Efficient Delivery of Macromolecules into Human Cells by Improving the Endosomal Escape Activity of Cell-Penetrating Peptides: Lessons Learned from dfTAT and its Analogs
Abstract
:1. Introduction
2. What Is dfTAT and How Does dfTAT-Mediated Delivery Work?
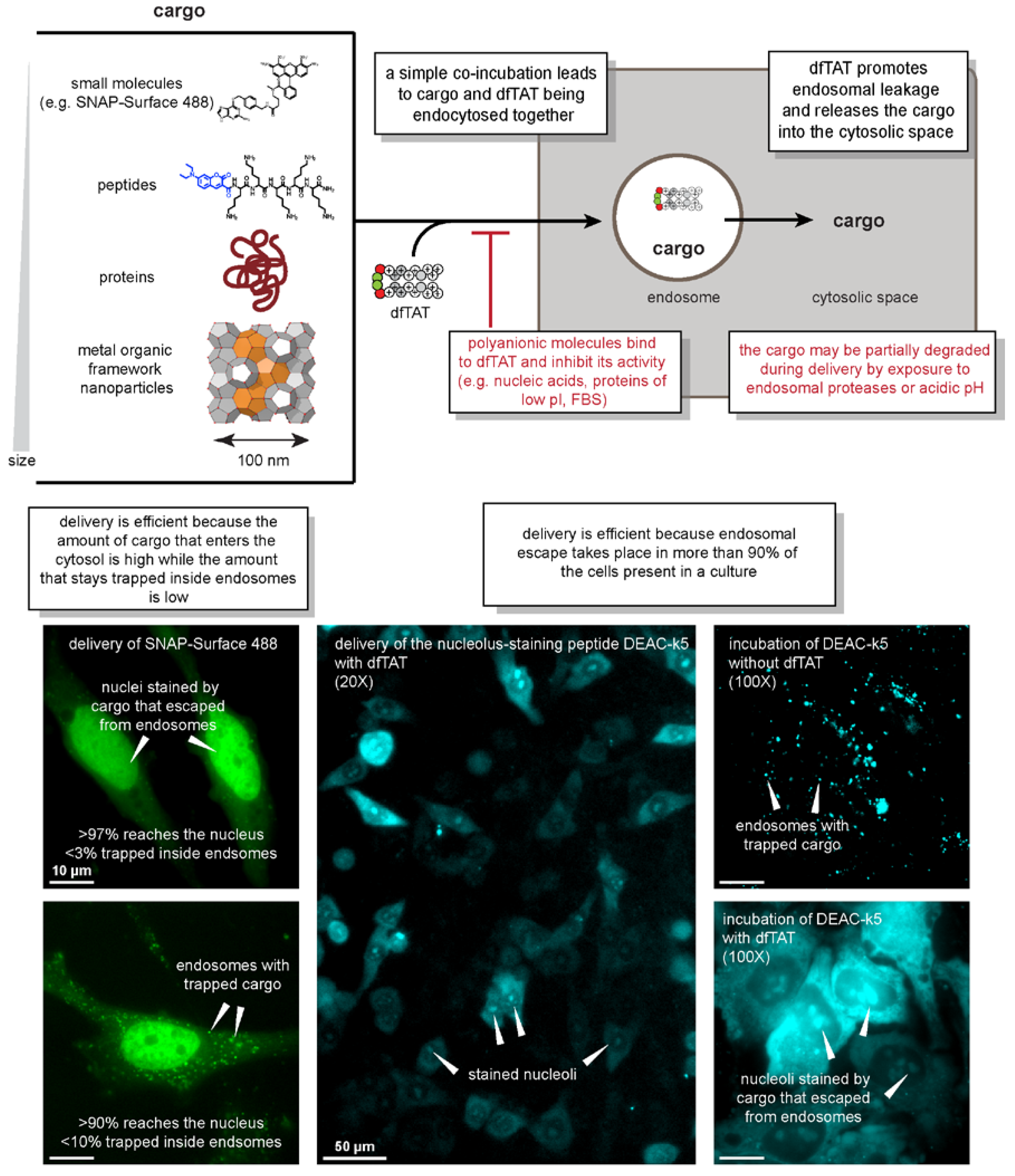
2.1. Structure and Activity of dfTAT
2.2. What Works
2.3. What Doesn’t Work
2.4. Characteristics of the Delivery Process
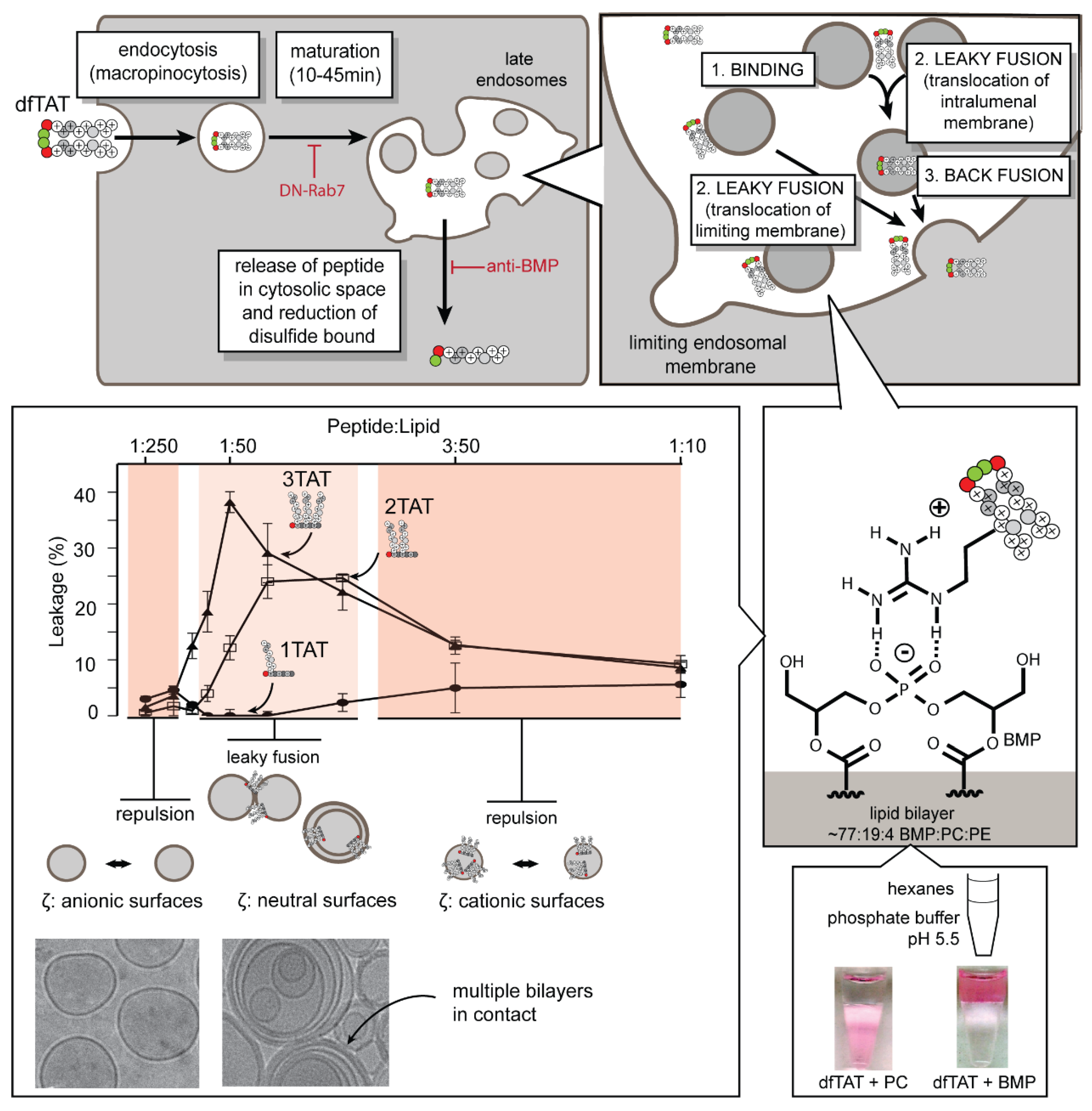
3. How Does dfTAT Get Inside Cells and Promote Endosomal Leakage?
3.1. Where Is Endosomal Escape Taking Place in the Cell?
3.2. What Cellular Factors Are Involved in Endosomal Leakage?
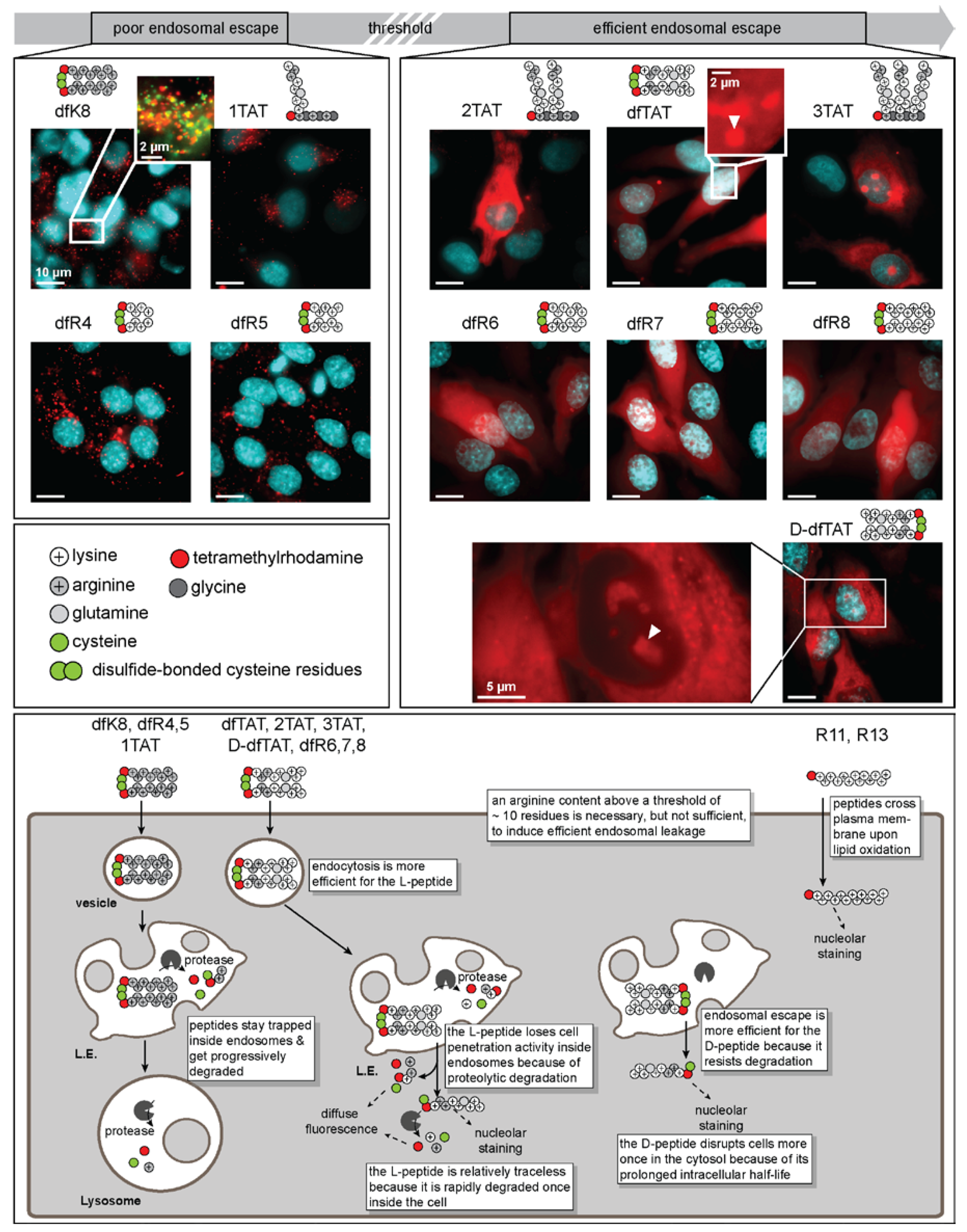
4. What Are the Features That Make dfTAT Active?
4.1. Cytosolic Penetration Is Dependent on Reaching a Threshold Guanidinium Density
4.2. Charge Density and Multimerization Play a Role in Successful Cytosolic Penetration
4.3. Is There More to dfTAT Activity than Arginine Content?
4.4. Do Chirality and Protease Degradation Impact Cell Penetration?
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Brooks, H.; Lebleu, B.; Vivès, E. Tat peptide-mediated cellular delivery: Back to basics. Adv. Drug Deliv. Rev. 2005, 57, 559–577. [Google Scholar] [CrossRef] [PubMed]
- Heitz, F.; Morris, M.C.; Divita, G. Twenty years of cell-penetrating peptides: From molecular mechanisms to therapeutics. Br. J. Pharmacol. 2009, 157, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Mager, I.; Eiriksdottir, E.; Langel, K.; El Andaloussi, S.; Langel, U. Assessing the uptake kinetics and internalization mechanisms of cell-penetrating peptides using a quenched fluorescence assay. Biochim. Biophys. Acta 2010, 1798, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Madani, F.; Lindberg, S.; Langel, U.; Futaki, S.; Graslund, A. Mechanisms of cellular uptake of cell-penetrating peptides. J. Biophys. 2011, 2011, 414729. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.T. Macropinocytosis: Searching for an endocytic identity and role in the uptake of cell penetrating peptides. J. Cell. Mol. Med. 2007, 11, 670–684. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Yamauchi, J.; Khalil, I.A.; Kajimoto, K.; Akita, H.; Harashima, H. Cell penetrating peptide-mediated systemic sirna delivery to the liver. Int. J. Pharm. 2011, 419, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-J.; Johnson, G.; Peltier, G.C.; Pellois, J.-P. A HA2-Fusion tag limits the endosomal release of its protein cargo despite causing endosomal lysis. Biochim. Biophys. Acta 2011, 1810, 752–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, G.A.; Muthukrishnan, N.; Pellois, J.-P. Photoinactivation of Gram positive and Gram negative bacteria with the antimicrobial peptide (KLAKLAK)(2) conjugated to the hydrophilic photosensitizer eosin Y. Bioconjug. Chem. 2013, 24, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Wharton, S.A.; Martin, S.R.; Ruigrok, R.W.; Skehel, J.J.; Wiley, D.C. Membrane fusion by peptide analogues of influenza virus haemagglutinin. J. Gen. Virol. 1988, 69, 1847–1857. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-J.; Johnson, G.; Pellois, J.-P. Modeling of the endosomolytic activity of HA2-TAT peptides with red blood cells and ghosts. Biochemistry 2010, 49, 7854–7866. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-J.; Erazo-Oliveras, A.; Pellois, J.-P. Delivery of macromolecules into live cells by simple co-incubation with a peptide. ChemBioChem 2010, 11, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Maiolo, J.R.; Ottinger, E.A.; Ferrer, M. Specific redistribution of cell-penetrating peptides from endosomes to the cytoplasm and nucleus upon laser illumination. J. Am. Chem. Soc. 2004, 126, 15376–15377. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, M.; Noguchi, H.; Lu, Y.F.; Tomizawa, K.; Michiue, H.; Li, S.T.; Hirose, K.; Bonner-Weir, S.; Matsui, H. Photo-acceleration of protein release from endosome in the protein transduction system. FEBS Lett. 2004, 572, 221–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selbo, P.K.; Weyergang, A.; Hogset, A.; Norum, O.J.; Berstad, M.B.; Vikdal, M.; Berg, K. Photochemical internalization provides time- and space-controlled endolysosomal escape of therapeutic molecules. J. Control. Release 2010, 148, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, D.; Muthukrishnan, N.; Johnson, G.; Erazo-Oliveras, A.; Lim, J.; Simanek, E.; Pellois, J.-P. Conjugation to the cell-penetrating peptide tat potentiates the photodynamic effect of carboxytetramethylrhodamine. PLoS ONE 2011, 6, e17732. [Google Scholar] [CrossRef] [PubMed]
- Muthukrishnan, N.; Johnson, G.; Lim, J.; Simanek, E.; Pellois, J.-P. Tat-mediated photochemical internalization results in cell killing by causing the release of calcium into the cytosol of cells. Biochim. Biophys. Acta 2012, 1820, 1734–1743. [Google Scholar] [CrossRef] [PubMed]
- Sung, M.; Poon, G.M.K.; Gariépy, J. The importance of valency in enhancing the import and cell routing potential of protein transduction domain-containing molecules. Biochim. Biophys. Acta 2006, 1758, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Lönn, P.; Kacsinta, A.D.; Cui, X.-S.; Hamil, A.S.; Kaulich, M.; Gogoi, K.; Dowdy, S.F. Enhancing endosomal escape for intracellular delivery of macromolecular biologic therapeutics. Sci. Rep. 2016, 6, 32301. [Google Scholar] [CrossRef] [PubMed]
- Arnusch, C.; Branderhorst, H.; de Kruijff, B.; Liskamp, R.M.J.; Breukink, E.; Pieters, R. Enhanced membrane pore formation by multimeric/oligomeric antimicrobial peptides. Biochemistry 2007, 46, 13437–13442. [Google Scholar] [CrossRef] [PubMed]
- Van Baal, I.; Malda, H.; Synowsky, S.; van Dongen, J.L.J.; Hackeng, T.; Merkx, M.; Meijer, E.W. Multivalent peptide and protein dendrimers using native chemical ligation. Angew. Chem. Int. Edit. 2005, 44, 5052–5057. [Google Scholar] [CrossRef] [PubMed]
