.R.; Nadelhoffer, K.J.; Hallett, R.A.; Aber, J.D. Forest ecosystem response to four years of chronic nitrate and sulfate additions to Bear Brooks Watershed, Maine, USA. For. Ecol. Manag. 1996, 84, 29–37. [Google Scholar] [CrossRef]
- Aber, J.D.; McDowell, W.; Nadelhoffer, K.J.; Magill, A.; Berntson, G.; Kamakea, M.; McNulty, S.; Currie, W.; Rusad, L.; Fernandez, I. Nitrogen saturation in temperate forest ecosystems: Hypotheses revisited. Bioscience 1998, 48, 921–934. [Google Scholar] [CrossRef]
- Lovett, G.M.; Goodale, C.L. A new conceptual model of nitrogen saturation based on experimental nitrogen addition to an oak forest. Ecosystems 2011, 14, 615–631. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Howarth, R.W. Nitrogen limitation on land and in the sea: How can it occur? Biogeochemistry 1991, 13, 87–115. [Google Scholar] [CrossRef]
- Lovett, G.M.; Weathers, K.C.; Arthur, M.A. Control of N loss from forested watersheds by soil carbon:nitrogen ratio and tree species composition. Ecosystems 2002, 5, 712–718. [Google Scholar] [CrossRef]
- Carroll, J.A.; Johnson, D.; Morecroft, M.; Taylor, A.; Caporn, S.J.M.; Lee, J.A. The effect of long-term nitrogen additions on the bryophyte cover of upland acidic grasslands. J. Bryol. 2000, 22, 83–89. [Google Scholar] [CrossRef]
- Haddad, N.M.; Haarstad, J.; Tilman, D. The effects of long-term nitrogen loading on grassland insect communities. Oecologia 2000, 124, 73–84. [Google Scholar] [CrossRef]
- Lamers, L.P.M.; Bobbink, R.; Roelofs, J.G.M. Natural nitrogen filter fails in polluted raised bogs. Glob. Change Biol. 2000, 6, 583–586. [Google Scholar] [CrossRef]
- Curtis, C.J.; Emmett, B.A.; Grant, H.; Kernan, M.; Reynolds, B.; Shilland, E. Nitrogen saturation in UK moorlands: The critical role of bryophytes and lichens in determining retention of atmospheric N deposition. J. Appl. Ecol. 2005, 42, 507–517. [Google Scholar] [CrossRef]
- Jones, M.L.M. Nitrogen Deposition in Upland Grasslands: Critical Loads, Management and Recovery. Ph.D. Thesis, University of Sheffield, Sheffield, UK, 2005. [Google Scholar]
- Van Wijk, M.T.; Clemmensen, K.E.; Shaver, G.R.; Williams, M.; Callaghan, T.V.; Chapin, F.S.; Cornelissen, J.H.C.; Gough, L.; Hobbie, S.E.; Jonasson, S.; et al. Long-term ecosystem level experiments at Toolik Lake, Alaska, and at Abisko, Northern Sweden: Generalizations and differences in ecosystem and plant type responses to global change. Glob. Change Biol. 2003, 10, 105–123. [Google Scholar] [CrossRef]
- Soudzilovskaia, N.A.; Onipchenko, V.G.; Cornelissen, J.H.C.; Aerts, R. Effects of fertilisation and irrigation on foliar afterlife in alpine tundra. J. Veg. Sci. 2007, 18, 755–766. [Google Scholar] [CrossRef]
- Shibata, H.; Kuboi, T.; Konohira, E.; Satoh, F.; Sasa, K. Retention processes of anthropogenic nitrogen deposition in a forest watershed in northern Japan, Proceedings of the 3rd International Nitrogen Conference, Beijing, China, October 2004; Zhu, Z., Minami, K., Xing, G., Eds.; Science Press USA: Princeeton Junction, NJ, USA, 2005; pp. 626–630.
