Hedgehog Promotes Production of Inhibitory Interneurons in Vivo and in Vitro from Pluripotent Stem Cells
Abstract
:1. Introduction
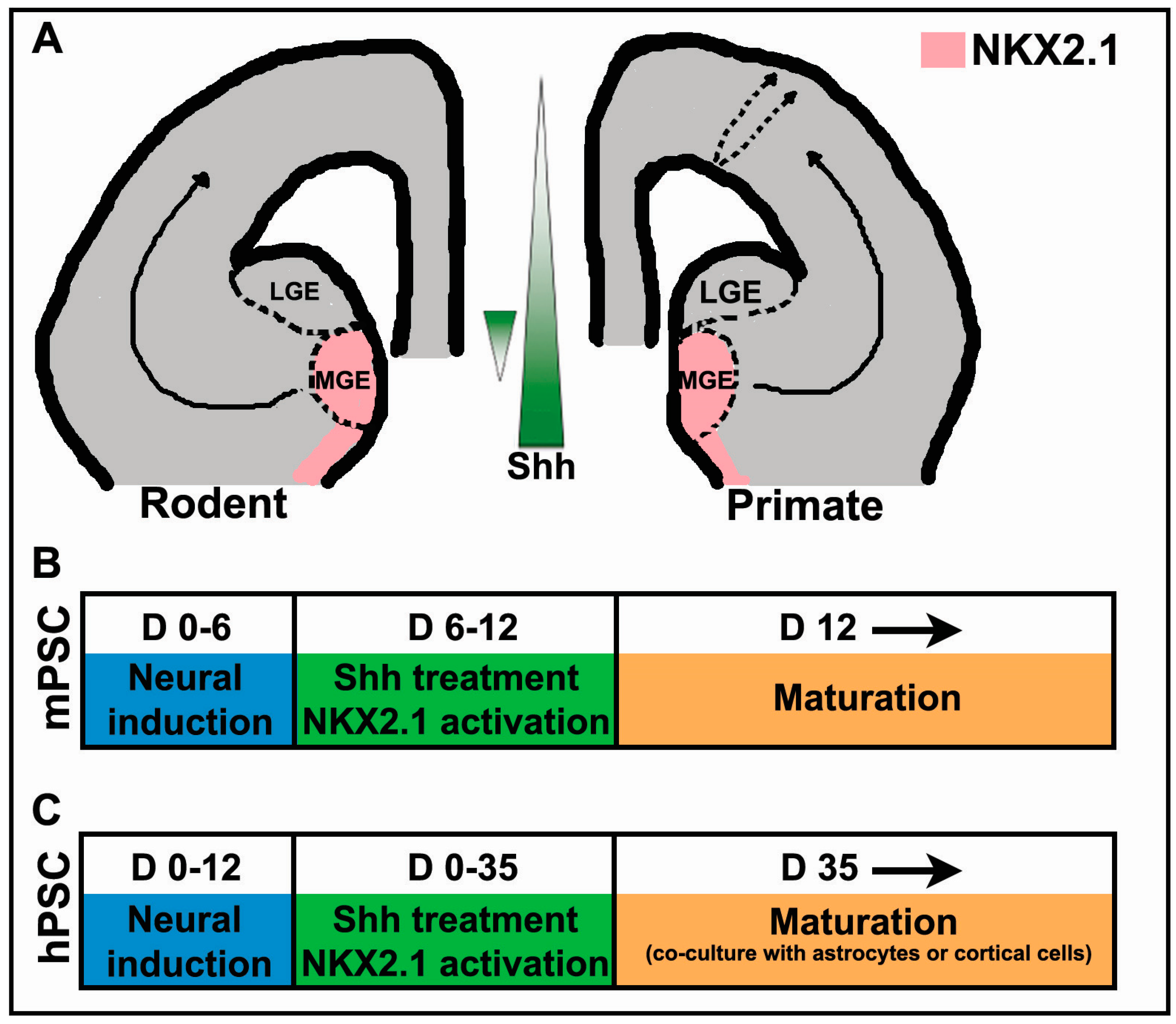
2. The Importance of Shh in Specification of Cortical Interneurons in Vivo
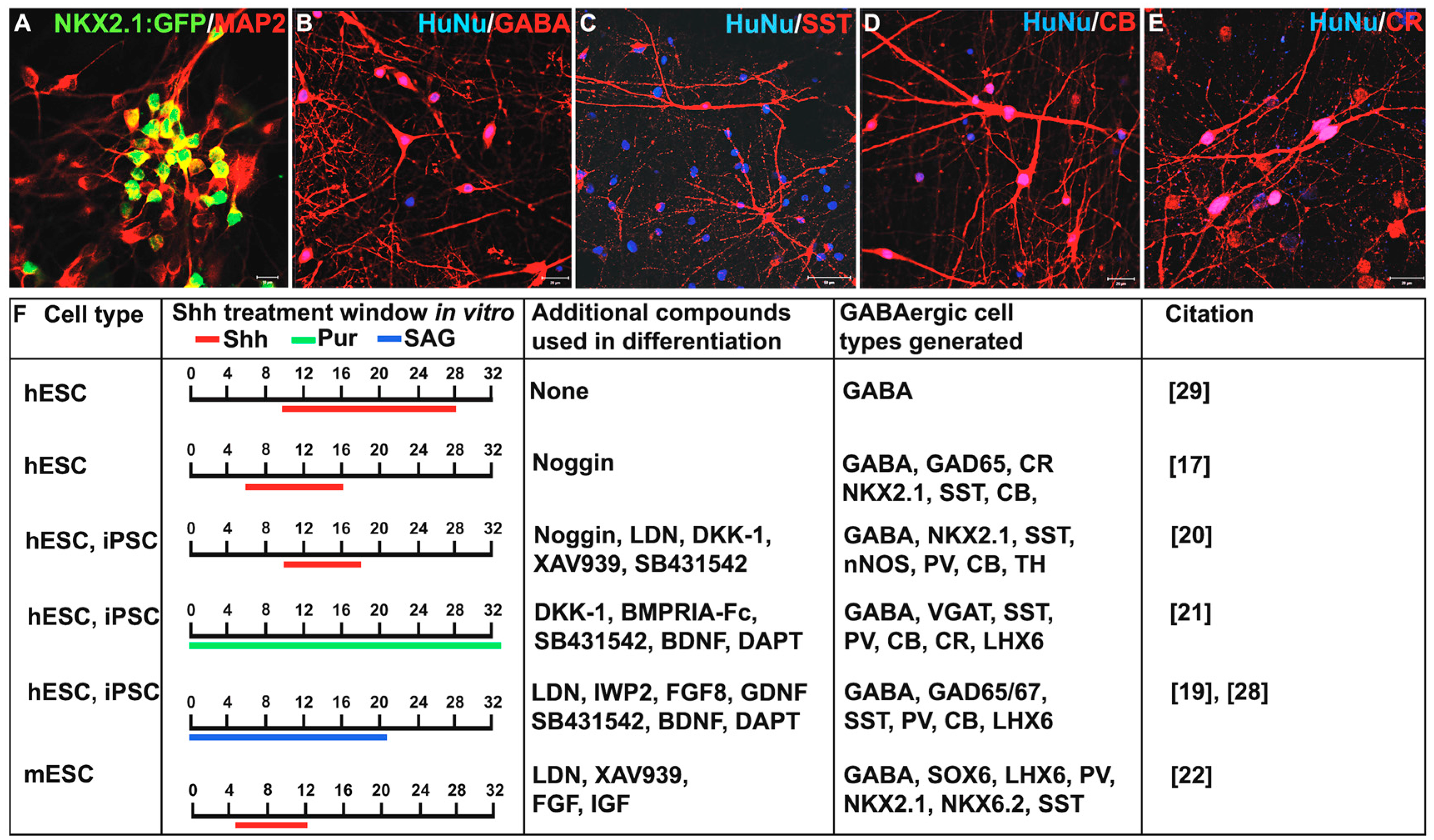
3. Shh Treatment for the Derivation of Interneurons from PSCs
4. Challenges to Future Clinical Applications Using PSC-Derived Interneurons
4.1. Human PSC-Derived Interneurons Require an Extended Maturation Timeline
4.2. Selective and Efficient Generation of Interneuron Subtypes
4.3. Genetic Modification of Cell Lines Used for Clinical Applications
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CNS | Central nervous system |
| SST | Somatostatin |
| PV | Parvalbumin |
| CR | Calretinin |
| CB | Calbindin |
| NPY | Neuropeptide-Y |
| VIP | Vasoactive intestinal peptide |
| GABA | Gamma-Aminobutyric acid |
| GABAergic | Gamma-aminobutyric acid-containing interneurons |
| GAD | Glutamic acid decarboxylase |
| nNOS | Neuronal nitric oxide synthase |
| ASD | Autism spectrum disorders |
| AD | Alzheimer’s disease |
| MGE | Medial ganglionic eminence |
| CGE | Caudal ganglionic eminence |
| LGE | Lateral ganglionic eminence |
| PSCs | Pluripotent stem cells |
| Shh | Sonic hedgehog |
| Smo | Smoothened |
| SAG | Smoothened agonist |
| VZ | Ventricular zone |
| SVZ | Subventricular zone |
| oSVZ | Outer subventricular zone |
| POA | Preoptic area |
| FACS | Fluorescence activated cell sorted |
| iPS | Induced pluripotent stem |
| iPSC | Induced pluripotent stem cell |
| ESC | Embryonic stem cell |
| sPSC | spontaneous postsynaptic currents |
| DCX | Doublecortin |
| PSA-NCAM | Polysialylated-neural cell adhesion molecule |
References
- Kepecs, A.; Fishell, G. Interneuron cell types are fit to function. Nature 2014, 505, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Corbin, J.G.; Butt, S.J. Developmental mechanisms for the generation of telencephalic interneurons. Dev. Neurobiol. 2011, 71, 710–732. [Google Scholar] [CrossRef] [PubMed]
- Vitalis, T.; Rossier, J. New insights into cortical interneurons development and classification: Contribution of developmental studies. Dev. Neurobiol. 2011, 71, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Wonders, C.P.; Anderson, S.A. The origin and specification of cortical interneurons. Nat. Rev. Neurosci. 