1. Introduction
The study of factors and processes affecting species range limits has a long history in ecology and evolution starting with Darwin, but there has been a recent resurgence of interest in range limits in part to understand some of the consequences of climate change [
1,
2,
3,
4]. Because most transplant experiments show poorer performance across range boundaries (Sexton
et al. 2009 [
2] for review), many range margin populations must face stressful environments that they are not adapted to. Therefore, understanding what prevents this adaptation may be key to understanding the development of range limits. Since there is often sufficient genetic variation for traits that matter within range margin populations, if there are also no barriers to dispersal, possible constraints on the process of adaptation include swamping gene flow from elsewhere in the range and tradeoffs. But because many range margin populations are also geographically and genetically isolated, it is thought that the study of range limit development should often focus on molecular, physiological or developmental tradeoffs [
5]. What kind of tradeoffs might be constraining low elevation trailing edge populations?
At low latitudinal or altitudinal “trailing edge” range limits, populations are thought to more commonly face both abiotic and biotic stressors compared to high altitudes or latitudes at leading edges where abiotic stressors predominate [
6]. Although there would be some exceptions to this pattern depending on latitude, altitude and local climate conditions, many cases should comply. For example, transplant experiments with the upland mustard species
Boechera stricta, a close relative of
Arabidopsis, resulted in lower survivorship across low elevation range boundaries that coincided with increased abiotic and biotic stressors such as decreased water availability and increased herbivory by generalist insect herbivores [
7]. Presumably, increased drought tolerance and chemical defense levels would allow upland species like
B. stricta to expand low elevation range boundaries. However, negative genetic correlations,
i.e., evolutionary tradeoffs, between glucosinolate (GS) toxin defense allocation and abiotic stress tolerances associated with low elevation range limits have been observed in
B. stricta [
7,
8,
9,
10].
One hypothesis for the evolutionary tradeoff involves natural selection acting on antagonistic plastic response pathways [
7,
11]. The process of adaptation often proceeds by modifying existing structures and pathways. Within ranges, stress response signal transduction pathways help plants to survive temporary challenges from abiotic and biotic stressors [
12]. Just across range boundaries, some of these same stressors increase in frequency; therefore, one would predict that adaptation to stressful environments across range limits would involve the up-regulation of stress response pathways such that the pathways and the traits that they regulate were expressed more frequently or stably. However, evolutionary models predict that a problem may arise when antagonistic response pathways are co-opted simultaneously for evolutionary change [
13]. For example, from work on
Arabidopsis it is well known that stress response pathways, such as Abscisic acid (ABA) signaling for coping with temporary challenges of abiotic stressors (e.g., drought), and Jasmonic acid (JA) signaling for coping with bouts of biotic stressors (e.g., herbivores) may negatively interfere with one another ([
14,
15,
16] for reviews). Thus, the simultaneous co-option of these antagonistic pathways for low elevation range expansion where organisms face both increased abiotic and biotic stressors may be problematic because of the crosstalk.
The problem is with negative pleiotropic and epistatic effects that may constrain evolution. Multiple signaling pathways often form networks involving regulatory genes—transcription factors (TFs)—that may interact to produce multiple positive and negative integrative effects. If natural selection acts on genetic variation in either the coding regions or the
cis regulatory regions of TFs involved in the networks, multiple pathways may be affected. Although some of the effects may be adaptive, many may be mal-adaptive. For example, the flowering time signaling network in
Arabidopsis consists of many positive and negative interactions among photoperiod, circadian clock, vernalization, autonomous and Gibberellic acid pathways. Epistatic interactions between the major flowering time network genes FRI and FLC, were one of the contributing factors in the maintenance of genetic variation in
Arabidopsis flowering time [
17]. Thus, by preventing fixation of alleles, the epistasis represents an evolutionary constraint. FRI and FLC are major TFs in the flowering time signaling network that allow large behavioral shifts involving many genes, but these major effects might impede evolution.
