The Role of Specific Chemokines in the Amelioration of Colitis by Appendicitis and Appendectomy
Abstract
:1. Introduction
- Global suppression of autophagy gene expression and gene-set expression [9].
- Late suppression of endothelin-related genes and gene-sets, specifically endothelins (ET-1 and ET-2), and endothelin converting enzyme B [10].
- Upregulation or downregulation of genes and gene-sets specific to interferon activity [11].
2. Results
2.1. Individual Distal Colonic Gene Expression of 40 Chemokine Genes Three Days Post-AA and 28 Days Post-AA
2.2. Individual Distal Colonic Gene Expression of 24 Described Chemokine Receptor Genes Three Days Post-AA and 28 Days Post-AA
2.3. Differentially Regulated Distal Colonic Gene Sets Associated with Differentially Regulated Individual Chemokine and Chemokine Receptor Genes 28 Days Post-AA
2.4. Differentially Regulated 28 Days Post-AA Gene-Sets Associated with Chemokines CCL7 and CCL8 Which Showed High-Fold-Change Gene Expression in the Distal Colon Three Days Post-AA
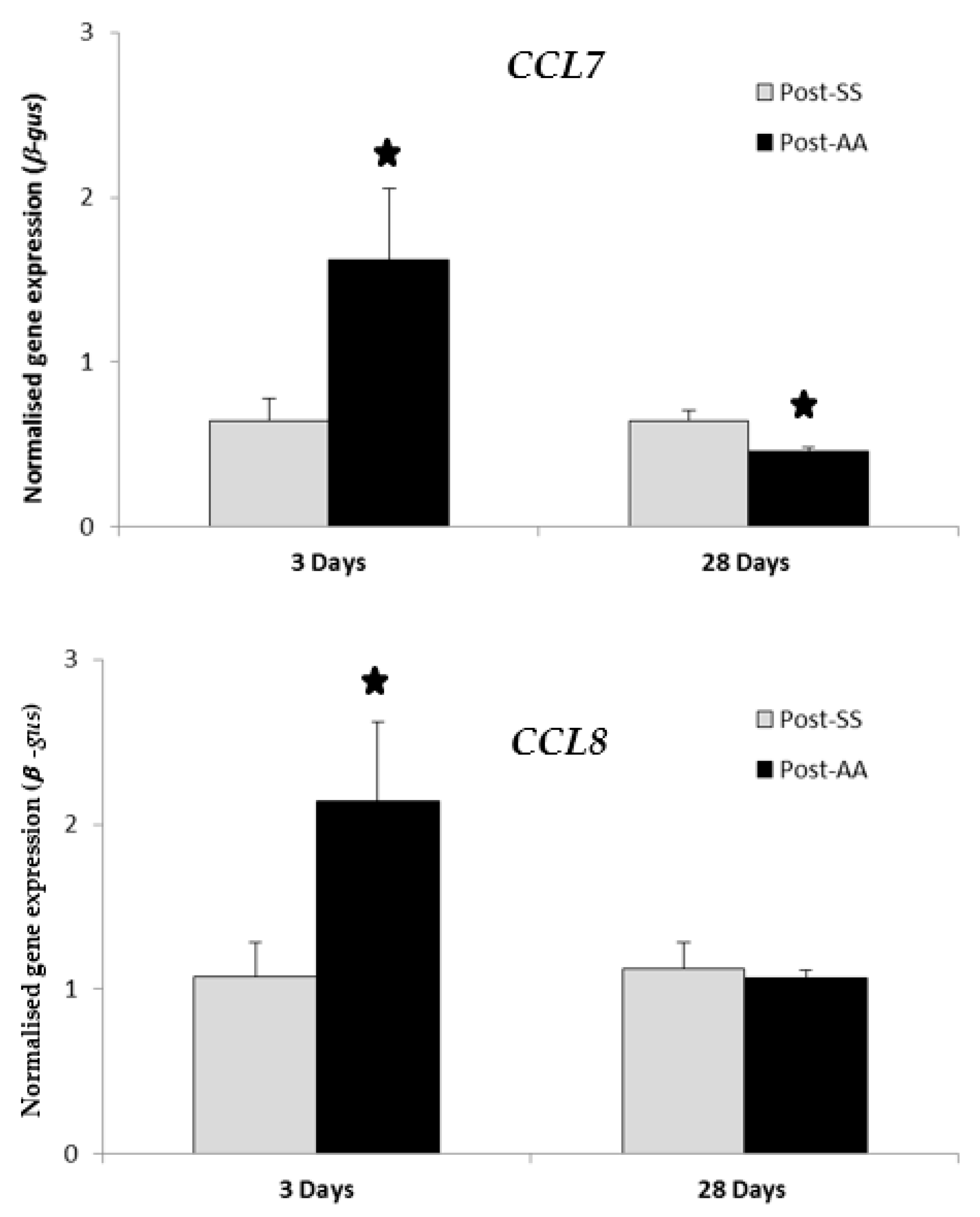
2.5. RT-PCR Expression Study of CCL7 and CCL8 Chemokines Three Days Post-AA and 28 Days Post-AA
3. Discussion
4. Materials and Methods
4.1. Animal Experiments
4.2. Processing of Colonic Specimens for RNA Extraction
4.3. Experimental Design of Microarray Study and the Affymetrix Array Process
4.4. Gene Set Enrichment Analysis and Enrichment of Chemokine and Chemokine Receptor Associated Gene Sets
4.5. Quantitative RT-PCR Expression of CCL7 and CCL8
4.6. Other Statistics Used
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Addiss, D.G.; Shaffer, N.; Fowler, B.S.; Tauxe, R.V. The epidemiology of appendicitis and appendectomy in the united states. Am. J. Epidemiol. 1990, 132, 910–925. [Google Scholar] [CrossRef] [PubMed]
- Koutroubakis, I.E.; Vlachonikolis, I.G.; Kouroumalis, E.A. Role of appendicitis and appendectomy in the pathogenesis of ulcerative colitis: A critical review. Inflamm. Bowel Dis. 2002, 8, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Lopez Ramos, D.; Gabriel, R.; Cantero Perona, J.; Moreno Otero, R.; Fernandez Bermejo, M.; Mate Jimenez, J. Association of MALTectomy (appendectomy and tonsillectomy) and inflammatory bowel disease: A familial case-control study. Rev. Esp. Enferm. Dig. 2001, 93, 303–314. [Google Scholar] [PubMed]