2006, 7, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Anderson, S.A. Development of cortical interneurons. Neuropsychopharmacology 2015, 40, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Shetty, A.K.; Bates, A. Potential of GABA-ergic cell therapy for schizophrenia, neuropathic pain, and alzheimers and parkinsons diseases. Brain Res. 2016, 1638, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Southwell, D.G.; Nicholas, C.R.; Basbaum, A.I.; Stryker, M.P.; Kriegstein, A.R.; Rubenstein, J.L.; Alvarez-Buylla, A. Interneurons from embryonic development to cell-based therapy. Science 2014, 344, 1240622. [Google Scholar] [CrossRef] [PubMed]
- Tyson, J.A.; Anderson, S.A. Gabaergic interneuron transplants to study development and treat disease. Trends Neurosci. 2014, 37, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Tricoire, L.; Pelkey, K.A.; Erkkila, B.E.; Jeffries, B.W.; Yuan, X.; McBain, C.J. A blueprint for the spatiotemporal origins of mouse hippocampal interneuron diversity. J. Neurosci. 2011, 31, 10948–10970. [Google Scholar] [CrossRef] [PubMed]
- Welagen, J.; Anderson, S. Origins of neocortical interneurons in mice. Dev. Neurobiol. 2011, 71, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Nery, S.; Fishell, G.; Corbin, J.G. The caudal ganglionic eminence is a source of distinct cortical and subcortical cell populations. Nat. Neurosci. 2002, 5, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Sussel, L.; Marin, O.; Kimura, S.; Rubenstein, J.L. Loss of NKX2.1 homeobox gene function results in a ventral to dorsal molecular respecification within the basal telencephalon: Evidence for a transformation of the pallidum into the striatum. Development 1999, 126, 3359–3370. [Google Scholar] [PubMed]
- Xu, Q.; Cobos, I.; de la Cruz, E.; Rubenstein, J.L.; Anderson, S.A. Origins of cortical interneuron subtypes. J. Neurosci. 2004, 24, 2612–2622. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Guo, L.; Moore, H.; Waclaw, R.R.; Campbell, K.; Anderson, S.A. Sonic hedgehog signaling confers ventral telencephalic progenitors with distinct cortical interneuron fates. Neuron 2010, 65, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Chedotal, A.; Rijli, F.M. Transcriptional regulation of tangential neuronal migration in the developing forebrain. Curr. Opin. Neurobiol. 2009, 19, 139–145. [Google Scholar] [CrossRef] [PubMed]
- DeBoer, E.M.; Anderson, S.A. Fate determination of cerebral cortical gabaergic interneurons and their derivation from stem cells. Brain Res. 2015. [Google Scholar] [CrossRef] [PubMed]
- Germain, N.D.; Banda, E.C.; Becker, S.; Naegele, J.R.; Grabel, L.B. Derivation and isolation of NKX2.1-positive basal forebrain progenitors from human embryonic stem cells. Stem Cells Dev. 2013, 22, 1477–1489. [Google Scholar] [CrossRef] [PubMed]
