The Spatiotemporal Dynamics of Forest–Heathland Communities over 60 Years in Fontainebleau, France
Abstract
:1. Introduction
2. Materials and Methods
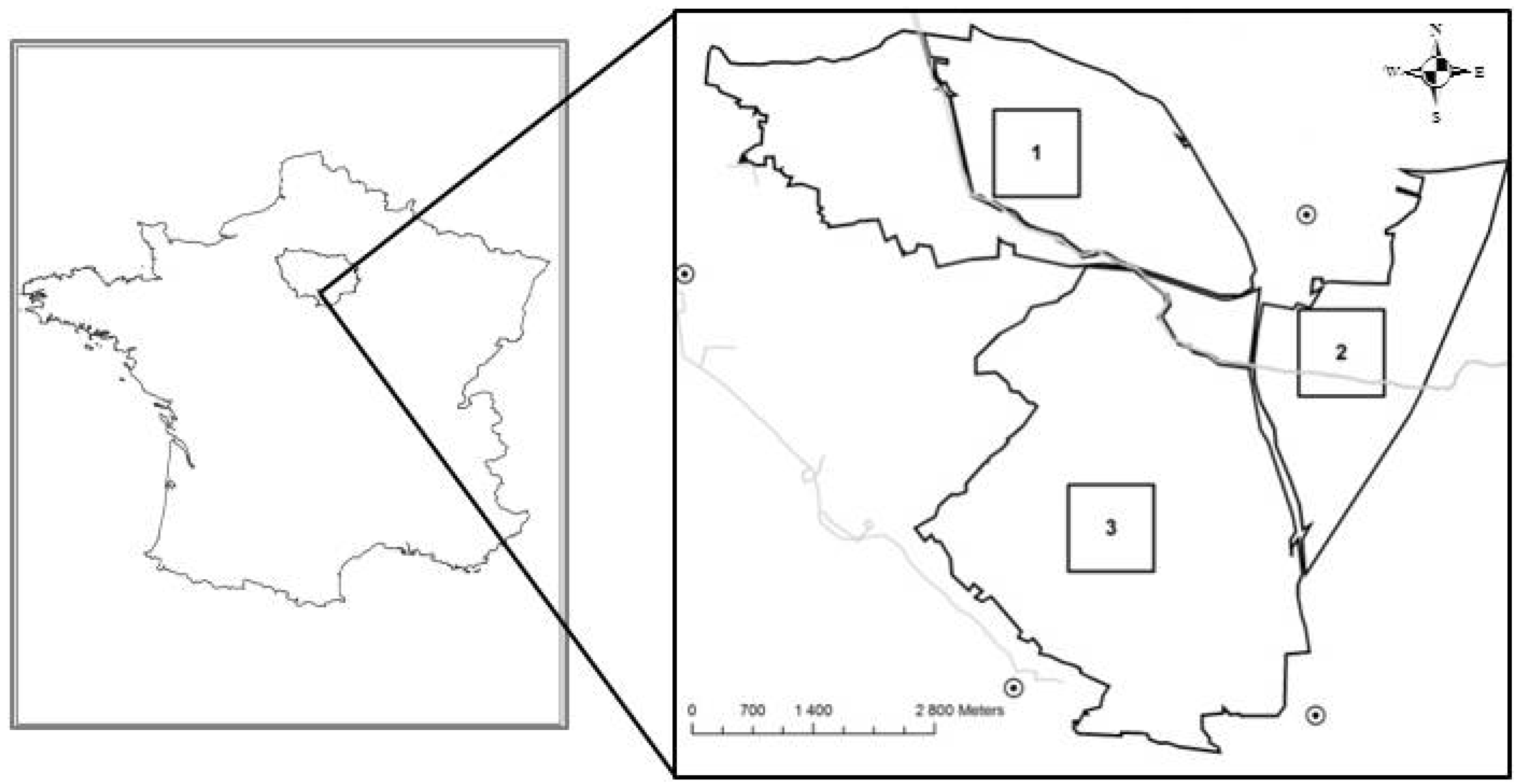
2.1. Study Site
2.2. Image Processing
2.2.1. Classification of Changes in Vegetation Cover
| Classes | Ranking Criteria | Land Cover | Abbreviations |
|---|---|---|---|
| Absence of vegetation | Bare Soil (sand) | S | |
| Low stratum | Absence of trees and the presence of low-lying shrubs | Heathland | Heath |
| Low and homogeneous stratum | Absence of trees | Lawn | L |
| Low and homogeneous stratum, presence of woody plants | Cover of forest trees is lower than 10%; Conifers species represent over 75% of the total tree cover | Conifer Woodland | CWL |
| Low and homogeneous stratum, presence of woody plants | Cover of forest trees is lower than 10%; Deciduous species represent over 75% of the total tree cover | Deciduous Woodland | DWL |
| High stratum is dominant, canopy is open | Cover of forest trees is greater than or equal to 10% and lower than 40%; Conifers species represent over 75% of the total tree cover | Thin Conifer Forest | CTF |
| High stratum is dominant, canopy is open | cover of forest trees is greater than or equal to 10% and lower than 40%; Deciduous species represent over 75% of the total tree cover | Thin Deciduous Forest | DTF |
| High stratum is dominant, canopy is open | Cover of forest trees is greater than or equal to 10% and lower than 40%; any group of trees reaches 75% of the total rate of canopy cover | Thin Mixed Forest (Conifer and Deciduous) | FMTF |