- Andersson, R.E.; Olaison, G.; Tysk, C.; Ekbom, A. Appendectomy and protection against ulcerative colitis. N. Engl. J. Med. 2001, 344, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Radford-Smith, G.L.; Edwards, J.E.; Purdie, D.M.; Pandeya, N.; Watson, M.; Martin, N.G.; Green, A.; Newman, B.; Florin, T.H. Protective role of appendicectomy on onset and severity of ulcerative colitis and Crohn’s disease. Gut 2002, 51, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Cheluvappa, R. A novel model of appendicitis and appendectomy to investigate inflammatory bowel disease pathogenesis and remediation. Biol. Proced. Online 2014, 16, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheluvappa, R.; Luo, A.S.; Grimm, M.C. T helper type 17 pathway suppression by appendicitis and appendectomy protects against colitis. Clin. Exp. Immunol. 2014, 175, 316–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheluvappa, R. Experimental appendicitis and appendectomy modulate the CCL20–CCR6 axis to limit inflammatory colitis pathology. Int. J. Colorectal Dis. 2014, 29, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Cheluvappa, R.; Luo, A.S.; Grimm, M.C. Autophagy suppression by appendicitis and appendectomy protects against colitis. Inflamm. Bowel Dis. 2014, 20, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Cheluvappa, R.; Eri, R.; Luo, A.S.; Grimm, M.C. Endothelin and vascular remodelling in colitis pathogenesis—Appendicitis and appendectomy limit colitis by suppressing endothelin pathways. Int. J. Colorectal Dis. 2014, 29, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- Cheluvappa, R.; Eri, R.; Luo, A.S.; Grimm, M.C. Modulation of interferon activity-associated soluble molecules by appendicitis and appendectomy limits colitis-identification of novel anti-colitic targets. J. Interferon Cytokine Res. 2015, 35, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Zlotnik, A.; Yoshie, O. Chemokines: A new classification system and their role in immunity. Immunity 2000, 12, 121–127. [Google Scholar] [CrossRef]
- Zlotnik, A.; Yoshie, O. The chemokine superfamily revisited. Immunity 2012, 36, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Baggiolini, M. Chemokines in pathology and medicine. J. Int. Med. 2001, 250, 91–104. [Google Scholar] [CrossRef] [Green Version]
- Dharmani, P.; Chadee, K. Biologic therapies against inflammatory bowel disease: A dysregulated immune system and the cross talk with gastrointestinal mucosa hold the key. Curr. Mol. Pharmacol. 2008, 1, 195–212. [Google Scholar] [CrossRef] [PubMed]
- Fiorino, G.; Rovida, S.; Correale, C.; Malesci, A.; Danese, S. Emerging biologics in the treatment of inflammatory bowel disease: What is around the corner? Curr. Drug Targets 2010, 11, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Stone, K.D.; Prussin, C.; Metcalfe, D.D. IgE, mast cells, basophils, and eosinophils. J. Allergy Clin. Immunol. 2010, 125, S73–S80. [Google Scholar] [CrossRef] [PubMed]