- Goulburn, A.L.; Alden, D.; Davis, R.P.; Micallef, S.J.; Ng, E.S.; Yu, Q.C.; Lim, S.M.; Soh, C.L.; Elliott, D.A.; Hatzistavrou, T.; et al. A targeted NKX2.1 human embryonic stem cell reporter line enables identification of human basal forebrain derivatives. Stem Cells 2011, 29, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.G.; Yao, R.; Monnell, T.; Cho, J.H.; Vasudevan, A.; Koh, A.; Peeyush, K.T.; Moon, M.; Datta, D.; Bolshakov, V.Y.; et al. Efficient specification of interneurons from human pluripotent stem cells by dorsoventral and rostrocaudal modulation. Stem Cells 2014, 32, 1789–1804. [Google Scholar] [CrossRef] [PubMed]
- Maroof, A.M.; Keros, S.; Tyson, J.A.; Ying, S.W.; Ganat, Y.M.; Merkle, F.T.; Liu, B.; Goulburn, A.; Stanley, E.G.; Elefanty, A.G.; et al. Directed differentiation and functional maturation of cortical interneurons from human embryonic stem cells. Cell Stem Cell 2013, 12, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, C.R.; Chen, J.; Tang, Y.; Southwell, D.G.; Chalmers, N.; Vogt, D.; Arnold, C.M.; Chen, Y.J.; Stanley, E.G.; Elefanty, A.G.; et al. Functional maturation of hpsc-derived forebrain interneurons requires an extended timeline and mimics human neural development. Cell Stem Cell 2013, 12, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Tyson, J.A.; Goldberg, E.M.; Maroof, A.M.; Xu, Q.; Petros, T.J.; Anderson, S.A. Duration of culture and sonic hedgehog signaling differentially specify pv versus sst cortical interneuron fates from embryonic stem cells. Development 2015, 142, 1267–1278. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Nagata, N.; Kurokawa, H.; Yamanaka, S. Ips cells: A game changer for future medicine. EMBO J. 2014, 33, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Kelava, I.; Lancaster, M.A. Stem cell models of human brain development. Cell Stem Cell 2016, 18, 736–748. [Google Scholar] [CrossRef] [PubMed]
- Mariani, J.; Coppola, G.; Zhang, P.; Abyzov, A.; Provini, L.; Tomasini, L.; Amenduni, M.; Szekely, A.; Palejev, D.; Wilson, M.; et al. Foxg1-dependent dysregulation of gaba/glutamate neuron differentiation in autism spectrum disorders. Cell 2015, 162, 375–390. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, Y.A.; Kemper, T.L.; Bauman, M.L.; Blatt, G.J. Parvalbumin-, calbindin-, and calretinin-immunoreactive hippocampal interneuron density in autism. Acta Neurol. Scand. 2010, 121, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Shetty, A.K. Progress in cell grafting therapy for temporal lobe epilepsy. Neurotherapeutics 2011, 8, 721–735. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, M.; Cho, J.H.; Leung, A.; Savvidis, G.; Ahn, S.; Moon, M.; Lee, P.K.; Han, J.J.; Azimi, N.; Kim, K.S.; et al. Hpsc-derived maturing gabaergic interneurons ameliorate seizures and abnormal behavior in epileptic mice. Cell Stem Cell 2014, 15, 559–573. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Weick, J.P.; Liu, H.; Krencik, R.; Zhang, X.; Ma, L.; Zhou, G.M.; Ayala, M.; Zhang, S.C. Medial ganglionic eminence-like cells derived from human embryonic stem cells correct learning and memory deficits. Nat. Biotechnol. 2013, 31, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Maisano, X.; Litvina, E.; Tagliatela, S.; Aaron, G.B.; Grabel, L.B.; Naegele, J.R. Differentiation and functional incorporation of embryonic stem cell-derived gabaergic interneurons in the dentate gyrus of mice with temporal lobe epilepsy. J. Neurosci. 2012, 32, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.G. The origins of cortical interneurons: Mouse versus monkey and human. Cereb. Cortex 2009, 19, 1953–1956. [Google Scholar] [CrossRef] [PubMed]
- Molnar, Z.; Butt, S.J. Best-laid schemes for interneuron origin of mice and men. Nat. Neurosci. 2013, 16, 1512–1514. [Google Scholar] [CrossRef] [PubMed]
- Hladnik, A.; Dzaja, D.; Darmopil, S.; Jovanov-Milosevic, N.; Petanjek, Z. Spatio-temporal extension in site of origin for cortical calretinin neurons in primates. Front. Neuroanat. 2014, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.V.; Lui, J.H.; Flandin, P.; Yoshikawa, K.; Rubenstein, J.L.; Alvarez-Buylla, A.; Kriegstein, A.R. Non-epithelial stem cells and cortical interneuron production in the human ganglionic eminences. Nat. Neurosci. 2013, 16, 1576–1587. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Wang, C.; Wang, L.; Zhou, X.; Tian, M.; Zhang, Q.; Zhang, Y.; Li, J.; Liu, Z.; Cai, Y.; et al. Subcortical origins of human and monkey neocortical interneurons. Nat. Neurosci. 2013, 16, 1588–1597. [Google Scholar] [CrossRef] [PubMed]
- Radonjic, N.V.; Ayoub, A.E.; Memi, F.; Yu, X.; Maroof, A.; Jakovcevski, I.; Anderson, S.A.; Rakic, P.; Zecevic, N. Diversity of cortical interneurons in primates: The role of the dorsal proliferative niche. Cell Rep. 2014, 9, 2139–2151. [Google Scholar] [CrossRef] [PubMed]
- Breunig, J.J.; Sarkisian, M.R.; Arellano, J.I.; Morozov, Y.M.; Ayoub, A.E.; Sojitra, S.; Wang, B.; Flavell, R.A.; Rakic, P.; Town, T. Primary cilia regulate hippocampal neurogenesis by mediating sonic hedgehog signaling. Proc. Natl. Acad. Sci. USA 2008, 105, 13127–13132. [Google Scholar] [CrossRef] [PubMed]
