A Comparative Study of Proteolytic Mechanisms during Leaf Senescence of Four Genotypes of Winter Oilseed Rape Highlighted Relevant Physiological and Molecular Traits for NRE Improvement
Abstract
:1. Introduction
2. Results
2.1. Changes in N and 15N Amounts during N Remobilization in the Source Leaf
| N fractions | Source of Variation | |||
|---|---|---|---|---|
| N Treatment (N) | Genotype (G) | N × G | ||
| FN | r | FG | FNxG | |
| N amount | 22.10 *** | 0.45 ** | 8.39 *** | 6.78 *** |
| 15N amount | 5.66 * | 0.29 * | 4.55 ** | 2.82 |
| Soluble protein amount | 5.95 * | 0.33 * | 1.28 | 1.14 |
| Amino acid amount | 44.62 *** | 0.63 *** | 5.31 ** | 3.74 * |
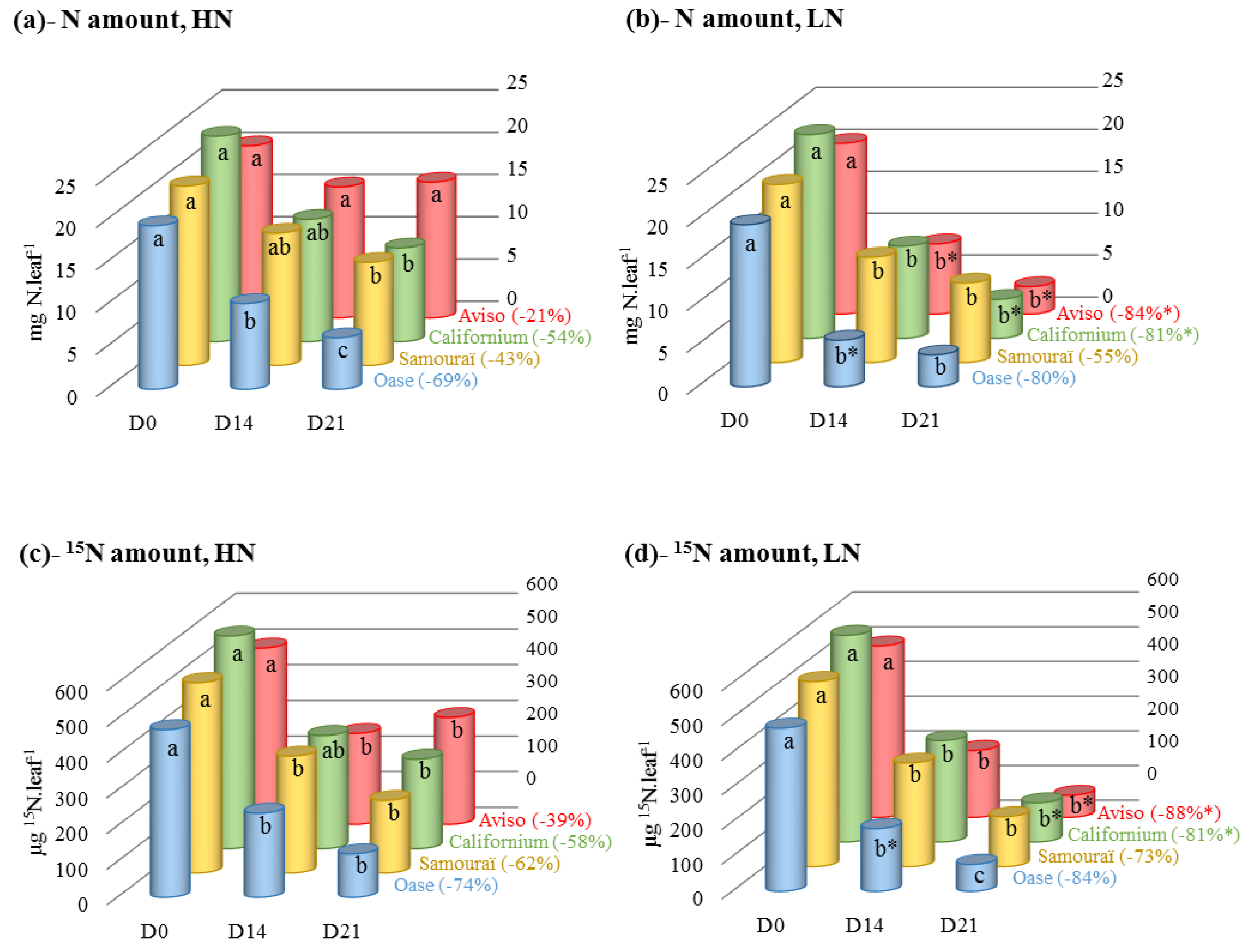
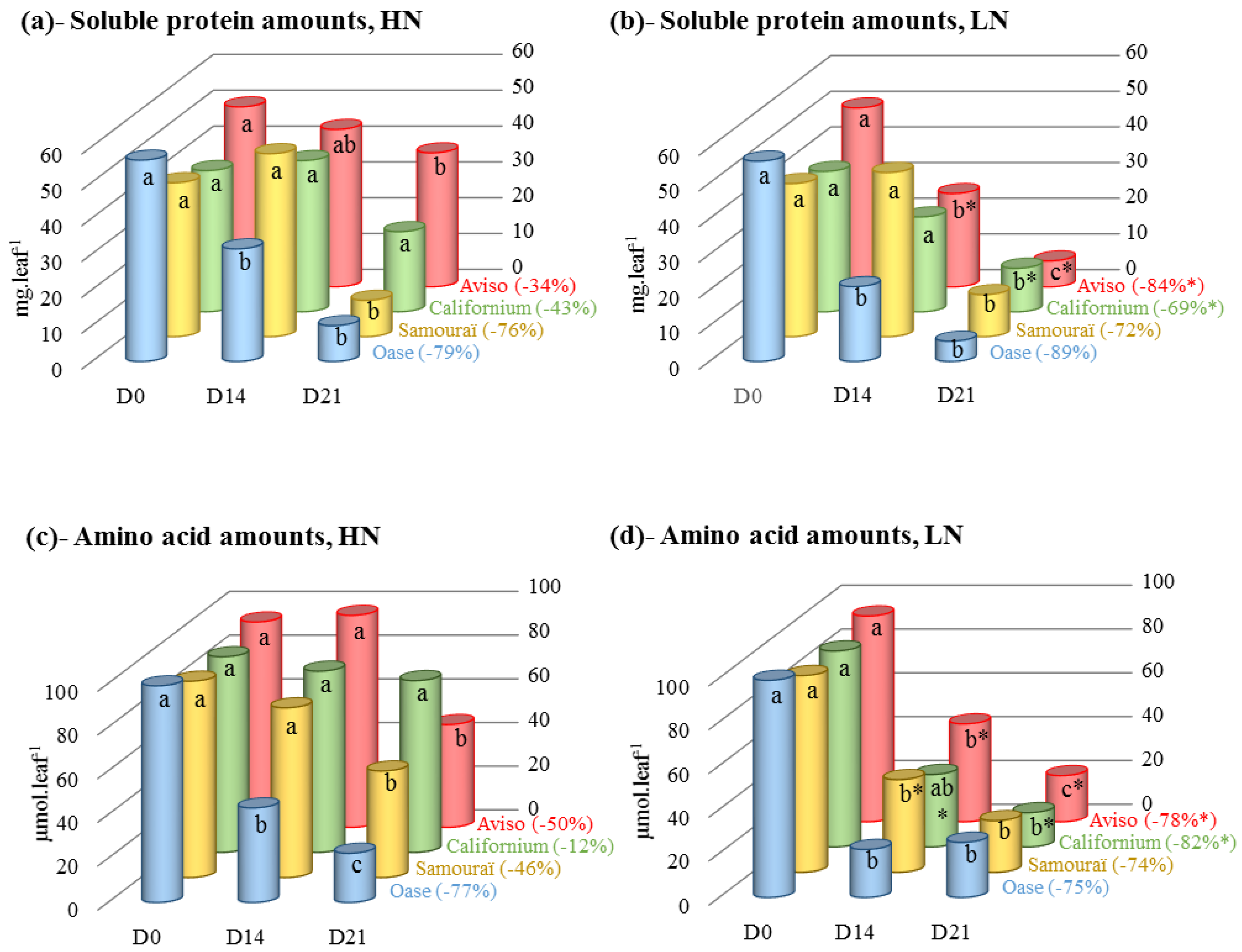
2.2. Changes in the Amounts of Soluble Proteins and Amino Acids during N Remobilization in Leaf
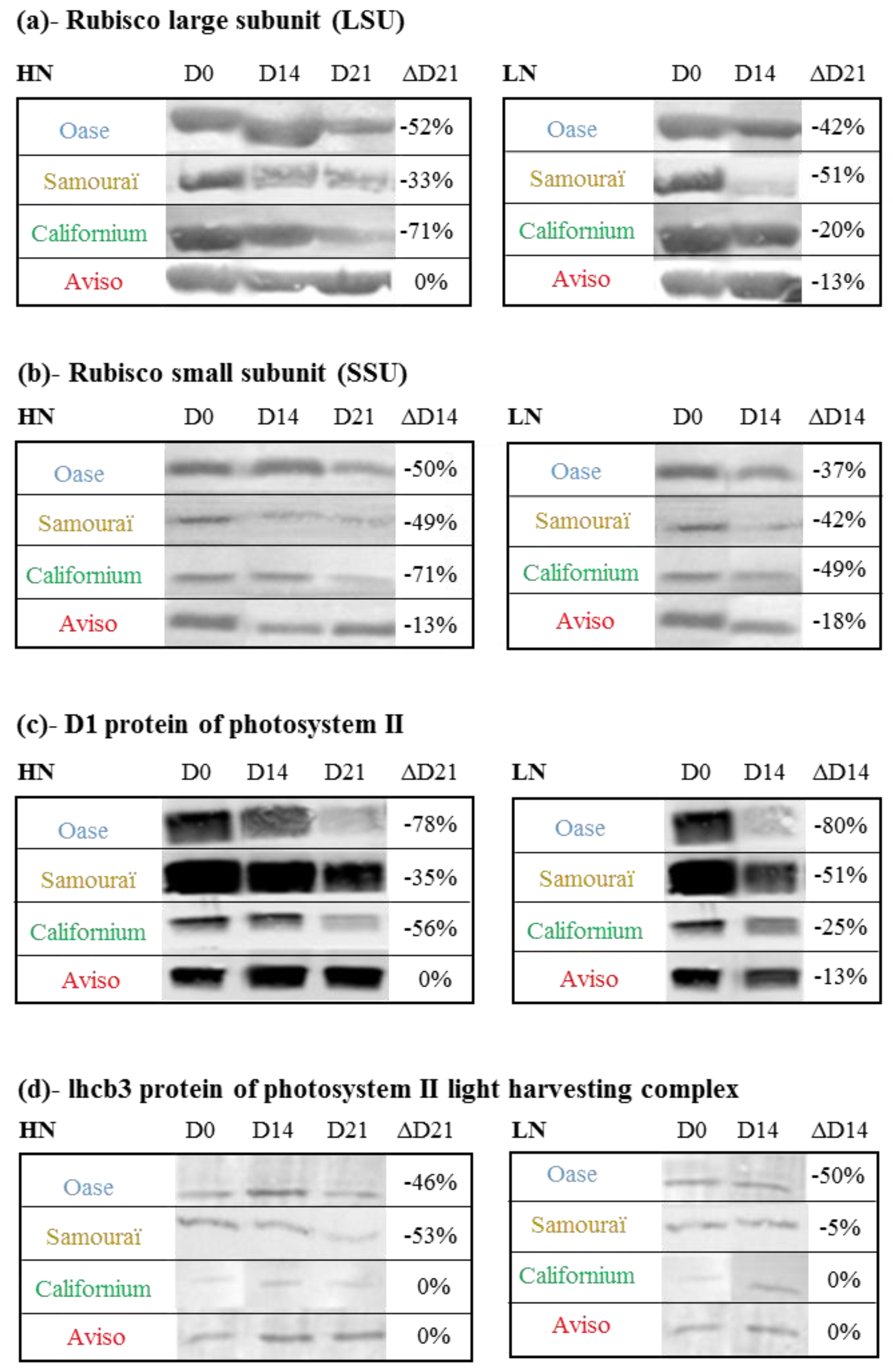
2.3. Impact of Nitrate Supply on the Abundance of Rubisco Subunits and Thylakoid-Bound Proteins of Photosystem II (D1 and lhcb3)
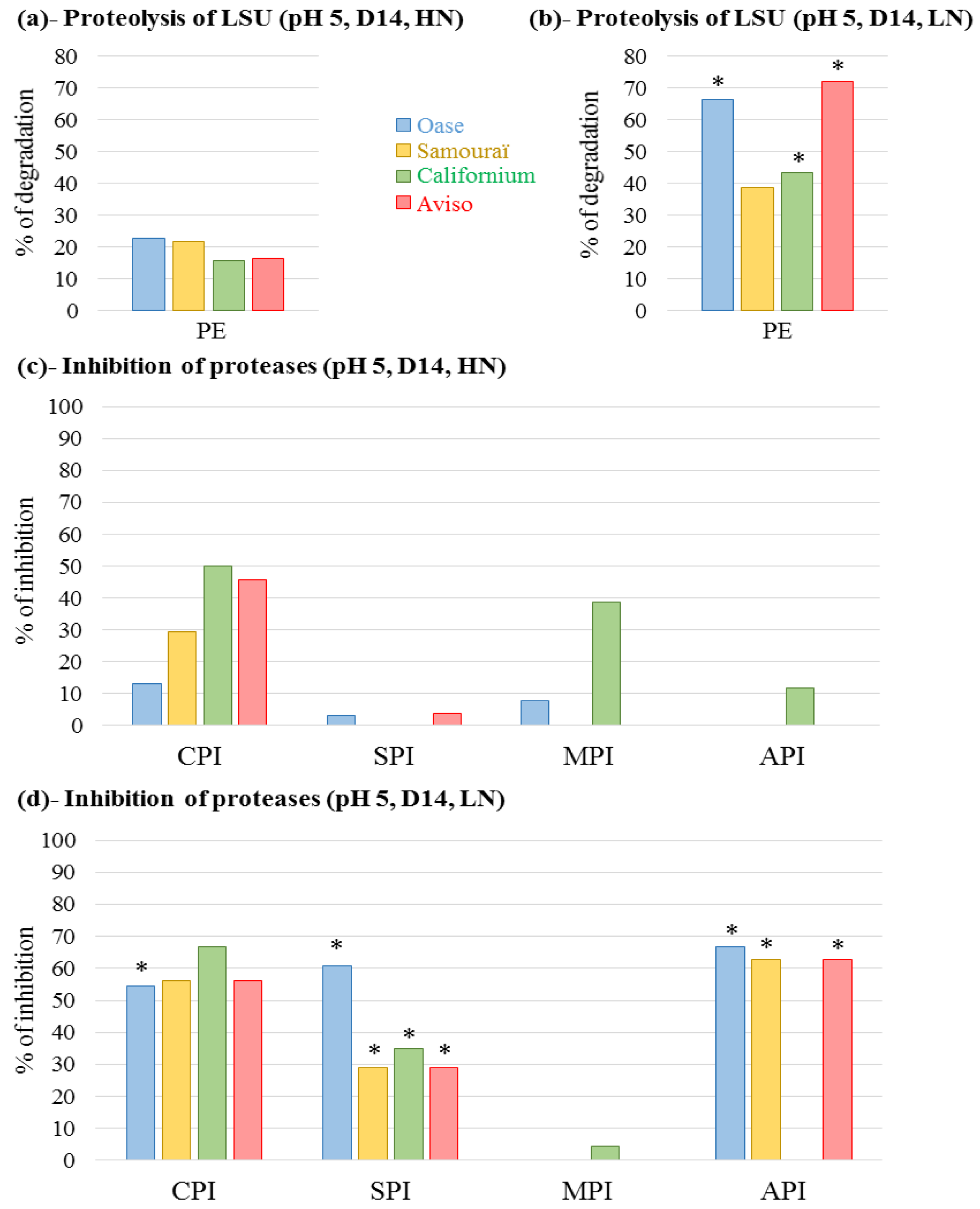
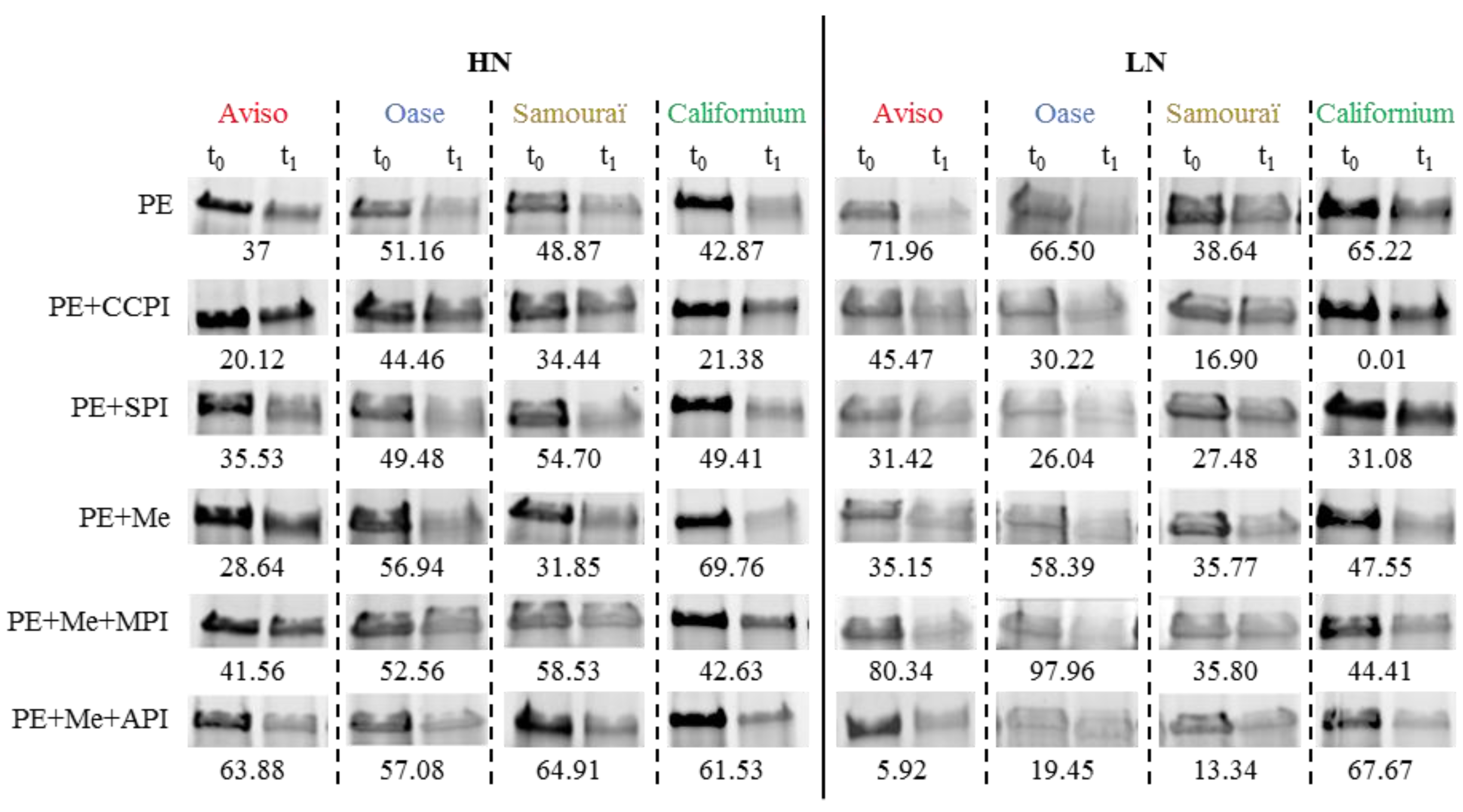
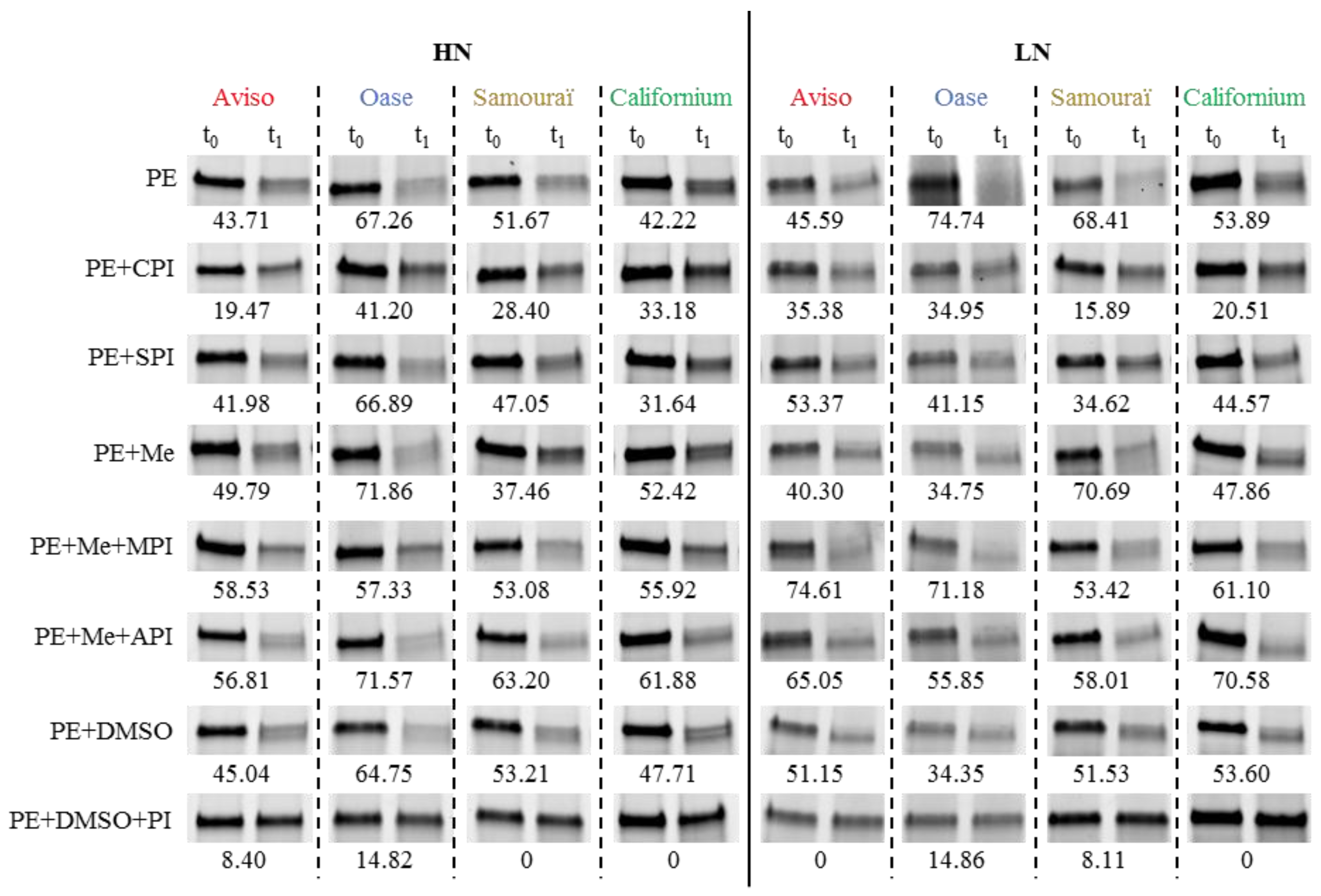
2.4. Proteolytic Activities Related to Rubisco LSU Degradation and Identification of the Classes of Proteases Involved in Remobilization of the Source Leaf Proteins
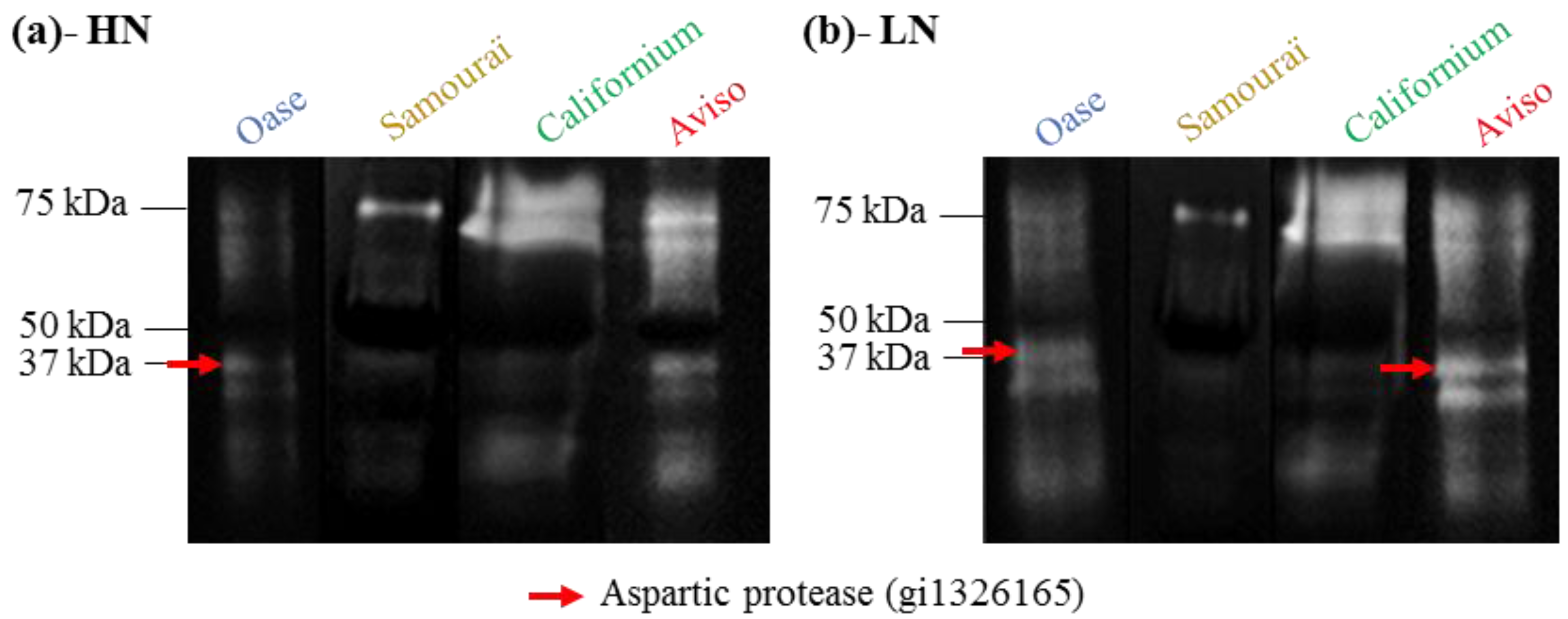
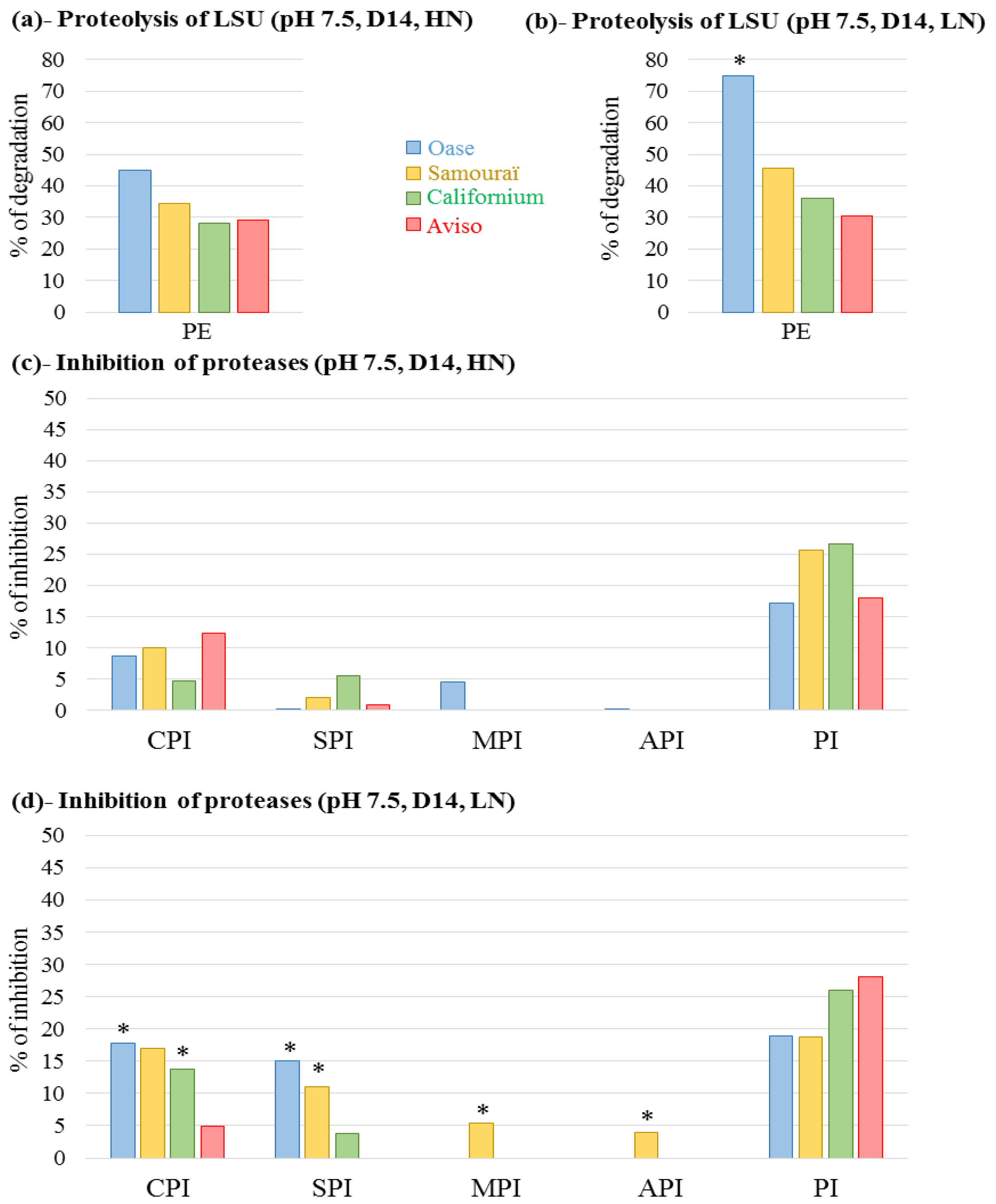
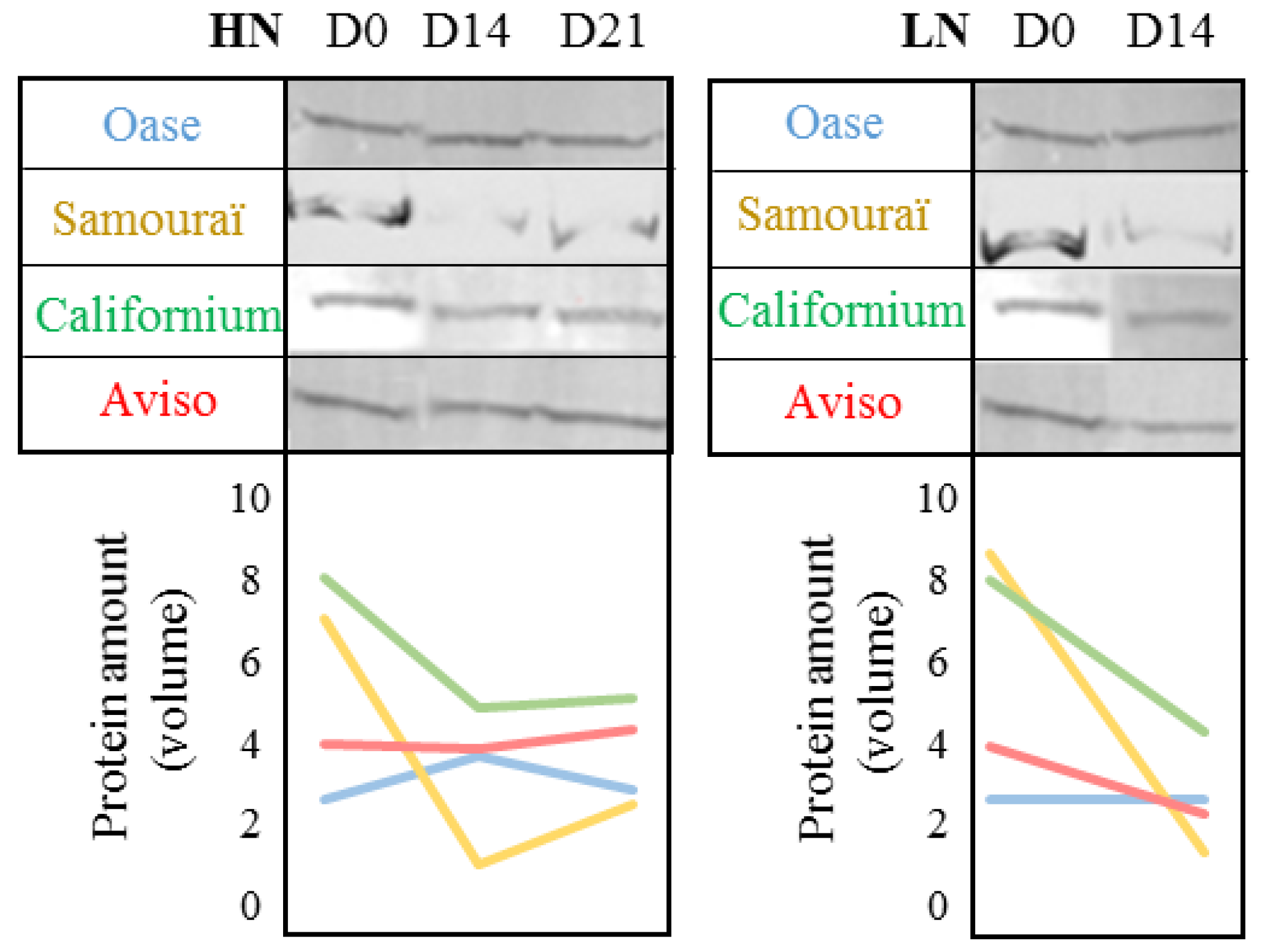
3. Discussion
3.1. The Improvement of Leaf NRE in Aviso and Californium in Response to LN Supply is Associated with a Higher Contribution of Acidic Proteases
3.2. The High Leaf NRE Observed in Oase, Irrespective of the Level of Nitrate Supply, Is Mainly Related to Efficient Proteolysis Mechanisms at Acidic pH
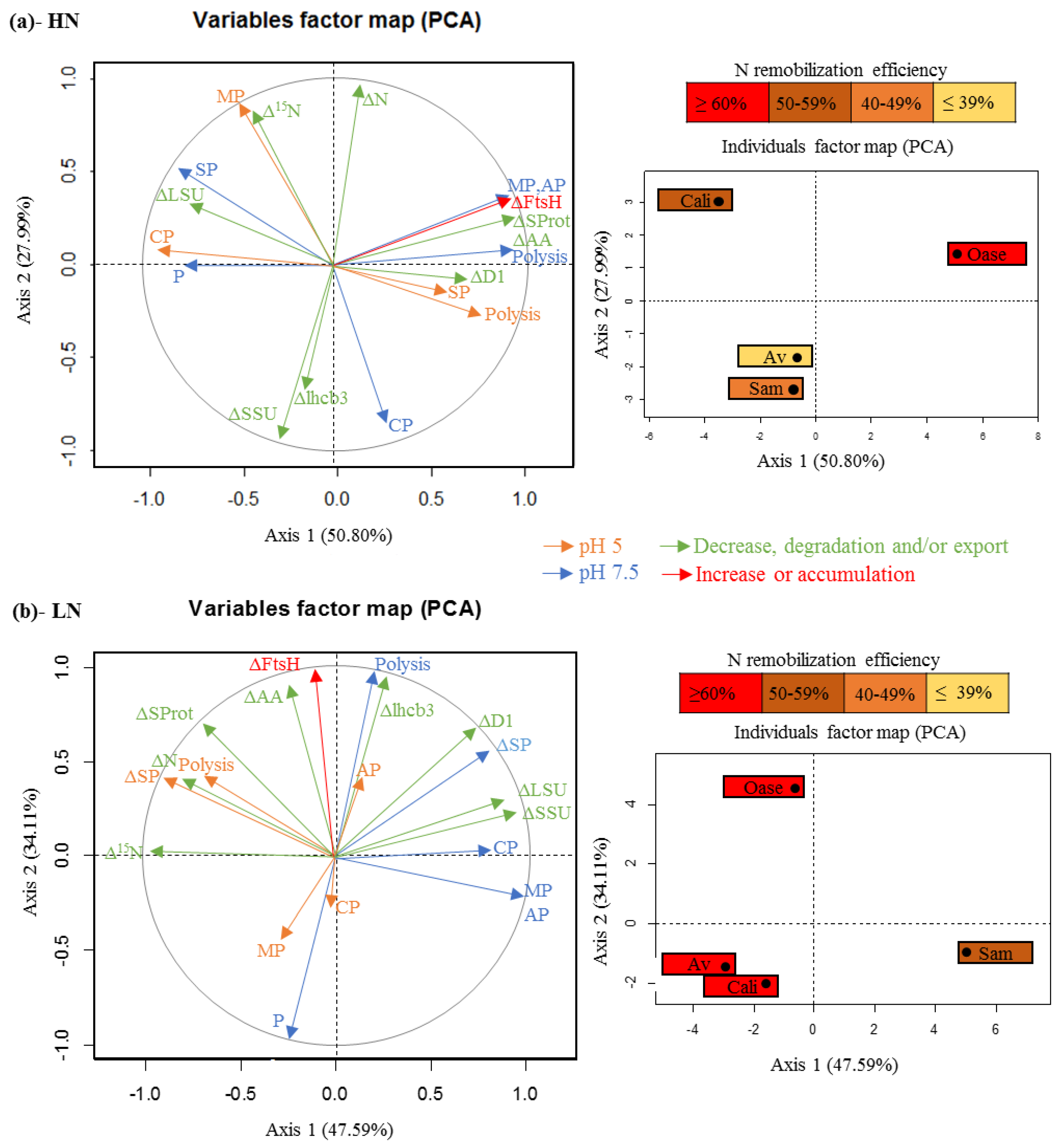
3.3. The Leaf NRE of Samouraï Is Limited between Proteolysis and Amino Acid Export
4. Experimental Section
4.1. Experimental Design
4.2. Quantification of Total N and 15N Amounts
4.3. Extraction and Quantification of Amino Acids
4.4. Extraction and Quantification of Soluble Proteins
4.5. Determination of Proteolytic Activities Using the Rubisco Large Subunit as Substrate
4.6. Detection of Proteolytic Activities by Zymograms
4.7. Immunodetection
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix
| Genotype/N Treatment | Score/Number of Peptides | Proteases Name (Organisms)/NCBI Accession No | Result of BLAST Protein-Protein (Brassica napus)/NCBI Accession No./% Identity | Result of BLAST Protein-Protein (Arabidopsis thaliana)/NCBI Accession No./% Identity |
|---|---|---|---|---|
| Aviso/LN | 49/7 | Aspartic proteases (Brassica napus)/gi|1326165 | BnaA09q47450D/gi|674898012/98% | AT1G11910/gi|222424506/95% |
| BnaC08g15160D/gi|674901714/93% | Aspartic proteinase/gi|1354272/93% | |||
| BnaA08g25040D/gi|674912117/92% | Aspartic proteinase A1/gi|15221141/92% | |||
| Oase/HN | 22/3 | BnaC04g19410D/gi|674924641/79% | Aspartic proteinase A2/gi|22330379/80% | |