- Yodoi, R.; Tamba, S.; Morimoto, K.; Segi-Nishida, E.; Nishihara, M.; Ichikawa, A.; Narumiya, S.; Sugimoto, Y. Rhoa/Rho kinase signaling in the cumulus mediates extracellular matrix assembly. Endocrinology 2009, 150, 3345–3352. [Google Scholar] [CrossRef] [PubMed]
- Yung, S.C.; Farber, J.M. Chapter 89—Chemokines. In Handbook of Biologically Active Peptides, 2nd ed.; Kastin, A.J., Ed.; Academic Press: Boston, MA, USA, 2013; pp. 656–663. [Google Scholar]
- Yoshie, O.; Matsushima, K. CCR4 and its ligands: From bench to bedside. Int. Immunol. 2015, 27, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.; Carter, S.; Hofer, M.J.; Campbell, I.L. Review: The chemokine receptor CXCR3 and its ligands CXCL9, CXCL10 and CXCL11 in neuroimmunity—A tale of conflict and conundrum. Neuropathol. Appl. Neurobiol. 2010, 36, 368–387. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nomiyama, H.; Osada, N.; Yoshie, O. A family tree of vertebrate chemokine receptors for a unified nomenclature. Dev. Comp. Immunol. 2011, 35, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Cheluvappa, R.; Luo, A.S.; Palmer, C.; Grimm, M.C. Protective pathways against colitis mediated by appendicitis and appendectomy. Clin. Exp. Immunol. 2011, 165, 393–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaser, A.; Ludwiczek, O.; Holzmann, S.; Moschen, A.R.; Weiss, G.; Enrich, B.; Graziadei, I.; Dunzendorfer, S.; Wiedermann, C.J.; Murzl, E.; et al. Increased expression of CCL20 in human inflammatory bowel disease. J. Clin. Immunol. 2004, 24, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.C.; Hansoul, S.; Nicolae, D.L.; Cho, J.H.; Duerr, R.H.; Rioux, J.D.; Brant, S.R.; Silverberg, M.S.; Taylor, K.D.; Barmada, M.M.; et al. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn’s disease. Nat. Gen. 2008, 40, 955–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson Ng, W.S.; Hampartzoumian, T.; Lloyd, A.R.; Grimm, M.C. A murine model of appendicitis and the impact of inflammation on appendiceal lymphocyte constituents. Clin. Exp. Immunol. 2007, 150, 169–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brazma, A. Minimum information about a microarray experiment (MIAME)—Successes, failures, challenges. Sci. World J. 2009, 9, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Brazma, A.; Hingamp, P.; Quackenbush, J.; Sherlock, G.; Spellman, P.; Stoeckert, C.; Aach, J.; Ansorge, W.; Ball, C.A.; Causton, H.C.; et al. Minimum information about a microarray experiment (MIAME)-toward standards for microarray data. Nat. Gen. 2001, 29, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Irizarry, R.A.; Bolstad, B.M.; Collin, F.; Cope, L.M.; Hobbs, B.; Speed, T.P. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003, 31, e15. [Google Scholar] [CrossRef] [PubMed]