- Angeles-Boza, A.M.; Erazo-Oliveras, A.; Lee, Y.-J.; Pellois, J.-P. Generation of endosomolytic reagents by branching of cell-penetrating peptides: Tools for the delivery of bioactive compounds to live cells in cis or trans. Bioconjug. Chem. 2010, 21, 2164–2167. [Google Scholar] [CrossRef] [PubMed]
- Erazo-Oliveras, A.; Najjar, K.; Dayani, L.; Wang, T.-Y.; Johnson, G.A.; Pellois, J.-P. Protein delivery into live cells by incubation with an endosomolytic agent. Nat. Methods 2014, 11, 861–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frankel, A.D.; Pabo, C.O. Cellular uptake of the tat protein from human immunodeficiency virus. Cell 1988, 55, 1189–1193. [Google Scholar] [CrossRef]
- Green, M.; Loewenstein, P.M. Autonomous functional domains of chemically synthesized human immunodeficiency virus TAT trans-activator protein. Cell 1988, 55, 1179–1188. [Google Scholar] [CrossRef]
- Vivès, E.; Brodin, P.; Lebleu, B. A truncated HIV-1 TAT protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J. Biol. Chem. 1997, 272, 16010–16017. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.; Erazo-Oliveras, A.; Pellois, J.P.; Zhou, H.C. High efficiency and long-term intracellular activity of an enzymatic nanofactory based on metal-organic frameworks. Nat. Commun. 2017, 8, 2075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erazo-Oliveras, A.; Najjar, K.; Truong, D.; Wang, T.Y.; Brock, D.J.; Prater, A.R.; Pellois, J.P. The late endosome and its lipid BMP act as gateways for efficient cytosolic access of the delivery agent dftat and its macromolecular cargos. Cell Chem. Biol. 2016, 23, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Najjar, K.; Erazo-Oliveras, A.; Brock, D.J.; Wang, T.Y.; Pellois, J.P. An l- to d-amino acid conversion in an endosomolytic analog of the cell-penetrating peptide TAT influences proteolytic stability, endocytic uptake, and endosomal escape. J. Biol. Chem. 2017, 292, 847–861. [Google Scholar] [CrossRef] [PubMed]