| BnaA03g59920D/gi|674908220/78% | Aspartic proteinase A3/gi|15233518/66% | |||
| Oase/LN | 132/12 | BnaA09g20460D/gi|674916523/67% | ||
| BnaC09g22820D/gi|674938912/67% |
References
- Good, A.G.; Shrawat, A.K.; Muench, D.G. Can less yield more? Is reducing nutrient input into the environment compatible with maintaining crop production? Trends Plant Sci. 2004, 9, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D. Global environmental impacts of agricultural expansion: The need for sustainable and efficient practices. Proc. Natl. Acad. Sci. USA 1999, 96, 5995–6000. [Google Scholar] [CrossRef] [PubMed]
- Rathke, G.W.; Christen, O.; Diepenbrock, W. Effects of nitrogen source and rate on productivity and quality of winter oilseed rape (Brassica napus L.) grown in different crop rotations. Field Crop. Res. 2005, 94, 103–113. [Google Scholar] [CrossRef]
- Sylvester-Bradley, R.; Kindred, D.R. Analysis nitrogen responses of cereals to prioritize routes to the improvement of nitrogen use efficiency. J. Exp. Bot. 2009, 60, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Laîné, P.; A. Ourry, A.; Macduff, J.; Boucaud, J.; Salette, J. Kinetic parameters of nitrate uptake by different catch crop species: Effects of low temperatures or previous nitrate starvation. Physiol. Plant. 1993, 88, 85–92. [Google Scholar] [CrossRef]
- Schjoerring, J.K.; Bock, J.G.H.; Gammelvind, L.; Jensen, C.R.; Mogensen, V.O. Nitrogen incorporation and remobilization in different shoot components of field-grown winter oilseed rape (Brassica napus L.) as affected by rate of nitrogen application and irrigation. Plant Soil 1995, 177, 255–264. [Google Scholar] [CrossRef]
- Svecnjak, Z.; Rengel, Z. Canola cultivars differ in nitrogen utilization efficiency at vegetative stage. Field Crop. Res. 2006, 97, 221–226. [Google Scholar] [CrossRef]
- Girondé, A.; Poret, M.; Etienne, P.; Trouverie, J.; Bouchereau, A.; Le Cahérec, F.; Leport, L.; Orsel, M.; Niogret, M.F.; Deleu, C.; et al. A profiling approach of the natural variability of foliar N remobilization at the rosette stage gives clues to understand the limiting processes involved in the low N use efficiency of winter oilseed rape. J. Exp. Bot. 2015. [Google Scholar] [CrossRef] [PubMed]
- Good, A.G.; Beatty, P.H. Biotechnological approaches to improving nitrogen use efficiency in plants: Alanine aminotransferase as a case study. In The Molecular and Physiological Basis of Nutrient Use Efficiency in Crops; Hawkesford, M.J., Barraclough, P., Eds.; John Wiley & Sons, Inc.: Oxford, UK, 2011; pp. 5–19. [Google Scholar]