- Storey, J.D.; Tibshirani, R. Statistical significance for genomewide studies. Proc. Natl. Acad. Sci. USA 2003, 100, 9440–9445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mootha, V.K.; Lindgren, C.M.; Eriksson, K.F.; Subramanian, A.; Sihag, S.; Lehar, J.; Puigserver, P.; Carlsson, E.; Ridderstrale, M.; Laurila, E.; et al. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Gen. 2003, 34, 267–273. [Google Scholar] [CrossRef] [PubMed]
| No. | Chemokine Gene | Other Names for Chemokine | Corresponding Chemokine Receptors | 3-Day Post-AA | 28-Day Post-AA | ||
|---|---|---|---|---|---|---|---|
| Fold-Change | p-Value | Fold-Change | p-Value | ||||
| C Chemokines | |||||||
| 1 | XCL1 | lymphoactin a, SCM-1a, ATAC | XCR1 | - | - | 1.03 | 0.709 |
| 2 | XCL2 | lymphoactin b, SCM-1b, ATAC | XCR1 | - | - | - | - |
| CC Chemokines | |||||||
| 1 | CCL1 | I-309 | CCR8 | 0.95 | 0.344 | 0.99 | 0.870 |
| 2 | CCL2 | MCP-1, MCAF | CCR2 | 1.48 | 0.347 | - | - |
| 3 | CCL3 | MIP-1α, LD78α | CCR1, CCR5 | 1.19 | 0.125 | - | - |
| 4 | CCL4 | MIP-1β, LAG-1, ACT-2 | CCR5 | 0.90 | 0.990 | 1.08 | 0.209 |
| 5 | CCL5 | RANTES | CCR1, CCR3, CCR5 | 0.91 | 0.537 | 1.22 | 0.048* |
| 6 | CCL7 | MCP-3 | CCR1, CCR2, CCR3 | 1.52# | 0.150 | 0.85 | 0.164 |
| 7 | CCL8 | MCP-2 | CCR3 | 1.87# | 0.349 | 1.18 | 0.368 |
| 8 | CCL11 | eotaxin | CCR3 | 1.27 | 0.059 | 1.07 | 0.458 |
| 9 | CCL13 | MCP-4 | CCR2, CCR3 | - | - | 0.95 | 0.721 |
| 10 | CCL14 | HCC-1 | CCR1 | - | - | - | - |
| 11 | CCL15 | HCC-2, Lkn-1, MIP-1d, MIP-5 | CCR1, CCR3 | - | - | - | - |
| 12 | CCL16 | HCC-4, LEC, LMC, LCC-1 | CCR1 | - | - | - | - |
| 13 | CCL17 | TARC | CCR4 | 1.02 | 0.864 | 0.83 | 0.047* |
| 14 | CCL18 | DC-CK1, PARC, AMAC-a, MIP-4 | ? | - | - | - | - |
| 15 | CCL19 | MIP-3β, ELC, exodus-3 | CCR7 | 0.98 | 0.667 | 0.95 | 0.554 |
| 16 | CCL20 | MIP-3α, LARC, exodus-1 | CCR6 | 0.60 | 0.028* | 0.63 | 0.023* |
| 17 | CCL21 | 6Ckine, SLC, exodus-2 | CCR7 | 1.14 | 0.359 | 1.11 | 0.353 |
| 18 | CCL22 | MDC, STCP-1 | CCR4 | 0.91 | 0.407 | 1.10 | 0.351 |
| 19 | CCL23 | MPIF-1, MIP-3, CKb-8 | CCR1 | - | - | - | - |
| 20 | CCL24 | MPIF-2, eotaxin-2, CKb-6 | CCR3 | 1.13 | 0.399 | 0.88 | 0.189 |
| 21 | CCL25 | TECK, MIP-4a | CCR9 | 0.90 | 0.285 | 0.90 | 0.566 |
| 22 | CCL26 | eotaxin-3 | CCR3 | 0.91 | 0.153 | 0.96 | 0.496 |
| 23 | CCL27 | Eskine, CTACK, ILC | CCR10 | 1.04 | 0.497 | 1.03 | 0.710 |
| CXC Chemokines | |||||||
| 1 | CXCL1 | GROa, MGSA-a | CXCR1, CXCR2 | 1.03 | 0.680 | 0.93 | 0.292 |
| 2 | CXCL2 | GROb, MGSA-b, MIP-2a | CXCR2 | 1.08 | 0.460 | 1.01 | 0.913 |
| 3 | CXCL3 | GROg, MGSA-g, MIP-2b | CXCR2 | 0.98 | 0.786 | 1.07 | 0.463 |
