Cytotoxicity of Different Concentrations of Three Root Canal Sealers on Human Mesenchymal Stem Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sealers
2.2. Preparation of the Extracts
2.3. Cell Culture
2.4. Test Groups
- AH Plus sealer group (AH Plus): Cells grown in medium conditioned by AH Plus sealer.
- Endosequence BC sealer group (BC): Cells grown in medium conditioned by Endosequence BC sealer.
- BioRoot RCS group (BR): Cells grown in medium conditioned by BioRoot sealer.
- Control group (C): Cells cultured in growth medium.
2.5. Cytotoxicity Assay
2.6. Cellular Morphology
2.7. Statistical Analysis
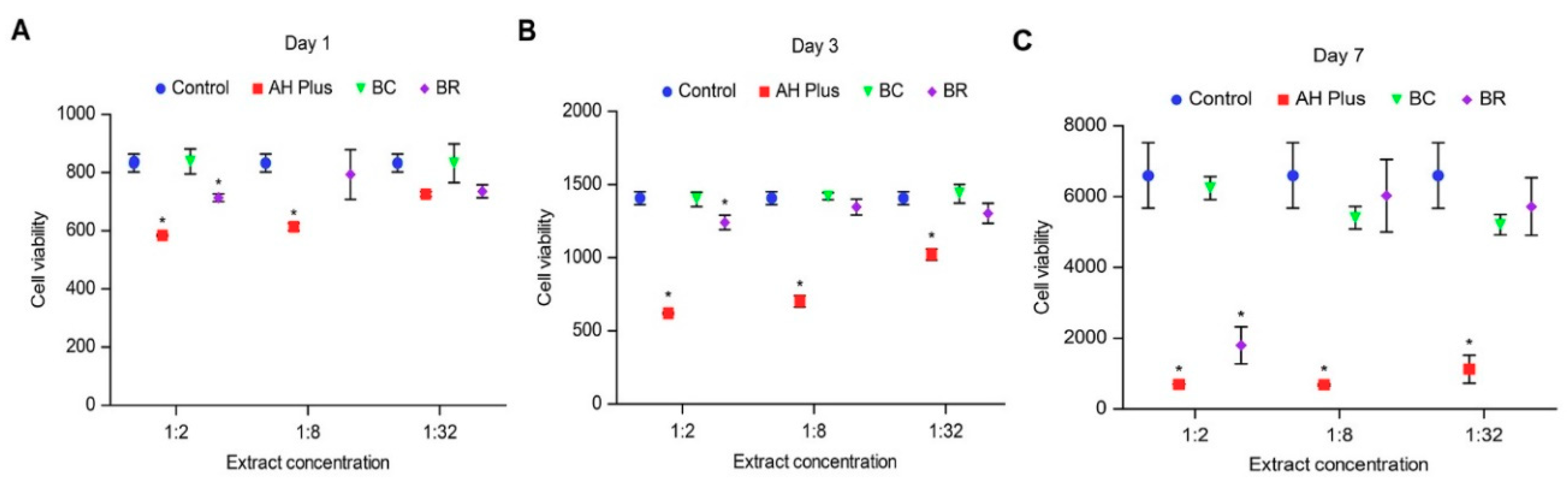
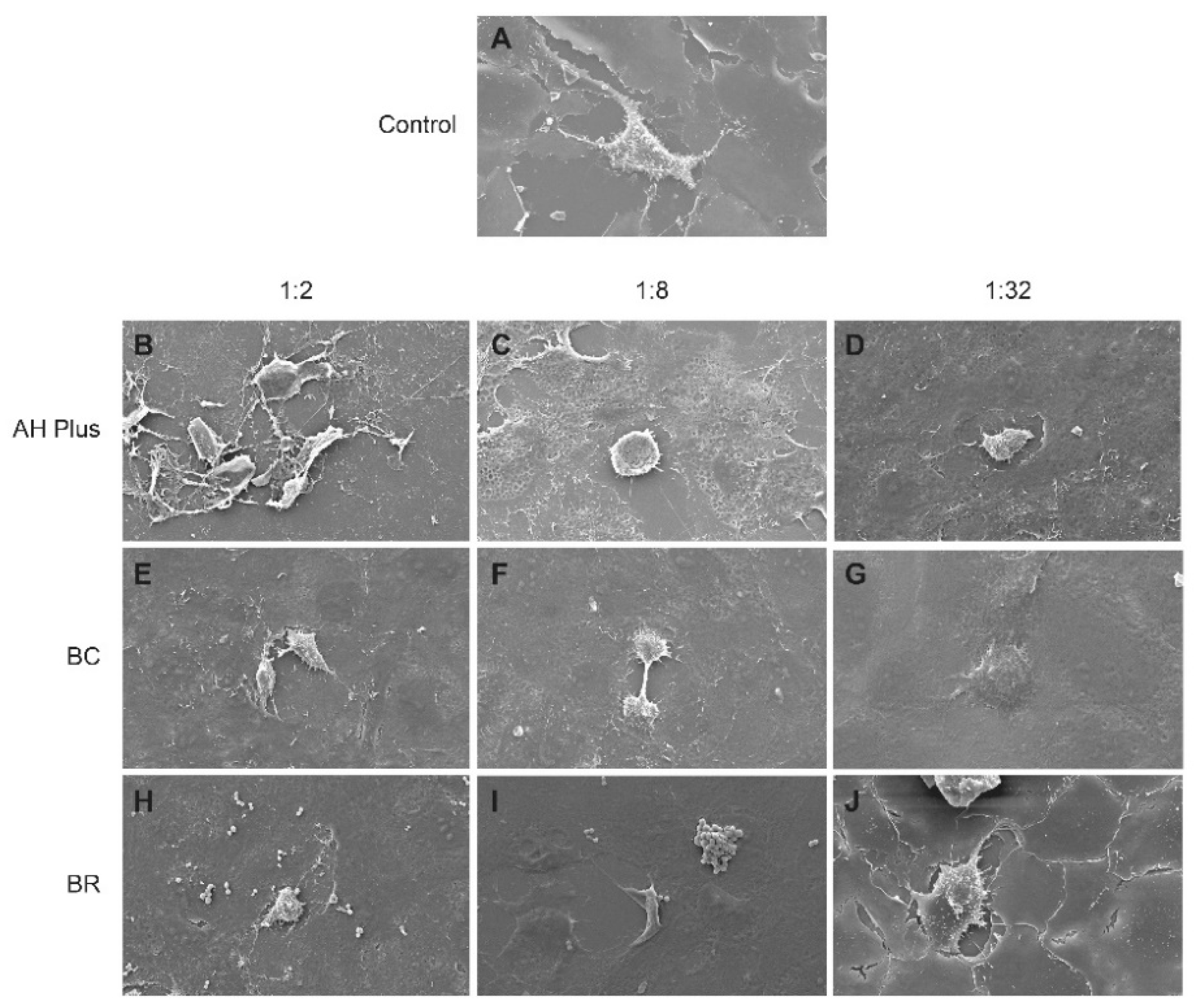
3. Results
3.1. Alamar Blue
3.2. Scanning Electron Microscope
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ørstavik, D. Materials used for root canal obturation: Technical, biological and clinical testing. Endod. Top. 2005, 12, 25–38. [Google Scholar] [CrossRef]
- Keiser, K.; Johnson, C.; Tipton, D. Cytotoxicity of mineral trioxide aggregate using human periodontal ligament fibroblasts. J. Endod. 2000, 26, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, N.K.; Caicedo, R.; Ritwik, P.; Moiseyeva, R.; Kawashima, I. Physicochemical basis of the biologic properties of mineral trioxide aggregate. J. Endod. 2005, 31, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Grazziotin-Soares, R.; Nekoofar, M.H.; Davies, T.; Hubler, R.; Meraji, N.; Dummer, P.M.H. Crystalline phases involved in the hydration of calcium silicate-based cements: Semi-quantitative Rietveld X-ray diffraction analysis. Aust. Endod. J. 2017. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.M.; Du, T.F.; Shen, Y.; Wang, Z.J.; Zheng, Y.F.; Haapasalo, M. In vitro cytotoxicity of calcium silicate-containing endodontic sealers. J. Endod. 