- Malagoli, P.; Laine, P.; Rossato, L.; Ourry, A. Dynamics of nitrogen uptake and mobilization in field-grown winter oilseed rape (Brassica napus) from stem extension to harvest: I. Global N flows between vegetative and reproductive tissues in relation to leaf fall and their residual N. Ann. Bot. 2005, 95, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Dejoux, J.F.; Recous, S.; Meynard, J.M.; Trinsoutrot, I.; Leterme, P. The fate of nitrogen from winter-frozen rapeseed leaves: Mineralization, fluxes to the environment and uptake by rapeseed crop in spring. Plant Soil 2000, 218, 257–272. [Google Scholar] [CrossRef]
- Noquet, C.; Avice, J.C.; Rossato, L.; Beauclair, P.; Henry, M.P.; Ourry, A. Effects of altered source-sink relationships on N allocation and vegetative storage protein accumulation in Brassica napus L. Plant Sci. 2004, 166, 1007–1018. [Google Scholar] [CrossRef]
- Malagoli, P.; Laine, P.; Rossato, L.; Ourry, A. Dynamics of nitrogen uptake and mobilization in field-grown winter oilseed rape (Brassica napus) from stem extension to harvest. II. An 15N-labelling-based simulation model of N partitioning between vegetative and reproductive tissues. Ann. Bot. 2005, 95, 1187–1198. [Google Scholar] [CrossRef] [PubMed]
- Peoples, M.B.; Dalling, M.J. The interplay between proteolysis and amino acids metabolism during senescence and nitrogen reallocation. In Senescence and Aging in Plants; Noodén, L.D., Leopold, A.C., Eds.; Academic Press: San Diego, CA, USA, 1988; pp. 181–217. [Google Scholar]
- Lohaus, G.; Moellers, C. Phloem transport of amino acids in two Brassica napus L. genotypes and one B. carinata genotype in relation to their seed protein content. Planta 2000, 211, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Lohaus, G.; Schwerdtfeger, M. Comparison of sugars, iridoid glycosides and amino acids in nectar and phloem sap of Maurandya barclayana, Lophospermum erubescens, and Brassica napus. PLoS ONE 2014, 9, e87689. [Google Scholar] [CrossRef] [PubMed]
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen uptake, assimilation and remobilization in plants: Challenges for sustainable and productive agriculture. Ann. Bot. 2010, 105, 1141–1157. [Google Scholar] [CrossRef] [PubMed]
- Tilsner, J.; Kassner, N.; Struck, C.; Lohaus, G. Amino acid contents and transport in oilseed rape (Brassica napus L.) under different nitrogen conditions. Planta 2005, 221, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Desclos, M.; Etienne, P.; Coquet, L.; Jouenne, T.; Bonnefoy, J.; Segura, R.; Reze, S.; Ourry, A.; Avice, J.C. A combined 15N tracing/proteomics study in Brassica napus reveals the chronology of proteomics events associated with N remobilisation during leaf senescence induced by nitrate limitation or starvation. Proteomics 2009, 9, 3580–3608. [Google Scholar] [CrossRef] [PubMed]
- Avice, J.C.; Etienne, P. Leaf senescence and nitrogen remobilization efficiency in oilseed rape (Brassica napus L.). J. Exp. Bot. 2014, 65, 3813–3824. [Google Scholar] [CrossRef] [PubMed]
- Gan, S.; Amasino, R.M. Making sense of senescence. Molecular genetic regulation and manipulation of leaf senescence. Plant Physiol. 1997, 113, 313–319. [Google Scholar] [PubMed]