| High stratum is dominant, canopy is closed and homogeneous | Cover of forest trees is greater than or equal to 40%; Conifers species represent over 75% of the total tree cover | Dense Conifer Forest | CFD |
| High stratum is dominant, canopy is closed and homogeneous | Cover of forest trees is greater than or equal to 40%; Deciduous species represent over 75% of the total tree cover | Dense Deciduous Forest | DFD |
| High stratum is dominant, canopy is closed and homogeneous | Cover of forest trees is greater than or equal to 40%; any group of trees reaches 75% of the total rate of canopy cover | Dense Mixed Forest (Conifer and Deciduous) | FMDF |
| Water | Water bodies | W |
2.2.2. Transition Matrices
2.3. Spatial Environmental Variables
2.3.1. Physiographic Variables
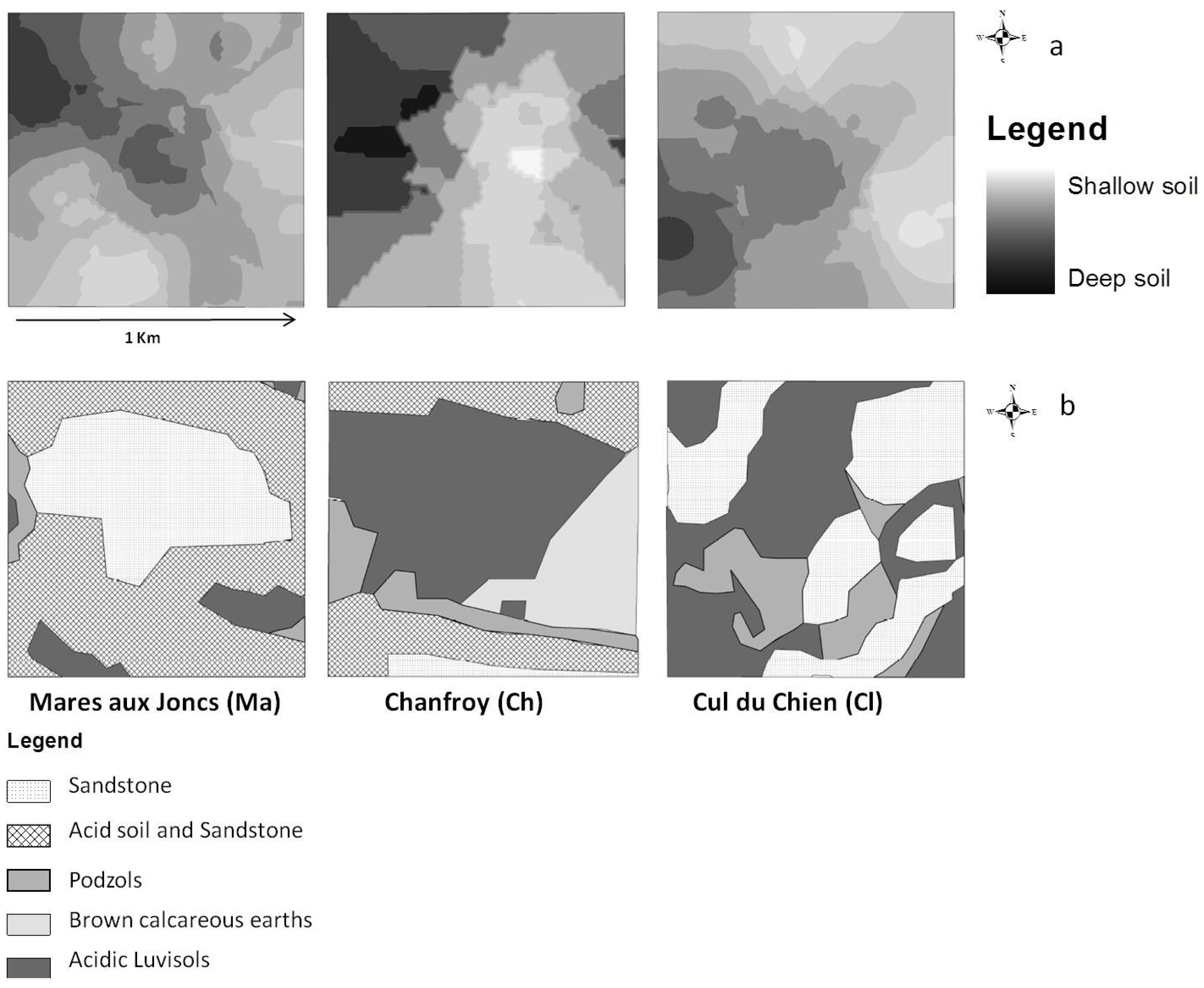
2.3.2. Soil Survey
- Soil depth data, acquired by field sampling. Measurements of soil depth were undertaken at 75 points, 25 points per site. A geostatistical study was conducted in order to obtain a raster map of soil depth based on the observation points [21]. To do this, we interpolated values at unobserved points using a kriging procedure. This method allows for the prediction of unknown values from data observed at known locations. Kriging uses variograms to express spatial variation and minimizes prediction errors by estimating the spatial distribution of predicted values [27,28]. Due to edge effects, the estimated values of soil depth at the plot edges might be subject to higher levels of uncertainty than other points