2015, 41, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Candeiro, G.T.; Moura-Netto, C.; D’Almeida-Couto, R.S.; Azambuja-Junior, N.; Marques, M.M.; Cai, S.; Gavini, G. Cytotoxicity, genotoxicity and antibacterial effectiveness of a bioceramic endodontic sealer. Int. Endod. J. 2015, 49, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Collado-Gonzalez, M.; Garcia-Bernal, D.; Onate-Sanchez, R.E.; Ortolani-Seltenerich, P.S.; Lozano, A.; Forner, L.; Llena, C.; Rodriguez-Lozano, F.J. Biocompatibility of three new calcium silicate-based endodontic sealers on human periodontal ligament stem cells. Int. Endod. J. 2017, 50, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Camps, J.; Jeanneau, C.; El Ayachi, I.; Laurent, P.; About, I. Bioactivity of a calcium silicate-based endodontic cement (BioRoot RCS): Interactions with human periodontal ligament cells in vitro. J. Endod. 2015, 41, 1469–1473. [Google Scholar] [CrossRef] [PubMed]
- Ricucci, D.; Langeland, K. Apical limit of root canal instrumentation and obturation, part 2. A histological study. Int. Endod. J. 1998, 31, 394–409. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.H.; Ding, S.J.; Hsu, T.Z.; Lee, Z.D.; Kao, C.T. Root canal sealers induce cytotoxicity and necrosis. J. Mater. Sci. Mater. Med. 2004, 15, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Zoufan, K.; Jiang, J.; Komabayashi, T.; Wang, Y.H.; Safavi, K.E.; Zhu, Q. Cytotoxicity evaluation of Gutta flow and endo sequence BC sealers. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 112, 657–661. [Google Scholar] [CrossRef] [PubMed]
- ISO. Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity; International Organization for Standardization: Geneva, Switzerland, 2009; pp. 10993–10995. [Google Scholar]
- Rodrigues, C.; Costa-Rodrigues, J.; Capelas, J.A.; Fernandes, M.H. Long-term dose- and time-dependent effects of endodontic sealers in human in vitro osteoclastogenesis. J. Endod. 2013, 39, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Elsafadi, M.; Manikandan, M.; Atteya, M.; Hashmi, J.A.; Iqbal, Z.; Aldahmash, A.; Alfayez, M.; Kassem, M.; Mahmood, A. Characterization of cellular and molecular heterogeneity of bone marrow stromal cells. Stem Cells Int. 2016, 2016, 9378081. [Google Scholar] [CrossRef] [PubMed]