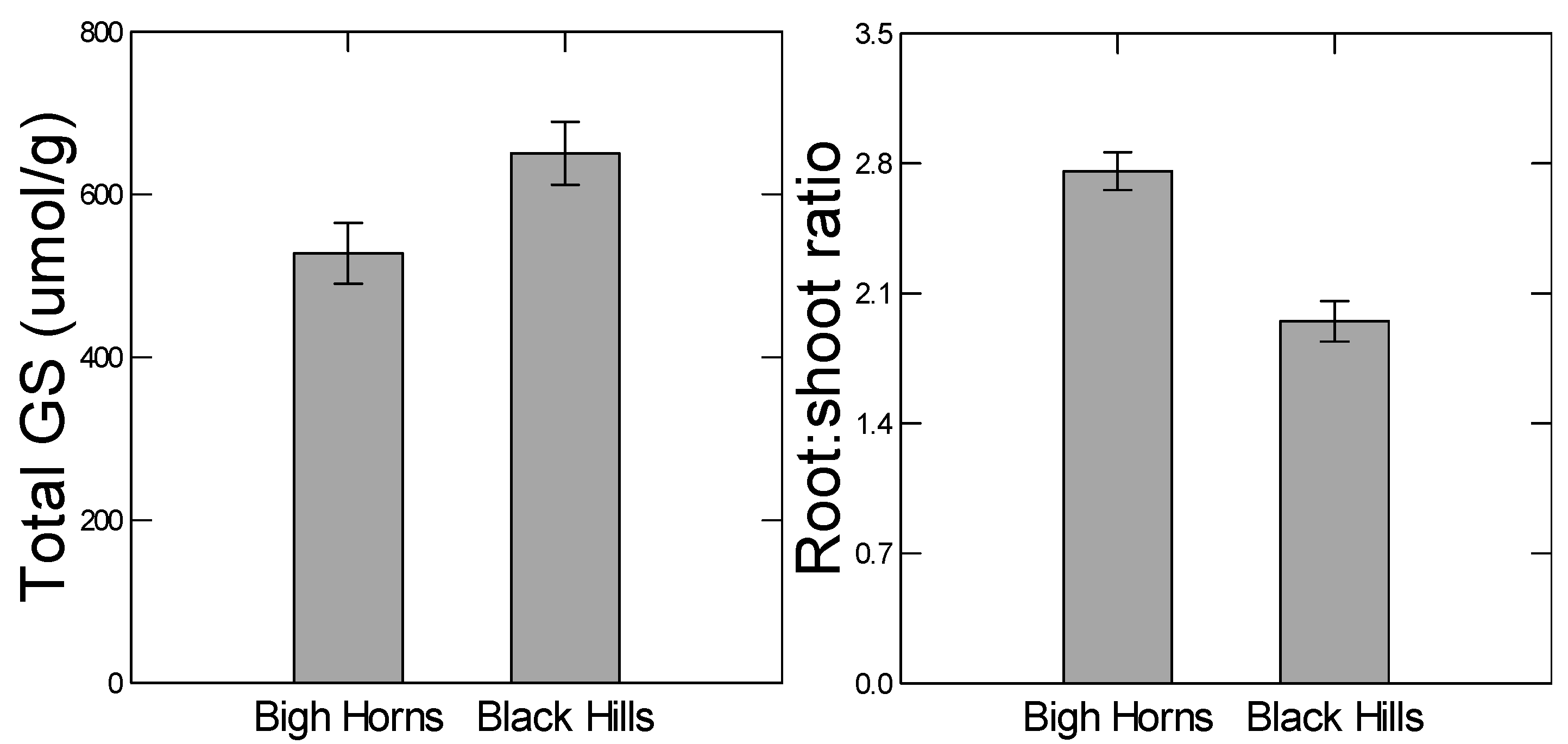
It has also been shown that high and low elevation populations of
B. stricta have diverged with respect to abiotic stress tolerance and glucosinolate defense levels as one would predict based on the above hypothesis; neither population had high values of both kinds of traits [
11]. In a common garden growth chamber experiment, the two populations showed genetic divergence for glucosinolate content (
F18, 701 = 7.101,
p < 0.001) and stress tolerance traits such as root:shoot ratio (
F5, 251 = 3.576,
p = 0.004). The high elevation population showed higher inherent root:shoot ratios, while the lower elevation population was higher in glucosinolate levels. Further, in experimental crosses between the populations, the two kinds of traits would not segregate independently of one another in the F2 generation (Siemens
et al., unpublished data [
18]:
F1, 599 = 65.987,
p < 0.001). Together, these results indicate a genetic tradeoff that probably involves negative pleiotropic or epistatic interactions.