- Ellis, R.J. The most abundant protein in the world. Trends Biochem. Sci. 1979, 4, 241–244. [Google Scholar] [CrossRef]
- Hörtensteiner, S.; Feller, U. Nitrogen metabolism and remobilization during senescence. J. Exp. Bot. 2002, 53, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.A.; Parry, M.A.J.; Mitchell, R.A.C.; Ahmad, A.; Abrol, Y.P. Photosynthesis and nitrogen-use efficiency. In Photosynthetic Nitrogen Assimilation and Associated Carbon and Respiratory Metabolism; Foyer, C.H., Noctor, G., Eds.; Springer: Dordrecht, The Netherlands, 2002; pp. 23–34. [Google Scholar]
- Makino, A.; Mae, T.; Ohira, K. Relation between nitrogen and ribulose-1,5-bisphosphate carboxylase in rice leaves from emergence through senescence. Plant Cell Physiol. 1984, 25, 429–437. [Google Scholar]
- Matile, P.; Hörtensteiner, S.; Thomas, H.; Kräutler, B. Chlorophyll breakdown in senescent leaves. Plant Physiol. 1996, 112, 1403–1409. [Google Scholar] [PubMed]
- Matile, P. Chloroplast senescence. In Crop Photosynthesis: Spatial and Temporal Determinants; Baker, N.R., Thomas, H., Eds.; Elsevier: Amsterdam, The Netherlands, 1992; pp. 413–440. [Google Scholar]
- Martínez, D.E.; Costa, M.L.; Guiamet, J.J. Senescence-associated degradation of chloroplast proteins inside and outside the organelle. Plant Biol. 2008, 10, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Coupe, S.A.; Sinclair, B.K.; Watson, L.M.; Heyes, J.A.; Eason, J.R. Identification of dehydratation-responsive cysteine proteases during post-harvest senescence of broccoli florets. J. Exp. Bot. 2003, 54, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Nakabayashi, K.; Ito, M.; Kiyosue, T.; Shinozaki, K.; Watanabe, A. Identification of clp genes expressed in senescing Arabidopsis leaves. Plant Cell Physiol. 1999, 40, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Luciński, R.; Misztal, L.; Samardakiewicz, S.; Jackowski, G. Involvement of Deg5 protease in wounding-related disposal of PsbF apoprotein. Plant Physiol. Biochem. 2011, 49, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Luciński, R.; Misztal, L.; Samardakiewicz, S.; Jackowski, G. The thylakoid protease Deg2 is involved in stress-related degradation of the photosystem II light-harvesting protein Lhcb6 in Arabidopsis thaliana. New Phytol. 2011, 192, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Sakamoto, W. Protein quality control in chloroplasts: A current model of D1 protein degradation in the photosystem II repair cycle. J. Biochem. 2009, 146, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Zienkiewics, M.; Ferenc, A.; Wasilewska, W.; Romanowska, E. High light stimulates Deg1-dependent cleavage of the minor LHCII antenna proteins CP26 and CP29 and the PsbS protein in Arabidopsis thaliana. Planta 2012, 235, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Fu, T.; Chen, N.; Guo, J.; Ma, J.; Zou, M.; Lu, C.; Zhang, L. The stromal chloroplast Deg7 protease participates in the repair of photosystem II after photoinhibition in Arabidopsis. Plant Physiol. 2010, 152, 1263–1273. [Google Scholar] [CrossRef] [PubMed]