within the plot boundaries. Three classes of soil depth were distinguished, in approximated accordance with observed soil horizons in this region [21]: shallow soils (0–20 cm), medium-depth soils (21–40 cm), and deep soils (greater than 40 cm). We used this interpolated map as a soil depth map as shown in Figure 2a.
2.4. Spatial Data Analysis
2.5. Description of Current Forest Structure
3. Results
3.1. Classification Accuracy Assessment
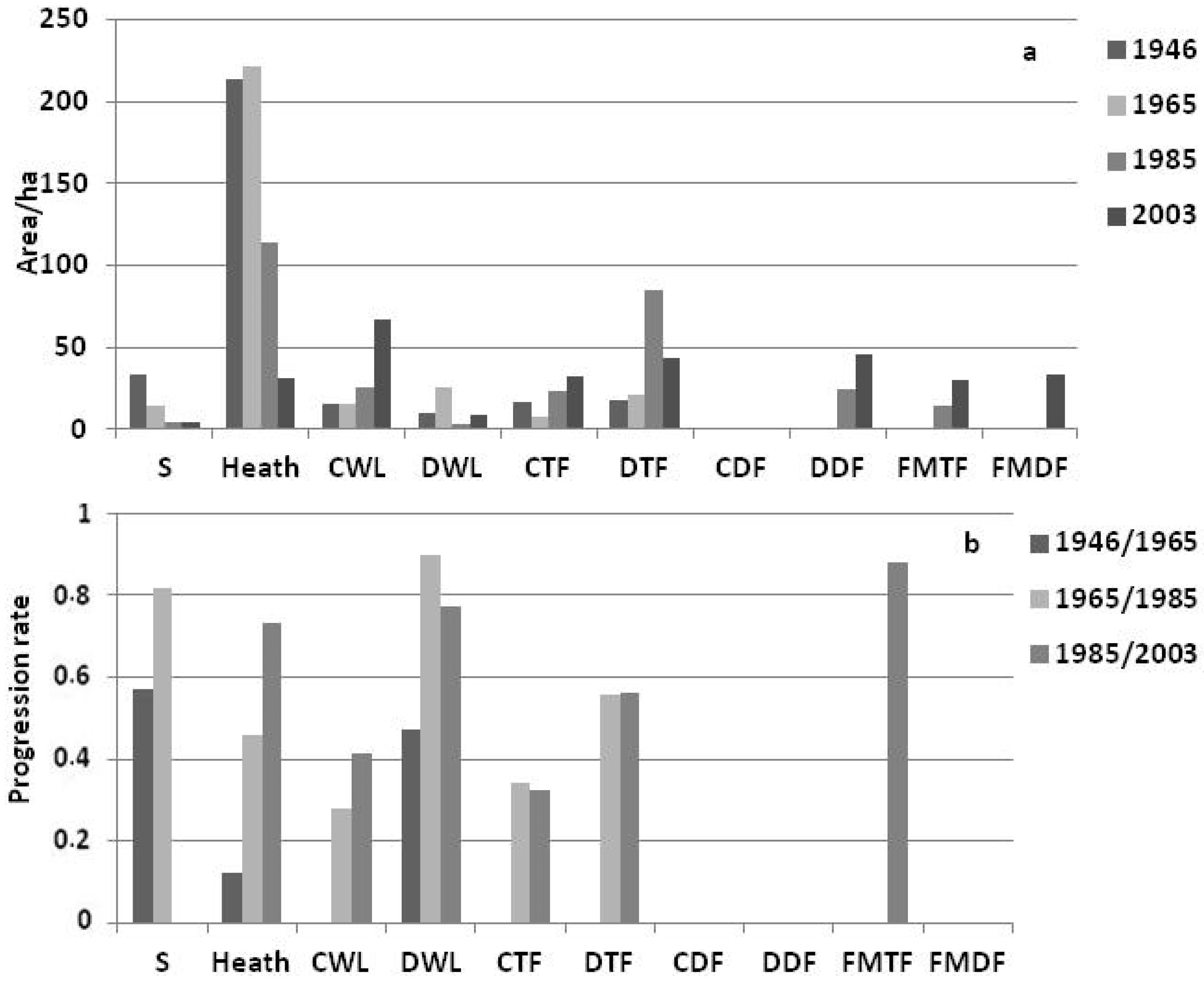
3.2. Landscape Dynamics
| 1965 | (a) | 1946 | ||||||||||
| Heath | CWL | DWL | CTF | DTF | CDF | DDF | FMTF | FMDF | S | L | ||
| Heath | 0.88 | 0.37 | 0.61 | 0.45 | 0.32 | |||||||
| CWL | 0.03 | 0.63 | 0.06 | |||||||||
| DWL | 0.07 | 0.53 | 0.16 | |||||||||
| CTF | 0.01 | 0.39 | 0.01 | |||||||||
| DTF | 0.02 | 0.47 | 0.54 | 0.03 | ||||||||
| CDF | 0.00 | |||||||||||
| DDF | 1.00 | |||||||||||
| FMTF | 0.00 | |||||||||||
| FMDF | 0.00 | |||||||||||
| S | 0.43 | |||||||||||
| L | 0.00 | |||||||||||
| 1985 | (b) | 1965 | ||||||||||
| Heath | CWL | DWL | CTF | DTF | CDF | DDF | FMTF | FMDF | S | L | ||
| Heath | 0.50 | 0.04 | 0.02 | 0.01 | 0.52 | |||||||
| CWL | 0.07 | 0.68 | ||||||||||
| DWL | 0.09 | |||||||||||
| CTF | 0.08 | 0.18 | 0.64 | 0.03 | ||||||||
| DTF | 0.23 | 0.76 | 0.11 | 0.43 | 0.26 | |||||||
| CDF | 0.00 | |||||||||||
| DDF | 0.06 | 0.14 | 0.24 | 0.29 | 1.00 | |||||||
| FMTF | 0.02 | 0.10 | 0.27 | 0.00 | ||||||||
| FMDF | 0.00 | |||||||||||
| S | 0.18 | |||||||||||
| L | 0.04 | 0.00 | ||||||||||
| 2003 | (c) | 1985 | ||||||||||
| Heath | CWL | DWL | CTF | DTF | CDF | DDF | FMTF | FMDF | S | L | ||