- Noguchi, M.; Yoshida, T. Tree regeneration in partially cut conifer–hardwood mixed forests in northern Japan: Roles of establishment substrate and dwarf bamboo. For. Ecol. Manage. 2004, 190, 335–344. [Google Scholar] [CrossRef]
- Toyooka, H. Sasa growing in Hokkaido as biomass resources. Bamboo J. 1983, 1, 22–24. [Google Scholar]
- Watanabe, T.; Fukuzawa, K.; Shibata, H. Temporal changes in litterfall, litter decomposition and their chemical composition in Sasa dwarf bamboo in a natural forest ecosystem of northern Japan. J. For. Res. 2013, 18, 129–138. [Google Scholar] [CrossRef]
- Ozawa, M.; Shibata, H.; Satoh, F.; Sasa, K. Effect of surface soil removal on dynamics of dissolved inorganic nitrogen in snow-dominated forest soil. Scientific World J. 2001, 1, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Shibata, H.; Ozawa, M.; Satoh, F.; Sasa, K. The effect of treatment for land surface during forest practice on soil nitrogen dynamics. J. Jpn. For. Soc. 2007, 89, 314–320. [Google Scholar] [CrossRef]
- Shibata, H.; Toda, H.; Fukushima, K.; Tanio, Y.; Takahashi, T.; Yoshida, T. Relationship between biogeochemical processes and forest management in Japanese forest ecosystems. J. Jpn. For. Soc. 2009, 91, 408–420. [Google Scholar] [CrossRef]
- Fukuzawa, K.; Shibata, H.; Takagi, K.; Nomura, M.; Kurima, N.; Fukazawa, T.; Satoh, F.; Sasa, K. Effect of clear-cutting on nitrogen leaching and fine root dynamics in a cool-temperate forested watershed in northern Japan. For. Ecol. Manage. 2006, 225, 257–261. [Google Scholar] [CrossRef]
- Fukuzawa, K.; Shibata, H.; Takagi, K.; Satoh, F.; Koike, T.; Sasa, K. Roles of dominant understory Sasa bamboo in carbon and nitrogen dynamics following canopy tree removal in a cool-temperate forest in northern Japan. Plant. Spec. Biol. 2015, 30, 104–115. [Google Scholar] [CrossRef]
- Chapin, F.S. The mineral nutrition of wild plants. Annu. Rev. Ecol. Syst. 1980, 11, 233–260. [Google Scholar] [CrossRef]
- Kikuzawa, K. A cost-benefit analysis of leaf habit and leaf longevity of trees and their geographical pattern. Am. Nat. 1991, 138, 1250–1263. [Google Scholar] [CrossRef]
- Killingbeck, K.T. Nutrients in senesced leaves: Keys to the search for potential resorption and resorption proficiency. Ecology 1996, 77, 1716–1727. [Google Scholar] [CrossRef]
- Magill, A.H.; Aber, J.D.; Hendricks, J.J.; Bowden, R.D.; Melillo, J.M.; Steudler, P.A. Biogeochemical response of forest ecosystems to simulated chronic nitrogen deposition. Ecol. Appli. 1997, 7, 402–415. [Google Scholar] [CrossRef]
- Magill, A.H.; Aber, J.D.; Berntson, G.M.; McDowell, W.H.; Nadelhoffer, K.J.; Melillo, J.M.; Steudler, P. Long-term nitrogen additions and nitrogen saturation in two temperate forests. Ecosystems 2000, 3, 238–253. [Google Scholar] [CrossRef]