Here, we compared the genetically diverged high and low elevation populations of B. stricta in a double challenge drought-herbivory growth chamber experiment to test the following predictions based on the central hypothesis that the evolutionary tradeoff derives from antagonistic plastic responses: (1) Drought stress inhibits herbivore-induced defense responses; (2) Induced abiotic stress responses are attenuated in the high elevation population that already shows high constitutive levels of the tolerance traits; (3) Likewise, herbivore-induced defense responses are attenuated in the low elevation population that already shows high basal levels of the defensive traits. In other words, predictions 2 and 3 state that high constitutive levels of traits would coincide with lower plasticity. Prediction 1 is just an expectation to determine whether antagonistic signaling exists.
3. Discussion
Many factors and processes, alone or in combination, may contribute to species range limits development by preventing adaptation to stressful environments. Some major factors include lack of genetic variation in range margin populations, barriers to dispersal, swamping gene flow from elsewhere within the range and various kinds of tradeoffs [
2]. Of these, relatively little is known about possible molecular, physiological or developmental tradeoffs [
5].
Here, we tested predictions from a hypothesis explaining the existence of an apparent genetic tradeoff between defense allocation and abiotic stress tolerance. This tradeoff may contribute to low elevation range limit development. The hypothesis states that antagonistic plastic response pathways may inhibit their simultaneous co-option for more stable expression that is needed for range expansion. If a plastic response pathway in a signaling network is co-opted in evolution for more stable expression, then one might predict that other pathways in the network would also be affected [
13]. To test this prediction, we compared two genetically diverged populations for their plastic responses to drought and herbivory. For example, the high elevation population from the Big Horn Mountains has diverged with respect to higher constitutive levels of some abiotic stress tolerant traits, such as root:shoot ratio (
Figure 2). Since the root:shoot ratio is in part regulated by ABA signaling [
21], we tested the prediction that ABA-regulated traits, such as stomatal aperture, would be less plastic in the high elevation population. We also expected there to be less gene expression in response to drought in the high elevation population. Similarly, we expected the low elevation population to be less plastic in defensive traits. In general, we predicted that relatively high constitutive levels of the functional traits would coincide with lower plasticity.
Although our central hypothesis involved evolutionary constraints caused by potentially antagonistic signaling pathways, we did not make direct measurement of the pathway hormones, making the link between the signaling and the ecological and evolutionary responses speculative. However the predictions that we tested did not necessitate direct measurement of the hormones that trigger the candidate pathways. Instead, we relied on existing literature that makes a link between the candidate signaling pathways, gene expression and the functional traits. The candidate signaling pathways that were invoked for the evolutionary tradeoff between chemical defense allocation and stress tolerance in
B. stricta were the jasmonic acid/ethylene (JA/ET) and the abscisic acid (ABA) pathways, respectively. These pathways are generally antagonistic to one another in
Arabidopsis, probably because a stress response under dry conditions takes precedence over defense that may function primarily against pathogens under moist conditions [
14,
15,
16,
20] for reviews). It is also known that JA/ET signaling regulates aliphatic glucosinolate (GS) toxin-induced defense responses in
Arabidopsis [
22,
23], induced resistance against generalist insect herbivores in another closely related species
Boechera divaricarpa [
24], and that GS are defensive against generalist insect herbivores as has also been found to be the case for
B. stricta (e.g., [
7,
19]). We therefore assumed that JA/ET signaling regulates aliphatic GS defense responses to generalist insect herbivores in
B. stricta. Likewise, ABA is a general stress response hormone, produced most notably in response to abiotic stressors, especially to drought or salinity, but also to other factors such as nutrient deficiency ([
21,
25] for reviews, [
26]). For example, in response to limiting supplies of water or soil nitrate levels and subsequent increases in ABA concentration or sensitivity, stomata close, leaves grow more slowly, and root growth is maintained and characterized by lateral root proliferation. Whether the genetic divergence between high and low elevation populations in levels of aliphatic GS or the various other functional traits associated with abiotic stress tolerance that we measured represents genetic divergence in the joint JA/ET regulatory pathway or ABA signaling was tested here indirectly without direct measurement of the hormones.