| Heath | 0.27 | |||||||||||
| CWL | 0.43 | 0.59 | 0.25 | |||||||||
| DWL | 0.07 | 0.23 | ||||||||||
| CTF | 0.10 | 0.19 | 0.60 | |||||||||
| DTF | 0.07 | 0.44 | ||||||||||
| CDF | 0.03 | |||||||||||
| DDF | 0.15 | 0.06 | 0.27 | 1.00 | ||||||||
| FMTF | 0.13 | 0.23 | 0.08 | 0.12 | 0.12 | |||||||
| FMDF | 0.37 | 0.15 | 0.07 | 0.88 | ||||||||
| S | 1.00 | |||||||||||
| L | 1.00 | |||||||||||

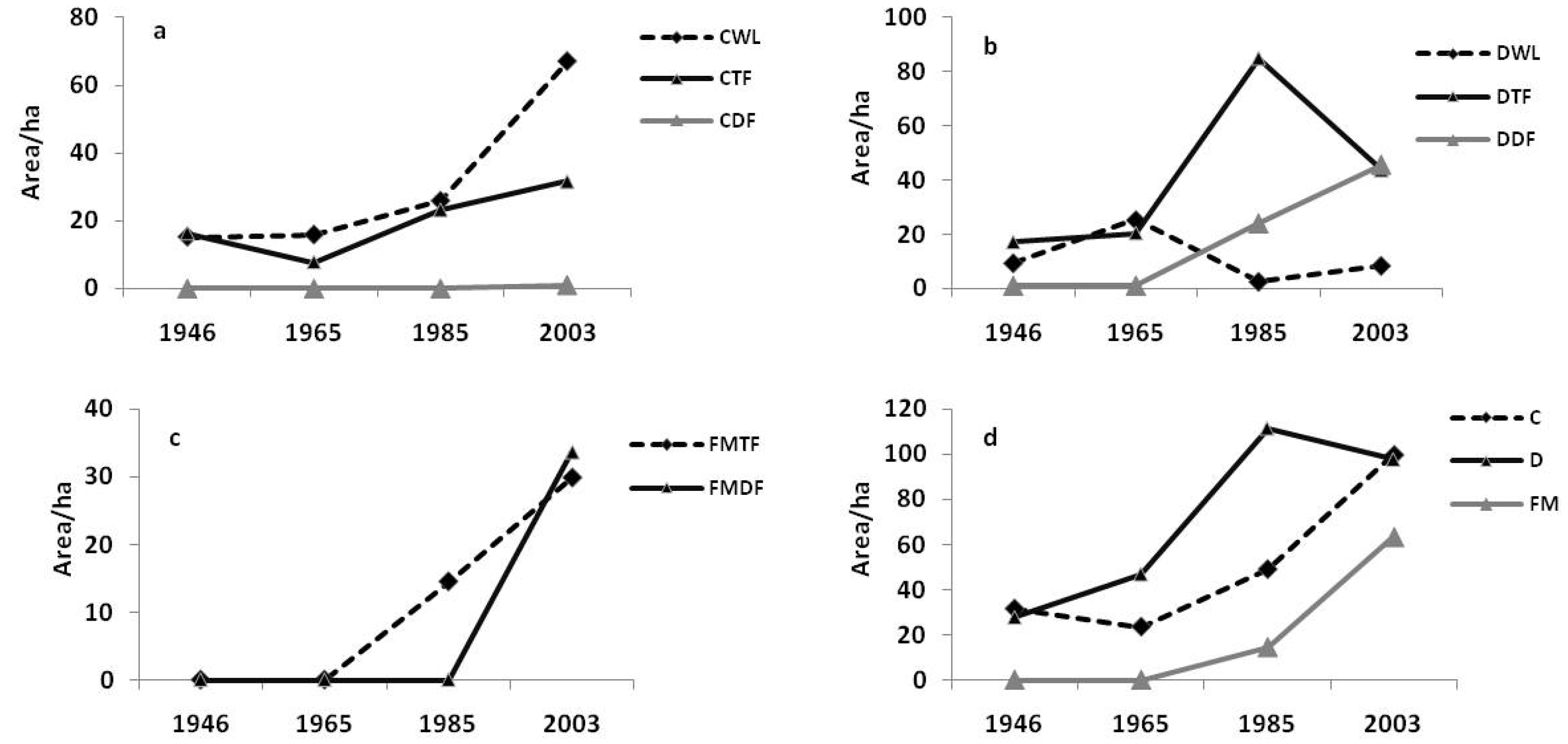
3.3. Influence of Spatial Environmental Variables on Forest Dynamics
3.4. Forest Structure and Species Composition
4. Discussion
5. Conclusion and Implications for Land Management
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Foley, J.A.; Defries, R.; Asner, G.P.; Barford, C.; Bonan, G.; Carpenter, S.R.; Chapin, F.S.; Coe, M.T.; Daily, G.C.; Gibbs, H.K.; et al. Global consequences of land use. Science 2005, 309, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Rockstrom, J.; Steffen, W.; Noone, K.; Persson, Å.; Chapin, F.S., III; Lambin, E.F.; Lenton, T.M.; Scheffer, M.; Folke, C.; Schellnhuber, H.J.; et al. A safe operating space for humanity. Nature 2009, 24, 472–475. [Google Scholar]
- EC Habitats Directive. Council Directive 92/43/EEC of 21 May 1992 on the Conservation of Natural Habitats and of Wild Fauna and Flora; European Commission: Brussels, Belgium, 1992. [Google Scholar]
- Ausden, M. Habitat Management for Conservation; A Handbook of Techniques; Oxford University Press: Oxford, UK, 2007; p. 384. [Google Scholar]