- Wagner, R.; Aigner, H.; Funk, C. FtsH proteases located in the plant chloroplast. Physiol. Plant. 2012, 145, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Zelisko, A.; Garcia-Lorenzo, M.; Jackowski, G.; Jansson, S.; Funk, C. AtFtsH6 is involved in the degradation of the light-harvesting complex II during high-light acclimation and senescence. Proc. Natl. Acad. Sci. USA 2005, 102, 13699–13704. [Google Scholar] [CrossRef] [PubMed]
- Wagner, R.; Aigner, H.; Pružinská, A.; Jänkänpää, H.J.; Jansson, S.; Funk, C. Fitness analyses of Arabidopsis thaliana mutants depleted of FtsH metalloproteases and characterization of three FtsH6 deletion mutants exposed to high light stress, senescence and chilling. New Phytol. 2011, 191, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Brushnell, T.P.; Brushnell, D.; Jagendorf, A.T. A purified zinc protease of pea chloroplasts, EP1, degrades the large subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase. Plant Physiol. 1993, 103, 585–591. [Google Scholar]
- Kato, Y.; Murakami, S.; Nakano, T.; Sato, F. CND41, a chloroplast DNA-binding protease, is involved in Rubisco degradation. Sci. Access. 2001, 3, 1–4. [Google Scholar]
- Kato, Y.; Yamamoto, Y.; Murakami, S.; Sato, F. Post-translational regulation of CND41 protease activity in senescent tobacco leaves. Planta 2005, 222, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Diaz, C.; Lemaître, T.; Christ, A.; Azzopardi, M.; Kato, Y.; Sato, F.; Morot-Gaudry, J.F.; Le Dily, F.; Masclaux-Daubresse, C. Nitrogen recycling and remobilization are differentially controlled by leaf senescence and development stage in Arabidopsis under low nitrogen nutrition. Plant Physiol. 2008, 147, 1437–1449. [Google Scholar] [CrossRef] [PubMed]
- Chiba, A.; Ishida, H.; Nishizawa, N.K.; Makino, A.; Mae, T. Exclusion of ribulose-1,5-bisphosphate carboxylase/oxygenase from chloroplasts by specific bodies in naturally senescing leaves of wheat. Plant Cell Physiol. 2003, 44, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Ishida, H.; Yoshimoto, K.; Izumi, M.; Reisen, D.; Yano, Y.; Makino, A.; Ohsumi, Y.; Hanson, M.R.; Mae, T. Mobilization of rubisco and stroma-localized fluorescent proteins of chloroplasts to the vacuole by an ATG gene-dependent autophagic process. Plant Physiol. 2008, 148, 142–155. [Google Scholar] [CrossRef] [PubMed]
- Ishida, H.; Izumi, M.; Wada, S.; Makino, A. Roles of autophagy in chloroplast recycling. Biochim. Biophys. Acta 2014, 1837, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Martinez, D.E.; Costa, M.L.; Gomez, F.M.; Otegui, M.; Guiamet, J.J. Senescence-associated vacuoles are involved in the degradation of chloroplast proteins in tobacco leaves. Plant J. 2008, 56, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Blumwald, E. Stress-induced chloroplast degradation in Arabidopsis is regulated via a process independent of autophagy and senescence-associated vacuoles. Plant Cell 2014, 26, 4875–4888. [Google Scholar] [CrossRef] [PubMed]