- Machold, R.; Hayashi, S.; Rutlin, M.; Muzumdar, M.D.; Nery, S.; Corbin, J.G.; Gritli-Linde, A.; Dellovade, T.; Porter, J.A.; Rubin, L.L.; et al. Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron 2003, 39, 937–950. [Google Scholar] [CrossRef]
- Palma, V.; Ruiz i Altaba, A. Hedgehog-Gli signaling regulates the behavior of cells with stem cell properties in the developing neocortex. Development 2004, 131, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Rowitch, D.H.; St-Jacques, B.; Lee, S.M.; Flax, J.D.; Snyder, E.Y.; McMahon, A.P. Sonic hedgehog regulates proliferation and inhibits differentiation of cns precursor cells. J. Neurosci. 1999, 19, 8954–8965. [Google Scholar] [PubMed]
- Willaredt, M.A.; Hasenpusch-Theil, K.; Gardner, H.A.; Kitanovic, I.; Hirschfeld-Warneken, V.C.; Gojak, C.P.; Gorgas, K.; Bradford, C.L.; Spatz, J.; Wolfl, S.; et al. A crucial role for primary cilia in cortical morphogenesis. J. Neurosci. 2008, 28, 12887–12900. [Google Scholar] [CrossRef] [PubMed]
- Briscoe, J.; Ericson, J. The specification of neuronal identity by graded sonic hedgehog signalling. Semin. Cell Dev. Biol. 1999, 10, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Hoch, R.V.; Rubenstein, J.L.; Pleasure, S. Genes and signaling events that establish regional patterning of the mammalian forebrain. Semin. Cell Dev. Biol. 2009, 20, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Flames, N.; Pla, R.; Gelman, D.M.; Rubenstein, J.L.; Puelles, L.; Marin, O. Delineation of multiple subpallial progenitor domains by the combinatorial expression of transcriptional codes. J. Neurosci. 2007, 27, 9682–9695. [Google Scholar] [CrossRef] [PubMed]
- Gelman, D.M.; Marin, O. Generation of interneuron diversity in the mouse cerebral cortex. Eur. J. Neurosci. 2010, 31, 2136–2141. [Google Scholar] [CrossRef] [PubMed]
- Gelman, D.; Griveau, A.; Dehorter, N.; Teissier, A.; Varela, C.; Pla, R.; Pierani, A.; Marin, O. A wide diversity of cortical gabaergic interneurons derives from the embryonic preoptic area. J. Neurosci. 2011, 31, 16570–16580. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Roby, K.D.; Callaway, E.M. Immunochemical characterization of inhibitory mouse cortical neurons: Three chemically distinct classes of inhibitory cells. J. Comp. Neurol. 2010, 518, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Gulacsi, A.; Anderson, S.A. Shh maintains NKX2.1 in the mge by a Gli3-independent mechanism. Cereb. Cortex 2006, 16 (Suppl. S1), i89–i95. [Google Scholar] [CrossRef] [PubMed]