- Thompson, D.B.A.; MacDonald, A.J.; Marsden, J.H.; Galbraith, C.A. Upland heather moorland in Great Britain: A review of international importance, vegetation change and some objectives for nature conservation. Biol. Conserv. 1995, 71, 163–178. [Google Scholar] [CrossRef]
- Usher, M.B. Management and diversity of arthropods in Calluna heathland. Biodivers. Conserv. 1992, 1, 63–79. [Google Scholar] [CrossRef]
- Usher, M.B.; Thompson, D.B.A. Variation in the upland heathland of Great Britain: Conservation importance. Biol. Conserv. 1993, 66, 69–81. [Google Scholar] [CrossRef]
- Webb, N.R. The traditional management of European heathlands. J. Appl. Ecol. 1998, 35, 987–990. [Google Scholar] [CrossRef]
- Webb, N.R. Atlantic heathland. In Davy Handbook of Ecological Restoration; Martin, R.P., Anthony, J., Eds.; Cambridge University Press: Cambridge, UK, 2008; Volumes 1 and 2, pp. 401–418. [Google Scholar]
- Price, E.A.C. Lowland Grassland and Heathland Habitats; Routledge: London, UK, 2003. [Google Scholar]
- Mobaied, S.; Riera, B.; Lalanne, A.; Baguette, M.; Machon, N. The use of diachronic spatial approaches and predictive modelling to study the vegetation dynamics of a managed heathland. Biodivers. Conserv. 2011, 20, 73–88. [Google Scholar] [CrossRef]
- Barker, C.G.; Power, S.A.; Bell, J.N.B.; Orme, C.D.L. Effects of habitat management on heathland response to atmospheric nitrogen deposition. Biol. Conserv. 2004, 120, 41–52. [Google Scholar] [CrossRef]
- Wamelink, G.W.W.; de Jong, J.J.; van Dobben, H.F.; van Wijk, M.N. Additional costs of nature management caused by deposition. Ecol. Econ. 2005, 52, 437–451. [Google Scholar] [CrossRef]