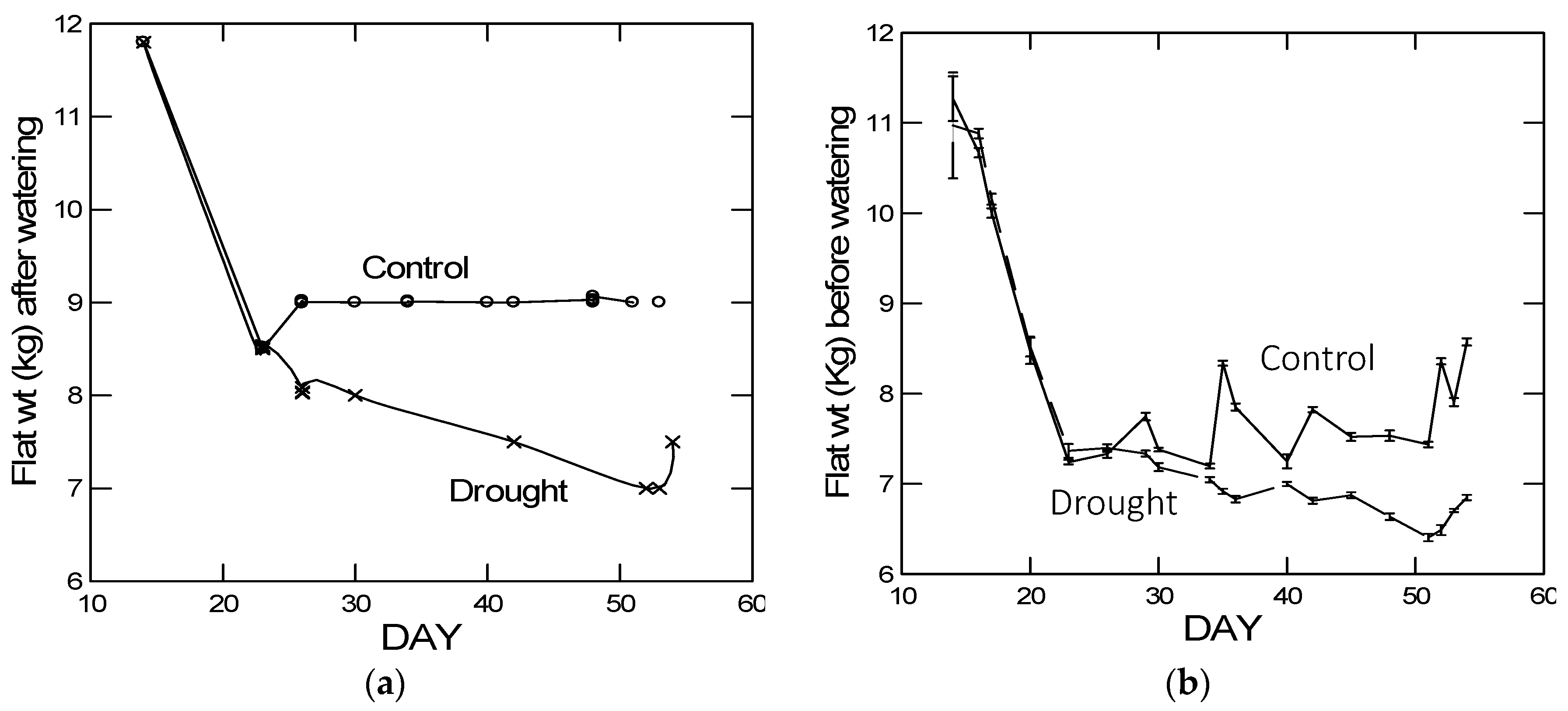
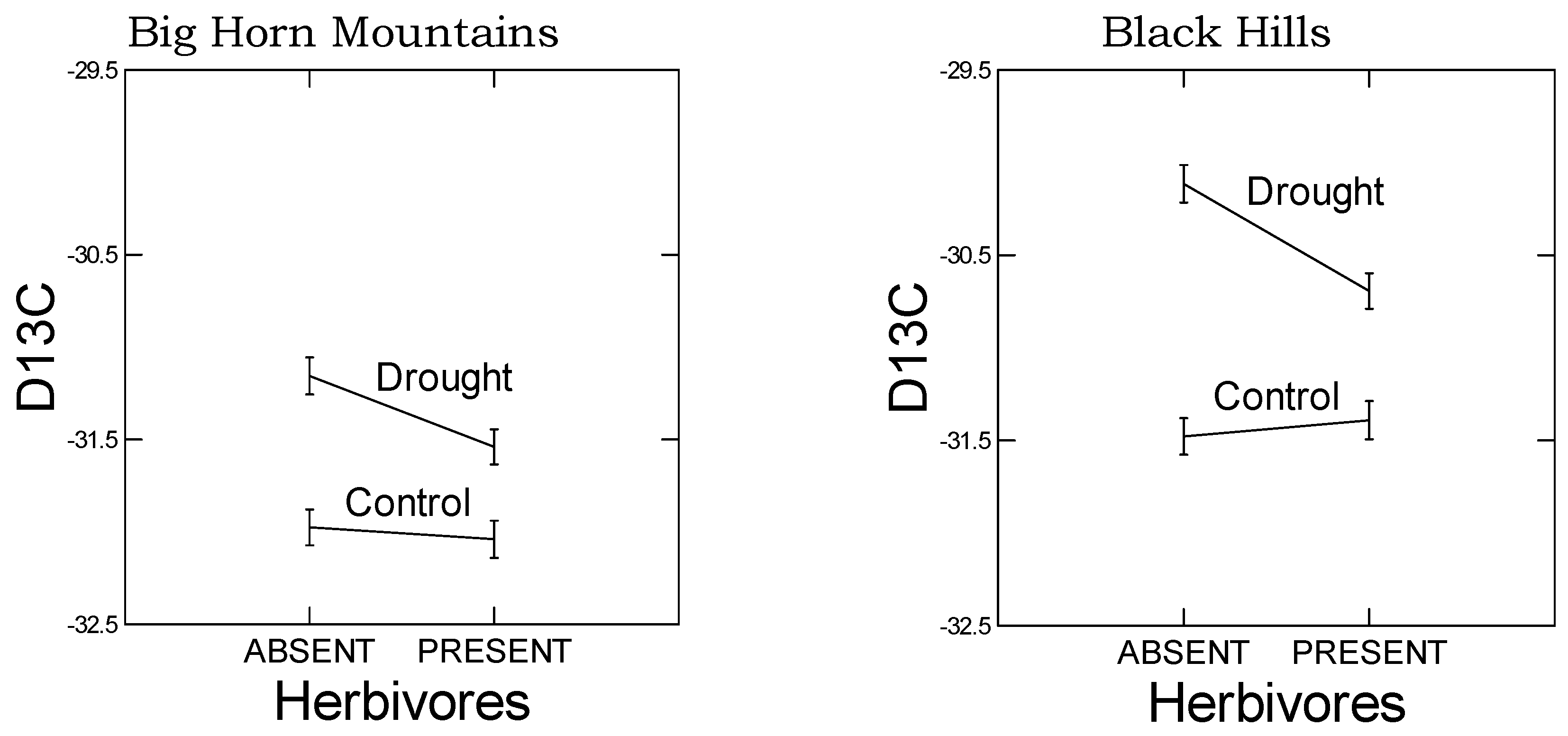
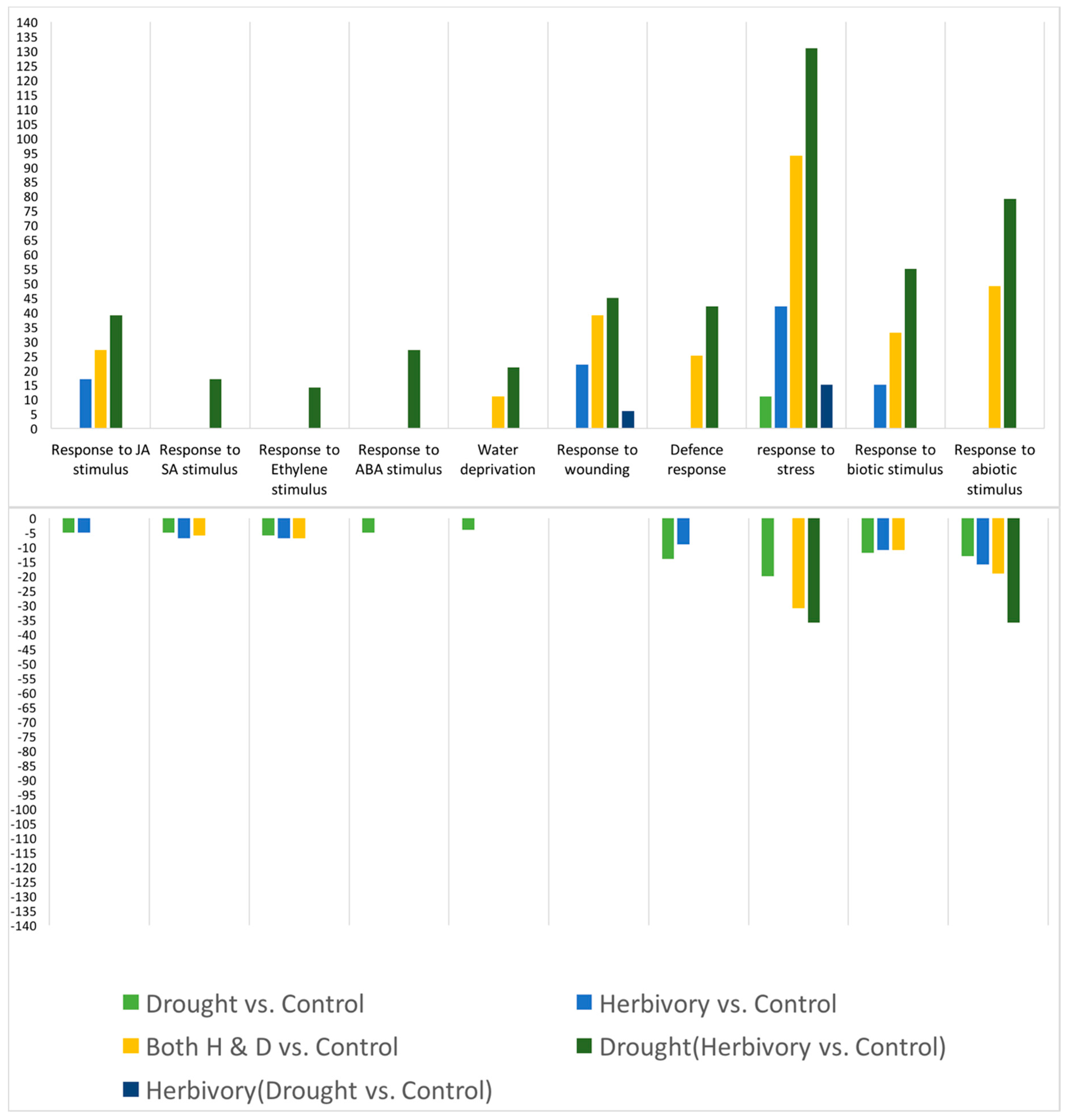
As predicted, in the high elevation population, we observed a reduced plastic stomatal aperture response to drought as measured by the carbon isotope ratio (
Figure 3). That is, for the carbon isotope ratio, the difference between drought and control treatments was smaller in the Big Horn Mountain population compared to the Black Hills population. However, less regulation on stomatal control is typical of alpine plants where water deficiency is usually not a problem [
27]. The drought-induced change in the root:shoot ratio was actually greater in the high elevation population, which was not predicted. However, the change was a decrease in the root:shoot ratio, which may not be mediated by ABA signaling. Under natural circumstances, root:shoot ratios increase in response to drought, but gradual water deprivation in small pots when watered from above is not conducive to increases in root:shoot ratio. Yet there was also no population-by-drought treatment interaction in the ANOVA for any of the four significant PCs constructed from the 10 putative abiotic stress tolerance response variables (
Table 5B). This interaction would be an indication of any differences in plasticity between the populations. It was not until we analyzed the gene expression data that there was relatively clear evidence of differential plasticity in response to drought between the populations. For the number of relevant up- and down-regulated genes, the high elevation Big Horn population was indeed less plastic to drought.
In a similar study, transcript profiles resulting from cDNA-AFLPs were compared between high and low elevation populations of
Boechera holboellii in a dry-down growth chamber experiment [
28]. The focus was on identifying candidate genes involved in local adaptation by noting population-specific expression patterns. Although the dry-down treatments resulted in differential expression patterns, only a couple of
Arabidopsis homologs were identified as candidates. Probably because of the method used for transcript profiling, there were too few gene expression differences between populations that were reported to evaluate predictions of our hypothesis.