- Rallu, M.; Corbin, J.G.; Fishell, G. Parsing the prosencephalon. Nat. Rev. Neurosci. 2002, 3, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Butt, S.J.; Sousa, V.H.; Fuccillo, M.V.; Hjerling-Leffler, J.; Miyoshi, G.; Kimura, S.; Fishell, G. The requirement of NKX2-1 in the temporal specification of cortical interneuron subtypes. Neuron 2008, 59, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Xu, Q.; Ocbina, P.J.; Anderson, S.A. NKX2.1 specifies cortical interneuron fate by activating lhx6. Development 2008, 135, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Wonders, C.P.; Anderson, S.A. Sonic hedgehog maintains the identity of cortical interneuron progenitors in the ventral telencephalon. Development 2005, 132, 4987–4998. [Google Scholar] [CrossRef] [PubMed]
- Butt, S.J.; Fuccillo, M.; Nery, S.; Noctor, S.; Kriegstein, A.; Corbin, J.G.; Fishell, G. The temporal and spatial origins of cortical interneurons predict their physiological subtype. Neuron 2005, 48, 591–604. [Google Scholar] [CrossRef] [PubMed]
- Radonjic, N.V.; Memi, F.; Ortega, J.A.; Glidden, N.; Zhan, H.; Zecevic, N. The role of sonic hedgehog in the specification of human cortical progenitors in vitro. Cereb. Cortex 2016, 26, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Wonders, C.P.; Taylor, L.; Welagen, J.; Mbata, I.C.; Xiang, J.Z.; Anderson, S.A. A spatial bias for the origins of interneuron subgroups within the medial ganglionic eminence. Dev. Biol. 2008, 314, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Gelman, D.M.; Martini, F.J.; Nobrega-Pereira, S.; Pierani, A.; Kessaris, N.; Marin, O. The embryonic preoptic area is a novel source of cortical gabaergic interneurons. J. Neurosci. 2009, 29, 9380–9389. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, G.; Hjerling-Leffler, J.; Karayannis, T.; Sousa, V.H.; Butt, S.J.; Battiste, J.; Johnson, J.E.; Machold, R.P.; Fishell, G. Genetic fate mapping reveals that the caudal ganglionic eminence produces a large and diverse population of superficial cortical interneurons. J. Neurosci. 2010, 30, 1582–1594. [Google Scholar] [CrossRef] [PubMed]
- Cauli, B.; Zhou, X.; Tricoire, L.; Toussay, X.; Staiger, J.F. Revisiting enigmatic cortical calretinin-expressing interneurons. Front. Neuroanat. 2014, 8, 52. [Google Scholar] [CrossRef] [PubMed]
- Zaitsev, A.V.; Gonzalez-Burgos, G.; Povysheva, N.V.; Kroner, S.; Lewis, D.A.; Krimer, L.S. Localization of calcium-binding proteins in physiologically and morphologically characterized interneurons of monkey dorsolateral prefrontal cortex. Cereb. Cortex 2005, 15, 1178–1186. [Google Scholar] [CrossRef] [PubMed]