- Buchanan-Wollaston, V.; Ainsworth, C. Leaf senescence in Brassica napus: Cloning of senescence related genes by subtractive hybridisation. Plant Mol. Biol. 1997, 33, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Stroeher, V.; Maclagan, J.L.; Good, A.G. Molecular cloning of a Brassica napus cysteine protease gene inducible by drought and low temperature stress. Physiol. Plant. 1997, 101, 389–397. [Google Scholar] [CrossRef]
- Noh, Y.S.; Amasino, R.M. Identification of a promoter region responsible for the senescence-specific expression of SAG12. Plant Mol. Biol. 1999, 41, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Desclos-Théveniau, M.; Coquet, L.; Jouenne, T.; Etienne, P. Proteomic analysis of residual proteins in blades and petioles of fallen leaves of Brassica napus. Plant Biolol. 2014, 17, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Roberts, I.N.; Caputo, C.; Criado, M.V.; Funk, C. Senescence-associated proteases in plants. Physiol. Plant. 2012, 145, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Avila-Ospina, L.; Moison, M.; Yoshimoto, K.; Masclaux-Daubresse, C. Autophagy, plant senescence, and nutrient recycling. J. Exp. Bot. 2014, 65, 3799–3811. [Google Scholar] [CrossRef] [PubMed]
- Otegui, M.; Noh, Y.S.; Martinez, D.E.; Petroff, M.G.V.; Staehelin, L.A.; Amasino, R.M.; Guiamet, J.J. Senescence-associated vacuoles with intense proteolytic activity develop in leaves of Arabidopsis and soybean. Plant J. 2005, 41, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Buchanan-Wollaston, V.; Page, T.; Harrison, E.; Breeze, E.; Lim, P.O.; Nam, H.G.; Lin, J.F.; Wu, S.H.; Swidzinski, J.; Ishizaki, K.; et al. Comparative transcriptome analysis reveals significant differences in gene expression and signalling pathways between developmental and dark/starvation-induced senescence in Arabidopsis. Plant J. 2005, 42, 567–585. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Mendoza, M.; Velasco-Arroyo, B.; Gonzalez-Melendi, P.; Martinez, M.; Diaz, I. C1A cysteine protease-cystatin interactions in leaf senescence. J. Exp. Bot. 2014, 65, 3825–3833. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.; Pan, S.; Zouhar, J.; Avila, E.L.; Girke, T.; Raikhel, N.V. The vegetative vacuole proteome of Arabidopsis thaliana reveals predicted and unexpected proteins. Plant Cell 2004, 16, 3285–3303. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, M.; Yang, D.H.; Andersson, B. Regulatory proteolysis of the major light-harvesting chlorophyll a/b protein of photosystem II by a light-induced membrane-associated enzymic system. Eur. J. Agron. 1995, 231, 503–509. [Google Scholar]