Similarly, because the low elevation Black Hills population was higher in constitutive levels of total glucosinolate content (
Figure 2), we predicted lower plastic responses to herbivory from that population. However, we did not detect any population differences in herbivore-induced glucosinolate measures (no indication of any herbivory-by-population interaction in the ANOVAs,
Table 4 and
Table 6). Many other studies have checked for negative genetic correlations between constitutive and herbivore-induced defense or resistance levels. This is because popular theory on the evolution of herbivore-induced defenses assumes resource allocation costs of constitutive defenses and cost-savings of inducible defenses ([
29] and references therein). The results have been mixed, probably because several factors may influence constitutive and induced defense levels [
30]. In response to herbivory, the low elevation Black Hills population was clearly less plastic in terms of the number of relevant up-regulated genes expressed, but this was not the case for the number of down-regulated genes.
Our predictions were based on the existence of antagonistic defense and abiotic stress tolerance plastic responses in
B. stricta, as has been well documented in
Arabidopsis [
20]. Specifically, we checked whether a glucosinolate defense response to the generalist insect herbivore
S. exigua was negatively affected under conditions of water deficiency. The assumption that we made was that under drought conditions, ABA signaling would interfere with JA/ET signaling to attenuate herbivore-induced defense responses [
14]. We found that an herbivore-induced GS ratio (BCGS/METGS) response was attenuated under conditions of drought. The BCGS/METGS ratio has been shown to cause resistance to generalist insect herbivores in
B. stricta [
19,
31] and therefore may be the most relevant GS response variable. That is, in these other studies, this GS ratio in
B. stricta has been fine mapped, and candidate genes have been transformed and near isogenic lines created and tested with positive results. Interestingly, feeding by
S. exigua caused GS concentrations to decrease, which was reversed under drought (
Figure 4; Note, BCGS and total GS concentrations are highly correlated r = 0.98 because of the relative abundance of BCGS). Because increased GS concentrations render plants more susceptible to specialists like flea beetles (e.g., [
32]), we suggest that the plants responded to feeding by increasing resistance to both generalists (by increasing GS ratio) and specialists (by decreasing total GS concentrations). If so, the drought treatments were antagonistic to herbivore-induced defense responses. To our knowledge, it is not known why the GS ratio is effective independent of total GS concentration.
In apparent contrast to the GS results, the gene expression results suggested that the
S. exigua larvae may down-regulate the
B. stricta induced defense response, but that this inhibition by the herbivore was blocked by the drought treatments. Of course, to verify this suggestion, direct hormone, proteome, metabolome and other experimental molecular and genetic analysis would help. Nonetheless, although an herbivore-induced JA response was evident, a defense response was notably absent (
Figure 6). Patterns of gene expression further suggested that the putative herbivore inhibition may be mediated by other pathways such as ET, SA and ABA. That herbivores may inhibit defense responses downstream of JA signaling via an ethylene burst was reported previously for feeding by the specialist herbivore
Manduca sexta on wild tobacco
Nicotiana attenuata [
33]. Here, we show that apparent signaling-mediated manipulation of plant defense responses by herbivores may be dependent on abiotic conditions. The mechanism of drought to block the herbivore inhibition of defense apparently involved further signaling crosstalk. However, in apparent agreement with the GS results, was the dependence of the CYP79 herbivore-induced response on the presence of drought. The
B. stricta homolog of CYP79 is involved in the GS-ratio response [
19].
A negative correlation was also observed between smaller stomatal apertures, as indicated by less negative carbon isotope ratios, and GS levels (
Figure S4). Interestingly, this pattern held for both BCGS and METGS, but the correlation was positive for GS ratio. In addition, these correlations were dependent on drought and herbivore treatments, the particulars of which differed between populations (
Table 7) and probably lie within populations. For example, in the low elevation Black Hills population, the correlations were dependent on the presence of drought and especially herbivory. What caused these correlations? These correlations may be dependent on the area of leaf tissue consumed, which we did not record, but which presumably would have influenced water loss and stomatal behavior. Alternatively, these correlations may reflect interactions between hormone-mediated response pathways.