| 4 | CXCL4 | PF4, oncostatin A | ? | - | - | - | - |
| 5 | CXCL5 | ENA-78 | CXCR2 | 1.53 | 0.376 | - | - |
| 6 | CXCL6 | GCP-2 | CXCR1, CXCR2 | - | - | 0.99 | 0.872 |
| 7 | CXCL7 | NAP-2, PPBP | CXCR2 | - | - | - | - |
| 8 | CXCL8 | IL-8, NAP-1, NAF, MDNCF | CXCR1, CXCR2 | - | - | - | - |
| 9 | CXCL9 | Mig | CXCR3 | 1.20 | 0.487 | 1.15 | 0.368 |
| 10 | CXCL10 | IP-10 | CXCR3 | 1.82 | 0.324 | 1.50 | 0.100 |
| 11 | CXCL11 | I-TAC | CXCR3 | 1.17 | 0.044 * | 1.16 | 0.043 * |
| 12 | CXCL12 | SDF-1α/β | CXCR4 | 1.21 | 0.111 | 1.12 | 0.171 |
| 13 | CXCL13 | BLC, BCA-1 | CXCR5 | 1.05 | 0.927 | 0.85 | 0.086 |
| 14 | CXCL14 | BRAK | ? | 1.13 | 0.215 | 0.83 | 0.085 |
| CX3C Chemokines | |||||||
| 1 | CX3CL1 | fractalkine | CX3CR1 | 1.00 | 0.995 | 0.89 | 0.161 |
| No. | Chemokine Receptor Gene | Other Names for Chemokine Receptor | 3-Day Post-AA | 28-Day Post-AA | ||
|---|---|---|---|---|---|---|
| Fold-Change | p-Value | Fold-Change | p-Value | |||
| Atypical Chemical Receptors (ACR) | ||||||
| 1 | ACKR1 | CCBP1, GPD, Dfy, CD234 | - | - | - | - |
| 2 | ACKR2 | CCR10, D6, CCR9 | - | - | - | - |
| 3 | ACKR3 | RDC1, GPR159, CXCR7 | - | - | 0.93 | 0.231 |
| 4 | ACKR4 | CCR11, CCBP2, VSHK1, CCX-CKR, PPR1 | - | - | 1.01 | 0.867 |
| 5 | CCRL2 | HCR, CRAM-B, CKRX, CRAM-A, ACKR5 | - | - | - | - |
| 6 | PITPNM3 | NIR1, RDGBA3, ACKR6 | - | - | - | - |
| C-C Motif Chemokine Receptors (CCR) | ||||||
| 1 | CCR1 | CKR-1, MIP1aR, CD191 | - | - | 0.88 | 0.294 |
| 2 | CCR2 | CC-CKR-2, CKR2, MCP-1-R, CD192, FLJ78302 | - | - | 1.05 | 0.477 |
| 3 | CCR3 | CC-CKR-3, CKR3, CD193 | - | - | 1.11 | 0.143 |
| 4 | CCR4 | CC-CKR-4, CMKBR4, CKR4, k5-5, ChemR13, CD194 | - | - | 0.99 | 0.864 |
| 5 | CCR5 | CKR-5, CC-CKR-5, CKR5, CD195, IDDM22 | - | - | 1.02 | 0.737 |
| 6 | CCR6 | CKR-L3, GPR-CY4, CMKBR6, GPR29, DRY-6, DCR2, BN-1, CD196 | - | - | 0.85 | 0.145 |
| 7 | CCR7 | BLR2, CDw197, CD197 | - | - | 1.13 | 0.226 |
| 8 | CCR8 | CY6, TER1, CKR-L1, GPR-CY6, CDw198 | - | - | 0.93 | 0.506 |
| 9 | CCR9 | GPR-9-6, CDw199 | - | - | 1.06 | 0.685 |
| 10 | CCR10 | - | - | 1.19 | 0.024 * | |
| C-X-C Motif Chemokine Receptors (CXCR) | ||||||
| 1 | CXCR1 | CKR-1, CDw128a, CD181 | - | - | - | - |
| 2 | CXCR2 | CMKAR2, CD182 | - | - | - | - |
| 3 | CXCR3 | CKR-L2, CMKAR3, IP10-R, MigR, CD183 | - | - | 0.93 | 0.419 |
| 4 | CXCR4 | LESTR, NPY3R, HM89, NPYY3R, D2S201E, fusin, HSY3RR, NPYR, CD184 | - | - | 1.13 | 0.373 |
| 5 | CXCR5 | MDR15, CD185 | - | - | 0.88 | 0.063 |
| 6 | CXCR6 | TYMSTR, STRL33, BONZO, CD186 | - | - | 1.14 | 0.101 |
| C-X-3-C Motif Chemokine Receptors (CX3CR) | ||||||
| 1 | CX3CR1 | CMKDR1, V28, CCRL1 | - | - | 1.13 | 0.218 |
| X-C motif chemokine receptors (XCR) | ||||||
