Suppression of mRNA Nanoparticle Transfection in Human Fibroblasts by Selected Interferon Inhibiting Small Molecule Compounds
Abstract
:1. Introduction
2. Results
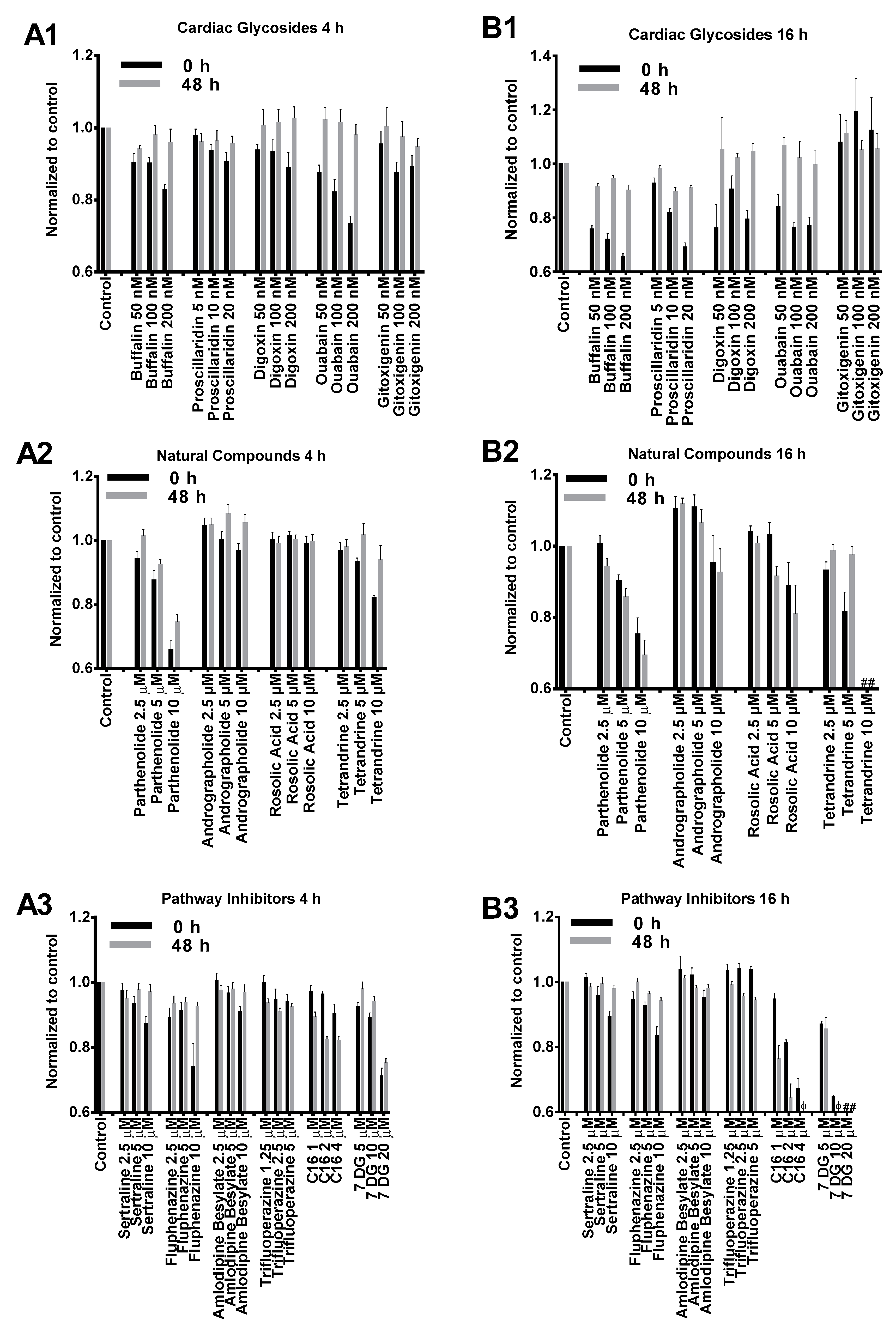
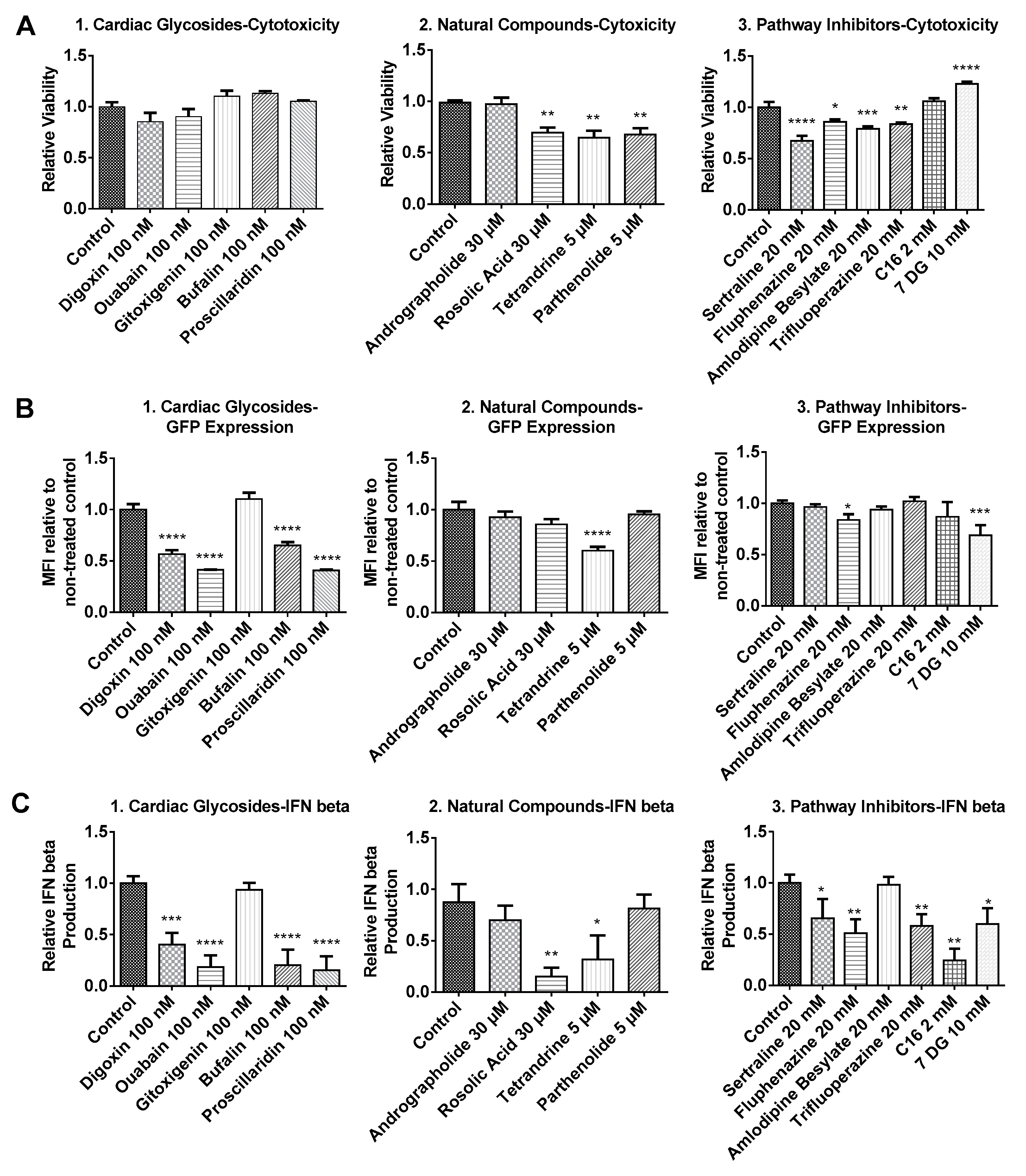
2.1. Cytotoxicity Profiles of Small Molecule Compounds
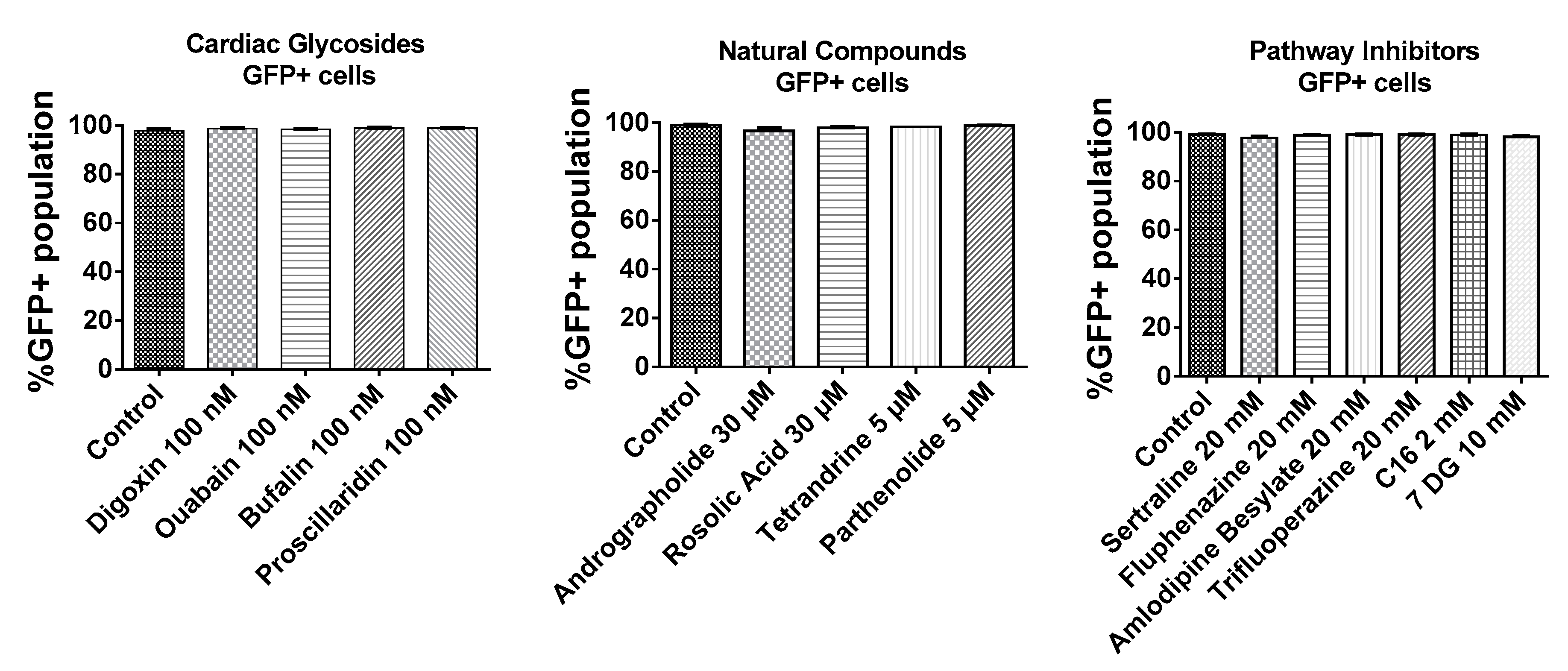
2.2. BJ Fibroblasts Transfected in the Presence of Small Molecules Express Less GFP Even with Reduced IFN Production
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. GFP mRNA Synthesis
4.3. Cell Culture and GFP mRNA Transfection
4.4. Cytotoxicity Assay
4.5. Production of IFN-β
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Weissman, D. mRNA transcript therapy. Expert Rev. Vaccines 2015, 14, 265–281. [Google Scholar] [CrossRef] [PubMed]