- Forsberg, J.; Ström, J.; Kieselbach, T.; Larsson, H.; Alexciev, K.; Engström, A.; Akerlund, H.E. Protease activities in the chloroplast capable of cleaving an LCHII N-terminal peptide. Physiol Plant. 2005, 123, 21–29. [Google Scholar] [CrossRef]
- Huesgen, P.F.; Schumann, H.; Adamska, I. Deg/HtrA proteases as components of a network for photosystem II quality control in chloroplasts and cyanobacteria. Res. Microbiol. 2009, 160, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Murakami, S.; Yamamoto, Y.; Chatani, H.; Kondo, Y.; Nakano, T.; Yokota, A.; Sato, F. The DNA-binding protease, CND41, and the degradation of ribulose-1,5-biphosphate carboxylase/oxygenase in senescent leaves of tobacco. Planta 2004, 220, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Gregersen, P.L.; Holm, P.B. Transcriptome analysis of senescence in the flag leaf of wheat (Triticum aestivum L.). Plant Biotechnol. J. 2007, 5, 192–206. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Kondo, Y.; Nakano, T.; Sato, F. Protease activity of CND41, a chloroplast nucleoid DNA-binding protein, isolated from cultured tobacco cells. FEBS Lett. 2000, 468, 15–18. [Google Scholar] [CrossRef]
- Damodaran, M.; Ananta-Narayanan, P. Enzymic proteolysis. Liberation of ammonia from proteins. Biochem. J. 1938, 32, 1877–1889. [Google Scholar] [CrossRef] [PubMed]
- Givan, C.V. Metabolic detoxification of ammonia in tissues of higher plants. Phytochemistry 1979, 18, 375–382. [Google Scholar] [CrossRef]
- Guo, Y.; Cai, Z.; Gan, S. Transcriptome of Arabidopsis leaf senenscence. Plant Cell Environ. 2004, 27, 521–549. [Google Scholar] [CrossRef]
- Park, J.H.; Oh, S.A.; Kim, Y.H.; Woo, H.R.; Nam, H.G. Differential expression of senescence-associated mRNAs during leaf senescence induced by different senescence-inducing factors in Arabidopis. Plant Mol. Biol. 1998, 37, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Saric, T.; Graef, C.I.; Goldberg, A.L. Pathway for degradation of peptides generated by proteasomes. A key role for thimet oligopeptidase and other metallopeptidases. J. Biol. Chem. 2004, 279, 46723–46732. [Google Scholar] [CrossRef] [PubMed]
- Reits, E.; Neijssen, J.; Herberts, C.; Benckhuijsen, W.; Janssen, L.; Drijhout, W.J.; Neefjes, J. A major role for TPPII in trimming proteasomal degradation products for MHC class I antigen presentation. Immunity 2004, 20, 495–506. [Google Scholar] [CrossRef]
- Polge, C.; Jaquinod, M.; Holzer, F.; Bourguignon, J.; Walling, L.; Brouquisse, R. Evidence for the existence in Arabidopsis thaliana of the proteasome proteolytic pathway. J. Biol. Chem. 2009, 284, 35412–35424. [Google Scholar] [CrossRef] [PubMed]
- Salon, C.; Bataillé, M.P.; Gallardo, K.; Jeudy, C.; Santoni, A.L.; Trouverie, J.; Voisin, A.S.; Avice, J.C. 34S and 15N labelling to model S and N flux in plants and determine the different components of N and S use efficiency. Methods Mol. Biol. 2014, 1090, 335–346. [Google Scholar] [PubMed]