- Huang, J.Y.; Zhu, X.G.; Yuan, Z.Y.; Song, S.H.; Li, X.; Li, L.H. Changes in nitrogen resorption traits of six temperate grassland species along a multi-level N addition gradient. Plant Soil. 2008, 306, 149–158. [Google Scholar] [CrossRef]
- Huang, J.Y.; Yu, H.L.; Wang, B.; Li, L.H.; Xiao, G.J.; Yuan, Z.Y. Nutrient resorption based on different estimations of five perennial herbaceous species from the grassland in inner Mongolia, China. J. Arid. Environ. 2012, 76, 1–8. [Google Scholar] [CrossRef]
- Loladze, I. Rising atmospheric CO2 and human nutrition: Toward globally imbalanced plant stoichiometry? Trends Ecol. Evol. 2002, 17, 457–461. [Google Scholar] [CrossRef]
- Reich, P.B.; Hobbie, S.E.; Lee, T.; Ellsworth, D.S.; West, J.B.; Tilman, D.; Knops, J.M.H.; Naeem, S.; Trost, J. Nitrogen limitation constrains sustainability of ecosystem response to CO2. Nature 2006, 440, 922–925. [Google Scholar] [CrossRef] [PubMed]
- Berg, B.; Matzner, E. Effect of N deposition on decomposition of plant litter and soil organic matter in forest ecosystem. Environ. Rev. 1997, 5, 1–25. [Google Scholar] [CrossRef]
- Hobbie, S.E. Contrasting effects of substrate and fertilizer nitrogen on the early stages of litter decomposition. Ecosystems 2005, 8, 644–656. [Google Scholar] [CrossRef]
- Nadelhoffer, K.; Downs, M.; Fry, B.; Magill, A.; Aber, J.D. Controls on N retention and exports in a forested watershed. Environ. Monit. Assess. 1999, 55, 187–210. [Google Scholar] [CrossRef]
- Nadelhoffer, K.J.; Emmett, B.A.; Gundersen, P.; Kjønaas, O.J.; Koopmans, C.J.; Schleppi, P.; Tietema, A.; Wright, R.F. Nitrogen deposition makes a minor contribution to carbon sequestration in temperate forests. Nature 1999, 398, 145–148. [Google Scholar] [CrossRef]
- Forest Soil Division. Classification of forest soil in Japan (1975). Bul. Gov. For. Exp. Sta. 1976, 280, 1–28. [Google Scholar]
- FAO. Soil Map of the World. Revised Legend; Reprinted with corrections 1990. World Soil Resources Reports 60; ISRIC: Wageningen, The Netherlands, 1997; p. 140. [Google Scholar]
- Wakamatsu, T.; Sato, K.; Takahashi, A.; Shibata, H. Proton budget for a Japanese cedar forest ecosystem. Water. Air. Soil. Poll. 2001, 130, 721–726. [Google Scholar] [CrossRef]
- Saitoh, T.; Seiwa, K.; Nishikawa, A. Effects of resource heterogeneity on nitrogen translocation within clonal fragments of Sasa palmata: An isotopic (15N) assessment. Ann. Bot. 2006, 98, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, T.; Seiwa, K. Physiological integration of clonal plants: Resource acquiring strategies in clonal fragments of a dwarf bamboo, Sasa palmata. Jpn. J. Ecol. 2007, 57, 229–237. (In Japanese) [Google Scholar]
- Giblin, A.E.; Laundre, J.A.; Nadelhoffer, K.J.; Shaver, G.R. Measuring nutrient availability in arctic soils using ion exchange resins: A field test. Soil. Sci. Soc. Am. J. 1994, 58, 1154–1162. [Google Scholar] [CrossRef]