- Authier, F.; Posner, B.I.; Bergeron, J.J. Endosomal proteolysis of internalized proteins. FEBS Lett. 1996, 389, 55–60. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, B.; Smed-Sorensen, A.; Cohn, L.; Chalouni, C.; Vandlen, R.; Lee, B.C.; Widger, J.; Keler, T.; Delamarre, L.; Mellman, I. Internalization and endosomal degradation of receptor-bound antigens regulate the efficiency of cross presentation by human dendritic cells. Blood 2012, 120, 2011–2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diment, S.; Stahl, P. Macrophage endosomes contain proteases which degrade endocytosed protein ligands. J. Biol. Chem. 1985, 260, 15311–15317. [Google Scholar] [PubMed]
- Kobayashi, T.; Stang, E.; Fang, K.S.; de Moerloose, P.; Parton, R.G.; Gruenberg, J. A lipid associated with the antiphospholipid syndrome regulates endosome structure and function. Nature 1998, 392, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Beuchat, M.H.; Chevallier, J.; Makino, A.; Mayran, N.; Escola, J.M.; Lebrand, C.; Cosson, P.; Kobayashi, T.; Gruenberg, J. Separation and characterization of late endosomal membrane domains. J. Biol. Chem. 2002, 277, 32157–32164. [Google Scholar] [CrossRef] [PubMed]
- Falguieres, T.; Luyet, P.P.; Bissig, C.; Scott, C.C.; Velluz, M.C.; Gruenberg, J. In vitro budding of intralumenal vesicles into late endosomes is regulated by Alix and Tsg101. Mol. Biol. Cell 2008, 19, 4942–4955. [Google Scholar] [CrossRef] [PubMed]
- Duchardt, F.; Fotin-Mleczek, M.; Schwarz, H.; Fischer, R.; Brock, R. A comprehensive model for the cellular uptake of cationic cell-penetrating peptides. Traffic 2007, 8, 848–866. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-Y.; Sun, Y.; Muthukrishnan, N.; Erazo-Oliveras, A.; Najjar, K.; Pellois, J.-P. Membrane oxidation enables the cytosolic entry of polyarginine cell-penetrating peptides. J. Biol. Chem. 2016, 291, 7902–7914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Najjar, K.; Erazo-Oliveras, A.; Mosior, J.; Whitlock, M.; Rostane, I.; Cinclair, J.; Pellois, J.-P. Unlocking endosomal entrapment with supercharged arginine-rich peptides. Bioconjug. Chem. 2017, 28, 2932–2941. [Google Scholar] [CrossRef] [PubMed]