- Fertuzinhos, S.; Krsnik, Z.; Kawasawa, Y.I.; Rasin, M.R.; Kwan, K.Y.; Chen, J.G.; Judas, M.; Hayashi, M.; Sestan, N. Selective depletion of molecularly defined cortical interneurons in human holoprosencephaly with severe striatal hypoplasia. Cereb. Cortex 2009, 19, 2196–2207. [Google Scholar] [CrossRef] [PubMed]
- Zecevic, N.; Chen, Y.; Filipovic, R. Contributions of cortical subventricular zone to the development of the human cerebral cortex. J. Comp. Neurol. 2005, 491, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Gaspard, N.; Bouschet, T.; Hourez, R.; Dimidschstein, J.; Naeije, G.; van den Ameele, J.; Espuny-Camacho, I.; Herpoel, A.; Passante, L.; Schiffmann, S.N.; et al. An intrinsic mechanism of corticogenesis from embryonic stem cells. Nature 2008, 455, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Zhang, X.; Johnson, M.A.; Wang, Z.B.; Lavaute, T.; Zhang, S.C. Coordination of sonic hedgehog and Wnt signaling determines ventral and dorsal telencephalic neuron types from human embryonic stem cells. Development 2009, 136, 4055–4063. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Kamiya, D.; Nishiyama, A.; Katayama, T.; Nozaki, S.; Kawasaki, H.; Watanabe, Y.; Mizuseki, K.; Sasai, Y. Directed differentiation of telencephalic precursors from embryonic stem cells. Nat. Neurosci. 2005, 8, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Guo, M.; Martins-Taylor, K.; Wang, X.; Zhang, Z.; Park, J.W.; Zhan, S.; Kronenberg, M.S.; Lichtler, A.; Liu, H.X.; et al. Specification of region-specific neurons including forebrain glutamatergic neurons from human induced pluripotent stem cells. PLoS ONE 2010, 5, e11853. [Google Scholar] [CrossRef] [PubMed]
- Danjo, T.; Eiraku, M.; Muguruma, K.; Watanabe, K.; Kawada, M.; Yanagawa, Y.; Rubenstein, J.L.; Sasai, Y. Subregional specification of embryonic stem cell-derived ventral telencephalic tissues by timed and combinatory treatment with extrinsic signals. J. Neurosci. 2011, 31, 1919–1933. [Google Scholar] [CrossRef] [PubMed]
- Chambers, S.M.; Fasano, C.A.; Papapetrou, E.P.; Tomishima, M.; Sadelain, M.; Studer, L. Highly efficient neural conversion of human Es and Ips cells by dual inhibition of smad signaling. Nat. Biotechnol. 2009, 27, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Au, E.; Ahmed, T.; Karayannis, T.; Biswas, S.; Gan, L.; Fishell, G. A modular gain-of-function approach to generate cortical interneuron subtypes from es cells. Neuron 2013, 80, 1145–1158. [Google Scholar] [CrossRef] [PubMed]