- Bormann, F.H.; Likens, G.E. Pattern and Processes in a Forested Ecosystem; Springer: New York, NY, USA, 1979. [Google Scholar]
- Goodale, C.L.; Aber, J.D. The long-term effects of land-use history on nitrogen cycling in northern hardwood forests. Ecol. Appli. 2001, 11, 253–267. [Google Scholar] [CrossRef]
- Chen, Y.; Högberg, P. Gross nitrogen mineralization rates still high 14 years after suspension of N input to a N-saturated forest. Soil. Biol. Biochem. 2006, 38, 2001–2003. [Google Scholar] [CrossRef]
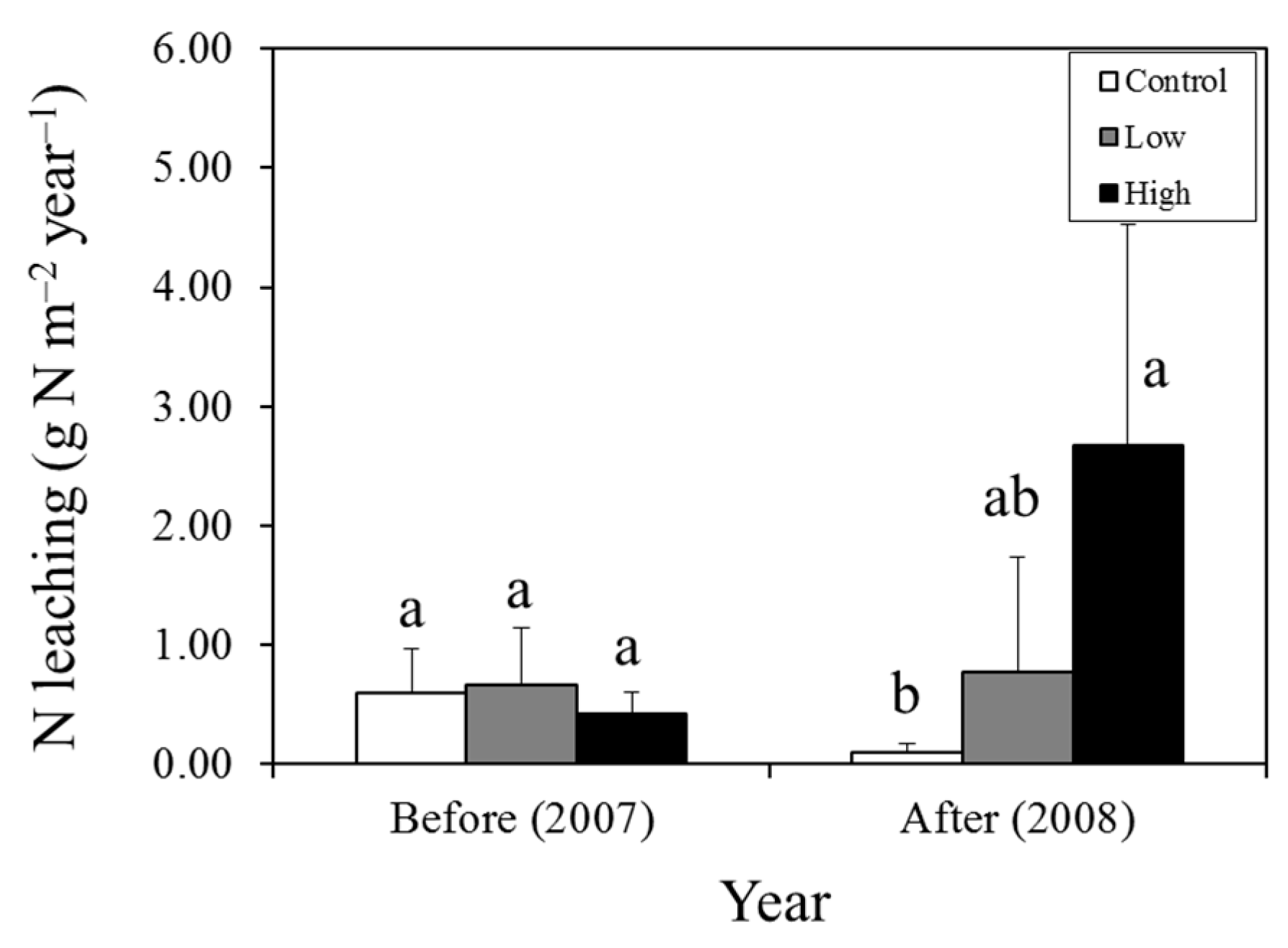
| Year | Control Plot | Low–N Plot | High–N Plot | Two–Way ANOVA | P Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||||||
| Total leaf number (number m–2) | |||||||||||
| 1 year After | 263 | 143 | aA (1) | 289 | 264 | aA | 619 | 372 | aA | Year (Y) | ns |
| 2 year After | 300 | 197 | bA | 275 | 116 | bA | 697 | 209 | aA | Treatment (T) | ˂0.01 |
| Interaction T × Y | ns | ||||||||||
| Total leaf biomass (g DW m–2) | |||||||||||
| 1 year After | 321 | 137 | aA | 297 | 205 | aA | 596 | 316 | aA | Year (Y) | ns |
| 2 year After | 218 | 108 | bA | 205 | 67 | bA | 512 | 167 | aA | Treatment (T) | ˂0.01 |
| Interaction T × Y | ns | ||||||||||
| Total leaf biomass N (g N m–2) | |||||||||||
| 1 year After | 6.1 | 2.7 | aA | 5.5 | 4.3 | aA | 11.4 | 6.2 | aA | Year (Y) | ns |
| 2 year After | 4.5 | 1.8 | bA | 3.9 | 1.3 | bA | 10.8 | 3.6 | aA | Treatment (T) | ˂0.01 |
| Interaction T × Y | ns | ||||||||||
| Current leaf number (number m–2) | |||||||||||
| 1 year After | 71 | 47 | aA | 107 | 148 | aA | 165 | 103 | aA | Year (Y) | ns |
| 2 year After | 155 | 64 | bA | 115 | 83 | bA | 300 | 90 | aA | Treatment (T) | ˂0.05 |
| Interaction T × Y | ns | ||||||||||
| Current leaf biomass (g DW m–2) | |||||||||||
| 1 year After | 90 | 44 | aA | 87 | 113 | aA | 129 | 87 | aA | Year (Y) | ns |
| 2 year After | 107 | 19 | aA | 80 | 56 | aA | 150 | 72 | aA | Treatment (T) | 0.0579 |
| Interaction T × Y | ns | ||||||||||
| Current leaf biomass N (g N m–2) | |||||||||||
| 1 year After | 1.9 | 1.0 | aA | 1.8 | 2.4 | aA | 3.4 | 2.1 | aA | Year (Y) | ns |
| 2 year After | 2.6 | 0.3 | bA | 1.9 | 1.3 | bA | 4.5 | 1.4 | aA | Treatment (T) | ˂0.05 |
| Interaction T × Y | ns | ||||||||||
| Previous leaf number (number m–2) | |||||||||||
| 1 year After | 192 | 100 | aA | 182 | 116 | aA | 454 | 271 | aA | Year (Y) | ns |
| 2 year After | 145 | 140 | bA | 160 | 53 | bA | 397 | 136 | aA | Treatment (T) | ˂0.01 |
| Interaction T × Y | ns | ||||||||||
| Previous leaf biomass (g DW m–2) | |||||||||||
| 1 year After | 231 | 96 | aA | 210 | 98 | aA | 446 | 230 | aA | Year (Y) | ns |
| 2 year After | 111 | 98 | bA | 125 | 42 | bA | 315 | 103 | aA | Treatment (T) | ˂0.01 |
| Interaction T × Y | ns | ||||||||||
| Previous leaf biomass N (g N m–2) | |||||||||||
| 1 year After | 4.1 | 1.8 | aA | 3.6 | 2.1 | aA | 8.0 | 4.1 | aA | Year (Y) | ns |