- Brock, D.J.; Kustigian, L.; Jiang, M.; Graham, K.; Wang, T.-Y.; Erazo-Oliveras, A.; Najjar, K.; Zhang, J.; Rye, H.; Pellois, J.-P. Efficient cell delivery mediated by lipid-specific endosomal escape of supercharged branched peptides. Traffic 2018, 19, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Scharf, B.; Clement, C.C.; Wu, X.X.; Morozova, K.; Zanolini, D.; Follenzi, A.; Larocca, J.N.; Levon, K.; Sutterwala, F.S.; Rand, J.; et al. Annexin A2 binds to endosomes following organelle destabilization by particulate wear debris. Nat. Commun. 2012, 3, 755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Castro, M.A.G.; Bunt, G.; Wouters, F.S. Cathepsin B launches an apoptotic exit effort upon cell death-associated disruption of lysosomes. Cell Death Discov. 2016, 2, 16012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allen, J.K.; Brock, D.J.; Kondow-McConaghy, H.M.; Pellois, J.-P. Efficient Delivery of Macromolecules into Human Cells by Improving the Endosomal Escape Activity of Cell-Penetrating Peptides: Lessons Learned from dfTAT and its Analogs. Biomolecules 2018, 8, 50. https://doi.org/10.3390/biom8030050
Allen JK, Brock DJ, Kondow-McConaghy HM, Pellois J-P. Efficient Delivery of Macromolecules into Human Cells by Improving the Endosomal Escape Activity of Cell-Penetrating Peptides: Lessons Learned from dfTAT and its Analogs. Biomolecules. 2018; 8(3):50. https://doi.org/10.3390/biom8030050
Chicago/Turabian StyleAllen, Jason K., Dakota J. Brock, Helena M. Kondow-McConaghy, and Jean-Philippe Pellois. 2018. "Efficient Delivery of Macromolecules into Human Cells by Improving the Endosomal Escape Activity of Cell-Penetrating Peptides: Lessons Learned from dfTAT and its Analogs" Biomolecules 8, no. 3: 50. https://doi.org/10.3390/biom8030050