- Colasante, G.; Lignani, G.; Rubio, A.; Medrihan, L.; Yekhlef, L.; Sessa, A.; Massimino, L.; Giannelli, S.G.; Sacchetti, S.; Caiazzo, M.; et al. Rapid conversion of fibroblasts into functional forebrain gabaergic interneurons by direct genetic reprogramming. Cell Stem Cell 2015, 17, 719–734. [Google Scholar] [CrossRef] [PubMed]
- Petros, T.J.; Maurer, C.W.; Anderson, S.A. Enhanced derivation of mouse ESC-derived cortical interneurons by expression of NKX2.1. Stem Cell Res. 2013, 11, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Tyson, J.A.; Anderson, S.A. The protracted maturation of human esc-derived interneurons. Cell Cycle 2013, 12, 3129–3130. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.A.; Classey, J.D.; Conde, F.; Lund, J.S.; Lewis, D.A. Synchronous development of pyramidal neuron dendritic spines and parvalbumin-immunoreactive chandelier neuron axon terminals in layer iii of monkey prefrontal cortex. Neuroscience 1995, 67, 7–22. [Google Scholar] [CrossRef]
- Fuchs, E.C.; Zivkovic, A.R.; Cunningham, M.O.; Middleton, S.; Lebeau, F.E.; Bannerman, D.M.; Rozov, A.; Whittington, M.A.; Traub, R.D.; Rawlins, J.N.; et al. Recruitment of parvalbumin-positive interneurons determines hippocampal function and associated behavior. Neuron 2007, 53, 591–604. [Google Scholar] [CrossRef] [PubMed]
- Patz, S.; Grabert, J.; Gorba, T.; Wirth, M.J.; Wahle, P. Parvalbumin expression in visual cortical interneurons depends on neuronal activity and trkb ligands during an early period of postnatal development. Cereb. Cortex 2004, 14, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Toth, K.; Magloczky, Z. The vulnerability of calretinin-containing hippocampal interneurons to temporal lobe epilepsy. Front. Neuroanat. 2014, 8, 100. [Google Scholar] [PubMed]
- Lee, S.; Hjerling-Leffler, J.; Zagha, E.; Fishell, G.; Rudy, B. The largest group of superficial neocortical gabaergic interneurons expresses ionotropic serotonin receptors. J. Neurosci. 2010, 30, 16796–16808. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anderson, N.C.; Chen, C.Y.; Grabel, L. Hedgehog Promotes Production of Inhibitory Interneurons in Vivo and in Vitro from Pluripotent Stem Cells. J. Dev. Biol. 2016, 4, 26. https://doi.org/10.3390/jdb4030026
Anderson NC, Chen CY, Grabel L. Hedgehog Promotes Production of Inhibitory Interneurons in Vivo and in Vitro from Pluripotent Stem Cells. Journal of Developmental Biology. 2016; 4(3):26. https://doi.org/10.3390/jdb4030026
Chicago/Turabian StyleAnderson, Nickesha C., Christopher Y. Chen, and Laura Grabel. 2016. "Hedgehog Promotes Production of Inhibitory Interneurons in Vivo and in Vitro from Pluripotent Stem Cells" Journal of Developmental Biology 4, no. 3: 26. https://doi.org/10.3390/jdb4030026