- Miletic, I.; Devcic, N.; Anic, I.; Borcic, J.; Karlovic, Z.; Osmak, M. The cytotoxicity of RoekoSeal and AH plus compared during different setting periods. J. Endod. 2005, 31, 307–309. [Google Scholar] [CrossRef] [PubMed]
- Barros, J.; Costa-Rodrigues, J.; Lopes, M.A.; Pina-Vaz, I.; Fernandes, M.H. Response of human osteoblastic and osteoclastic cells to AH plus and pulp canal sealer containing quaternary ammonium polyethylenimine nanoparticles. J. Endod. 2014, 40, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Corral Nunez, C.M.; Bosomworth, H.J.; Field, C.; Whitworth, J.M.; Valentine, R.A. Biodentine and mineral trioxide aggregate induce similar cellular responses in a fibroblast cell line. J. Endod. 2014, 40, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Alkahtani, A.; Alkahtany, S.M.; Mahmood, A.; Elsafadi, M.A.; Aldahmash, A.M.; Anil, S. Cytotoxicity of QMix™ endodontic irrigating solution on human bone marrow mesenchymal stem cells. BMC Oral Health 2014, 14, 27. [Google Scholar] [CrossRef] [PubMed]
- Rampersad, S.N. Multiple applications of alamar blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors 2012, 12, 12347–12360. [Google Scholar] [CrossRef] [PubMed]
- Cohen, B.I.; Pagnillo, M.K.; Musikant, B.L.; Deutsch, A.S. Formaldehyde evaluation from endodontic materials. Oral Health 1998, 88, 37–39. [Google Scholar] [PubMed]
- Zhang, W.; Li, Z.; Peng, B. Effects of iRoot SP on mineralization-related genes expression in MG63 cells. J. Endod. 2010, 36, 1978–1982. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.W.; Lee, S.Y.; Kang, S.K.; Kum, K.Y.; Kim, E.C. In vitro biocompatibility, inflammatory response, and osteogenic potential of 4 root canal sealers: Sealapex, sankin apatite root sealer, MTA fillapex, and iRoot SP root canal sealer. J. Endod. 2014, 40, 1642–1648. [Google Scholar] [CrossRef] [PubMed]