- Calvo, L.; Alonso, I.; Marcos, E.; de Luis, E. Effects of cutting and nitrogen deposition on biodiversity in Cantabrian heathlands. Appl. Veg. Sci. 2007, 10, 43–52. [Google Scholar] [CrossRef]
- Fagundez, J. Heathlands confronting global change: Drivers of biodiversity loss from past to future scenarios. Ann. Bot. 2013, 111, 151–172. [Google Scholar] [CrossRef] [PubMed]
- Miles, J. Vegetation Dynamics; Chapman and Hall: London, UK, 1979. [Google Scholar]
- Evans, F.C.; Dhal, E. The vegetational structure of an abandoned field in southeastern Michigan and its relations to environmental factors. Ecology 1955, 36, 685–706. [Google Scholar] [CrossRef]
- Dargie, T.C.D. An ordination analysis of vegetation patterns on topoclimate gradients in South-East Spain. J. Biogeogr. 1987, 14, 197–211. [Google Scholar] [CrossRef]
- Badano, E.I.; Cavieres, L.A.; Molina-Montenegro, M.A.; Quiroz, C.L. Slope aspect influences plant association patterns in the Mediterranean matorral of central Chile. J. Arid Environ. 2005, 62, 93–108. [Google Scholar] [CrossRef]
- Tilman, D.; Kareiva, P. Spatial Ecology: The Role of Space in Population Dynamics and Interspecific Interactions; Princeton University: Princeton, NJ, USA, 1997; p. 368. [Google Scholar]
- Mobaied, S.; Ponge, J.F.; Salmon, S.; Lalanne, A.; Riera, B. Influence of the spatial variability of soil type and tree colonization on the dynamics of Molinia caerulea (L.) Moench in managed heathland. Ecol. Complex. 2012, 11, 118–125. [Google Scholar] [CrossRef]