| 2 year After | 1.9 | 1.7 | bA | 2.1 | 0.6 | bA | 6.3 | 2.4 | aA | Treatment (T) | ˂0.01 |
| Interaction T × Y | ns | ||||||||||
| Soil inorganic N (NH4+ and NO3–) amount (g m–2) | |||||||||||
| 1 year After | 5.43 | 1.48 | aA | 3.92 | 0.91 | aA | 3.94 | 0.73 | aA | Year (Y) | ˂0.01 |
| 2 year After | 3.17 | 1.33 | aA | 2.06 | 0.53 | aB | 3.17 | 0.83 | aA | Treatment (T) | ns |
| Interaction T × Y | ns | ||||||||||
| Collected Year of Used Litter | Control | Low–N | High–N | One–Way ANOVA P Value | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| Initial leaf litter N concentration (mg g–1) | |||||||
| October (2007) | 10.4 | 1.8 | 11.2 | 3.0 | 8.6 | 2.2 | ns |
| October (2008) | 12.1 | 1.7 | 11.2 | 1.3 | 12.4 | 1.1 | ns |
| Collected Year of Used Litter | Decomposition Period | Control | Low-N | High-N | One-Way ANOVA P Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||||||
| Dry mass remaining (%) | |||||||||||
| October (2007) | November 2007 to 2008 | 74.1 | 4.5 | a (1) | 75.9 | 3.0 | a | 74.2 | 3.3 | a | ns |
| October (2008) | November 2008 to 2009 | 72.1 | 3.3 | a | 71.5 | 4.3 | a | 69.7 | 4.4 | a | ns |
| N mass remaining (%) | |||||||||||
| October (2007) | November 2007 to 2008 | 108.8 | 22.1 | b | 106.5 | 28.9 | b | 140.6 | 39.3 | a | ˂0.05 |
| October (2008) | November 2008 to 2009 | 82.9 | 17.3 | b | 103.6 | 20.0 | a | 92.9 | 11.4 | ab | ˂0.05 |
| Component | Low-N Plot | Net Change (g N m–2) | High-N Plot | Net Change (g N m−2) | ||
|---|---|---|---|---|---|---|
| Before | After | Before | After | |||
| N input–output (g N m−2 year−1) | ||||||
| N input | 0 | 5 | 5 | 0 | 15 | 15 |
| N leaching | 0.66 | 0.77 | 0.11 | 0.42 | 2.67 | 2.25 |
| N retention | 4.89 | 12.75 | ||||
| N storage (g N m−2) | ||||||
| Aboveground biomass N | 15.68 | 9.26 | −6.42 | 13.36 | 19.12 | 5.76 |
| Belowground biomass N | 4.59 | 7.29 | 2.71 | 5.54 | 8.12 | 2.58 |
| N mass remaining in litter | 0.85 | 1.18 | 0.33 | 1.37 | 1.73 | 0.36 |
| Soil inorganic N | 4.10 | 3.92 | −0.18 | 3.50 | 3.94 | 0.44 |
| Estimated N storage | 8.11 | 2.72 | ||||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, T.; Fukuzawa, K.; Shibata, H. Short-Term Response of Sasa Dwarf Bamboo to a Change of Soil Nitrogen Fertility in a Forest Ecosystem in Northern Hokkaido, Japan. Plants 2016, 5, 19. https://doi.org/10.3390/plants5020019
Watanabe T, Fukuzawa K, Shibata H. Short-Term Response of Sasa Dwarf Bamboo to a Change of Soil Nitrogen Fertility in a Forest Ecosystem in Northern Hokkaido, Japan. Plants. 2016; 5(2):19. https://doi.org/10.3390/plants5020019
Chicago/Turabian StyleWatanabe, Tsunehiro, Karibu Fukuzawa, and Hideaki Shibata. 2016. "Short-Term Response of Sasa Dwarf Bamboo to a Change of Soil Nitrogen Fertility in a Forest Ecosystem in Northern Hokkaido, Japan" Plants 5, no. 2: 19. https://doi.org/10.3390/plants5020019