- Loushine, B.A.; Bryan, T.E.; Looney, S.W.; Gillen, B.M.; Loushine, R.J.; Weller, R.N.; Pashley, D.H.; Tay, F.R. Setting properties and cytotoxicity evaluation of a premixed bioceramic root canal sealer. J. Endod. 2011, 37, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Cornelio, A.L.; Rodrigues, E.M.; Salles, L.P.; Mestieri, L.B.; Faria, G.; Guerreiro-Tanomaru, J.M.; Tanomaru-Filho, M. Bioactivity of MTA plus, biodentine and an experimental calcium silicate-based cement on human osteoblast-like cells. Int. Endod. J. 2017, 50, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, E.M.; Gomes-Cornelio, A.L.; Soares-Costa, A.; Salles, L.P.; Velayutham, M.; Rossa-Junior, C.; Guerreiro-Tanomaru, J.M.; Tanomaru-Filho, M. An assessment of the overexpression of BMP-2 in transfected human osteoblast cells stimulated by mineral trioxide aggregate and biodentine. Int. Endod. J. 2017, 50 (Suppl. S2), e9–e18. [Google Scholar] [CrossRef] [PubMed]
- Zanini, M.; Sautier, J.M.; Berdal, A.; Simon, S. Biodentine induces immortalized murine pulp cell differentiation into odontoblast-like cells and stimulates biomineralization. J. Endod. 2012, 38, 1220–1226. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wang, J.; Zhai, W.; Wang, Z.; Chang, J. The self-setting properties and in vitro bioactivity of tricalcium silicate. Biomaterials 2005, 26, 6113–6121. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsubait, S.A.; Al Ajlan, R.; Mitwalli, H.; Aburaisi, N.; Mahmood, A.; Muthurangan, M.; Almadhri, R.; Alfayez, M.; Anil, S. Cytotoxicity of Different Concentrations of Three Root Canal Sealers on Human Mesenchymal Stem Cells. Biomolecules 2018, 8, 68. https://doi.org/10.3390/biom8030068
Alsubait SA, Al Ajlan R, Mitwalli H, Aburaisi N, Mahmood A, Muthurangan M, Almadhri R, Alfayez M, Anil S. Cytotoxicity of Different Concentrations of Three Root Canal Sealers on Human Mesenchymal Stem Cells. Biomolecules. 2018; 8(3):68. https://doi.org/10.3390/biom8030068
Chicago/Turabian StyleAlsubait, Sara A., Reem Al Ajlan, Hala Mitwalli, Nour Aburaisi, Amer Mahmood, Manikandan Muthurangan, Randa Almadhri, Musaad Alfayez, and Sukumaran Anil. 2018. "Cytotoxicity of Different Concentrations of Three Root Canal Sealers on Human Mesenchymal Stem Cells" Biomolecules 8, no. 3: 68. https://doi.org/10.3390/biom8030068