- James, S.A.; Irene, M.; Johannes, R. Small-Format Aerial Photography: Principles, Techniques and Geoscience Applications; Elsevier Science: Trier, Germany, 2010; p. 268. [Google Scholar]
- Derrière, N.; Lucas, S. La Forêt Française en 2005 Résultats de la Première Campagne Nationale Annuelle; IFN: Saint-Mandé, France, 2006; p. 8. [Google Scholar]
- Ross, S.L.; John, G.L. Remote Sensing and GIS Accuracy Assessment; U.S. Environmental Protection Agency (EPA): Washington, DC, USA, 2011; p. 320.
- IDRISI, version Andes 32; Clark University: Worcester, OH, USA, 1987–2006.
- ESRI ArcGIS, version 9.2; Environmental Systems Research Institute Inc.: Redlands, CA, USA, 2006.
- Isaaks, E.H.; Srivastava, R.M. An Introduction to Applied Geostatistics; Oxford University Press: New York, NY, USA, 1989; p. 561. [Google Scholar]
- Krige, D. A statical problem to some basic minim valuation problems on the Witwatersrand. J. Chem. Metall. Min. Soc. S. Afr. 1951, 52, 119–139. [Google Scholar]
- Institut Géographique National (France). Soil Map of Forêt de Trois Pignons ONF/ED EDR25©IGN2006 [map]; Technical report for Forêt Domaniale des Trois Pignons; Office National des Forêts: Paris, France, 2006.
- Pontius, R.G. Quantification error versus location error in comparison of categorical maps. Photogramm. Eng. Remote Sens. 2000, 66, 1011–1016. [Google Scholar]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Visser, H.; de Nijs, T. The map comparison kit MCK software. Environ. Modell. Softw. 2006, 21, 346–358. [Google Scholar] [CrossRef]
- Map Comparison Kit 3. Available online: www.riks.nl (accessed on 21 February 2014).