- Rosen, H. A modified ninhydrin colorimetric analysis for amino acids. Arch. Biochem. Biophys. 1957, 67, 10–15. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Girondé, A.; Etienne, P.; Trouverie, J.; Bouchereau, A.; Le Cahérec, F.; Leport, L.; Orsel, M.; Niogret, M.F.; Nesi, N.; Deleu, C.; et al. The contrasting management of two oilseed rape genotypes reveals the mechanisms of proteolysis associated with leaf N remobilization and the respective contributions of leaves and stems to N storage and remobilisation during seed filling. BMC Plant Biol. 2015, 15, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laemmli, U.K. Cleavage of structural proteins during the heat bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, F.; Cejudo, F.J. Pattern of endoproteolysis following wheat grain germination. Physiol. Plant. 1995, 95, 253–259. [Google Scholar] [CrossRef]
- Matrix Science. Available online: http://www.matrixscience.com (accessed on 15 January 2015).
- Brassica Genome Gateway. Available online: http://brassica.bbsrc.ac.uk (accessed on 15 January 2015).
- Desclos, M.; Dubousset, L.; Etienne, P.; Le Caherec, F.; Satoh, H.; Bonnefoy, J.; Ourry, A.; Avice, J.C. A proteomic profiling approach to reveal a novel role of Brassica napus drought 22 kD/water-soluble chlorophyll-binding protein in young leaves during nitrogen remobilization induced by stressful conditions. Plant Physiol. 2008, 147, 1830–1844. [Google Scholar] [CrossRef] [PubMed]
- Mechin, V.; Consoli, L.; Le Guilloux, M.; Damerval, C. An efficient solubilization buffer for plant proteins focused in immobilized pH gradients. Proteomics 2003, 3, 1299–1302. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Girondé, A.; Poret, M.; Etienne, P.; Trouverie, J.; Bouchereau, A.; Le Cahérec, F.; Leport, L.; Niogret, M.-F.; Avice, J.-C. A Comparative Study of Proteolytic Mechanisms during Leaf Senescence of Four Genotypes of Winter Oilseed Rape Highlighted Relevant Physiological and Molecular Traits for NRE Improvement. Plants 2016, 5, 1. https://doi.org/10.3390/plants5010001
Girondé A, Poret M, Etienne P, Trouverie J, Bouchereau A, Le Cahérec F, Leport L, Niogret M-F, Avice J-C. A Comparative Study of Proteolytic Mechanisms during Leaf Senescence of Four Genotypes of Winter Oilseed Rape Highlighted Relevant Physiological and Molecular Traits for NRE Improvement. Plants. 2016; 5(1):1. https://doi.org/10.3390/plants5010001
Chicago/Turabian StyleGirondé, Alexandra, Marine Poret, Philippe Etienne, Jacques Trouverie, Alain Bouchereau, Françoise Le Cahérec, Laurent Leport, Marie-Françoise Niogret, and Jean-Christophe Avice. 2016. "A Comparative Study of Proteolytic Mechanisms during Leaf Senescence of Four Genotypes of Winter Oilseed Rape Highlighted Relevant Physiological and Molecular Traits for NRE Improvement" Plants 5, no. 1: 1. https://doi.org/10.3390/plants5010001