| 2 | XCR1 | GPR5, CCXCR1 | - | - | 1.13 | 0.375 |
| Gene | Upregulated Gene-Sets in AA | No. of Enriched Genes | FDR q-val | Downregulated Gene-Sets in AA | No. of Enriched Genes | FDR q-val |
|---|---|---|---|---|---|---|
| Differentially Regulated Chemokine Gene | ||||||
| CCL5 | UPREG 7 gene-sets | DOWNREG 1 gene-sets | ||||
| BOYLAN_MULTIPLE_MYELOMA_C_D_DN | 253 | 0.036 | MONNIER_POSTRADIATION_TUMOR_ESCAPE_UP | 357 | 0.009 | |
| BOYLAN_MULTIPLE_MYELOMA_PCA1_UP | 100 | 0.016 | ||||
| LIANG_SILENCED_BY_METHYLATION_2 | 31 | 0.002 | ||||
| MAHADEVAN_RESPONSE_TO_MP470_UP | 17 | 0.001 | ||||
| SABATES_COLORECTAL_ADENOMA_DN | 260 | 0.016 | ||||
| SEITZ_NEOPLASTIC_TRANSFORMATION_BY_8P_DELETION_UP | 68 | 0.000 | ||||
| WIELAND_UP_BY_HBV_INFECTION | 86 | 0.002 | ||||
| CCL17 | UPREG 0 gene-sets | DOWNREG 0 gene-sets | ||||
| - | - | |||||
| CCL20 | UPREG 1 gene-sets | DOWNREG 0 gene-sets | ||||
| LIANG_SILENCED_BY_METHYLATION_2 | 31 | 0.002 | - | |||
| CXCL11 | UPREG 6 gene-sets | DOWNREG 0 gene-sets | ||||
| MAHADEVAN_RESPONSE_TO_MP470_UP | 17 | 0.001 | - | |||
| RADAEVA_RESPONSE_TO_IFNA1_UP | 30 | 0.009 | ||||
| SANA_RESPONSE_TO_IFNG_UP | 56 | 0.000 | ||||
| SEITZ_NEOPLASTIC_TRANSFORMATION_BY_8P_DELETION_UP | 68 | 0.000 | ||||
| UROSEVIC_RESPONSE_TO_IMIQUIMOD | 15 | 0.023 | ||||
| WIELAND_UP_BY_HBV_INFECTION | 86 | 0.002 | ||||
| DIFFERENTIALLY REGULATED CHEMOKINE RECEPTOR GENE | ||||||
| CCR10 | UPREG 0 gene-sets | DOWNREG 1 gene-sets | ||||
| - | SPIELMAN_LYMPHOBLAST_EUROPEAN_VS_ASIAN_UP | 46 | 0.034 | |||
| Gene | Upregulated Gene-Sets in AA | No. of Enriched Genes | FDR q-val | Downregulated Gene-Sets in AA | No. of Enriched Genes | FDR q-val |
|---|---|---|---|---|---|---|
| CCL7 | UPREG 0 gene-sets | DOWNREG 1 gene-sets | ||||
| - | BERENJENO_TRANSFORMED_BY_RHOA_UP | 495 | 0.006 | |||
| CCL8 | UPREG 2 gene-sets | DOWNREG 0 gene-sets | ||||
| SABATES_COLORECTAL_ADENOMA_DN | 260 | 0.016 | - | |||
| UROSEVIC_RESPONSE_TO_IMIQUIMOD | 15 | 0.023 | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheluvappa, R.; Thomas, D.G.; Selvendran, S. The Role of Specific Chemokines in the Amelioration of Colitis by Appendicitis and Appendectomy. Biomolecules 2018, 8, 59. https://doi.org/10.3390/biom8030059
Cheluvappa R, Thomas DG, Selvendran S. The Role of Specific Chemokines in the Amelioration of Colitis by Appendicitis and Appendectomy. Biomolecules. 2018; 8(3):59. https://doi.org/10.3390/biom8030059
Chicago/Turabian StyleCheluvappa, Rajkumar, Dennis G. Thomas, and Selwyn Selvendran. 2018. "The Role of Specific Chemokines in the Amelioration of Colitis by Appendicitis and Appendectomy" Biomolecules 8, no. 3: 59. https://doi.org/10.3390/biom8030059