- Braun-Blanquet, J. Plant Sociology; McGraw-Hill Book Company: New York, NY, USA, 1932; p. 438. [Google Scholar]
- Milan, C.; Zdenka, O. Plot sizes used for phytosociological sampling of European vegetation. J. Veg. Sci. 2003, 14, 563–570. [Google Scholar]
- Gimingham, C.H. Ecology of Heathland; Chapman and Hall: London, UK, 1972; p. 266. [Google Scholar]
- Walker, L.R.; Walker, J.; Hobbs, R.J. Linking Restoration and Ecological Succession; Springer: New York, NY, USA, 2007. [Google Scholar]
- Gimingham, C.H. A reappraisal of the cyclical processes in Calluna heathland. Vegetation 1988, 77, 61–64. [Google Scholar] [CrossRef]
- Grime, J.P.; Hodgson, J.G.; Hunt, R. Comparative Plant Ecology: A Functional Approach to Common British Species; Unwin Hyman: London, UK, 1988; p. 742. [Google Scholar]
- Rameau, J.C.; Mansion, D.; Dumé, G. Flore Forestière Française; Institut pour le Développement Forestier: Dijon, France, 1993; p. 2421. [Google Scholar]
- Debussche, M.; Lepart, J. Establishment of woody plants in Mediterranean old fields: Opportunity in space and time. Landsc. Ecol. 1992, 6, 133–145. [Google Scholar] [CrossRef]
- Debain, S.; Curt, T.; Lepart, J.; Prevosto, B. Reproductive variability in Pinus sylvestris in southern France: Implications for invasion. J. Veg. Sci. 2003, 4, 509–516. [Google Scholar] [CrossRef]
- Richardson, D.M. Ecology and Biogeography of Pinus; Cambridge University Press: Cambridge, UK, 1988; p. 546. [Google Scholar]
- Scherer-Lorenzen, M.; Körner, C.; Schulze, E.D. Temperate and boreal systems. In Forest Diversity and Function; Springer: Berlin, Germany, 2005; pp. 377–390. [Google Scholar]
- Cañellas, I.; Martínez García, F.; Montero, G. Silviculture and dynamics of Pinus sylvestris L. stands in Spain. Investig. Agrar. Sist. Recur. For. 2000, 1, 233–253. [Google Scholar]
- Bessemoulin, P.; Mestre, O. Le réchauffement climatique sur le siècle en France. Fr. Chang. Glob. 2001, 12, 32–34. [Google Scholar]
- Canellas, C.; Gibelin, A.L.; Lassègues, P.; Kerdoncuff, M.; Dandin, P.; Simon, P. Les normales climatiques spatialisées Aurelhy 1981–2010: Températures et précipitations. La Météorol. 2014, 85. [Google Scholar] [CrossRef]
- Moisselin, J.M. Les précipitations en France au XXème siècle. Fr. Chang. Glob. 2002, 13, 57–62. [Google Scholar]
- Dulamsuren, C.; Hauck, M.; Bader, M.; Oyungerel, S.; Osokhjargal, D.; Nyambayar, S.; Leuschner, C. The different strategies of Pinus sylvestris and Larix sibirica to deal with summer drought in a northern Mongolian forest-steppe ecotone suggest a future superiority of pine in a warming climate. Can. J. For. Res. 2009, 39, 2520–2528. [Google Scholar] [CrossRef]
- Menzel, A.; Fabian, P. Growing season extended in Europe. Nature 1999, 397, 659–661. [Google Scholar] [CrossRef]
- Rocchini, D.; Foody, D.G.; Nagendra, H.; Ricotta, C.; Anand, M.; He, K.S.; Amici, V.; Kleinschmit, B.; Forster, M.; Schmidtlein, S.; et al. Uncertainty in ecosystem mapping by remote sensing. Comput. Geosci. 2013, 50, 128–135. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mobaied, S.; Machon, N.; Lalanne, A.; Riera, B. The Spatiotemporal Dynamics of Forest–Heathland Communities over 60 Years in Fontainebleau, France. ISPRS Int. J. Geo-Inf. 2015, 4, 957-973. https://doi.org/10.3390/ijgi4020957
Mobaied S, Machon N, Lalanne A, Riera B. The Spatiotemporal Dynamics of Forest–Heathland Communities over 60 Years in Fontainebleau, France. ISPRS International Journal of Geo-Information. 2015; 4(2):957-973. https://doi.org/10.3390/ijgi4020957
Chicago/Turabian StyleMobaied, Samira, Nathalie Machon, Arnault Lalanne, and Bernard Riera. 2015. "The Spatiotemporal Dynamics of Forest–Heathland Communities over 60 Years in Fontainebleau, France" ISPRS International Journal of Geo-Information 4, no. 2: 957-973. https://doi.org/10.3390/ijgi4020957