- Van der Jeught, K.; Joe, P.T.; Bialkowski, L.; Heirman, C.; Daszkiewicz, L.; Liechtenstein, T.; Escors, D.; Thielemans, K.; Breckpot, K. Intratumoral administration of mRNA encoding a fusokine consisting of IFN-beta and the ectodomain of the TGF-beta receptor II potentiates antitumor immunity. Oncotarget 2014, 5, 10100–10113. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Convertine, A.J.; Stayton, P.S.; Bryers, J.D. Multifunctional triblock copolymers for intracellular messenger RNA delivery. Biomaterials 2012, 33, 6868–6876. [Google Scholar] [CrossRef] [PubMed]
- Phua, K.K. Towards Targeted Delivery Systems: Ligand Conjugation Strategies for mRNA Nanoparticle Tumor Vaccines. J. Immunol. Res. 2015, 2015, 680620. [Google Scholar] [CrossRef] [PubMed]
- Phua, K.K.; Boczkowski, D.; Dannull, J.; Pruitt, S.; Leong, K.W.; Nair, S.K.; Phua, K.K. Towards Targeted Delivery Systems: Ligand Conjugation Strategies for mRNA Nanoparticle Tumor Vaccines. J. Immunol. Res. 2015, 2015, 680620. [Google Scholar] [CrossRef] [PubMed]
- Phua, K.K.L.; Liu, Y.; Sim, S.H. Non-linear enhancement of mRNA delivery efficiencies by influenza A derived NS1 protein engendering host gene inhibition property. Biomaterials 2017, 133, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Fricke, J.; Kavanagh, D.G.; Irvine, D.J. In vitro and in vivo mRNA delivery using lipid-enveloped pH-responsive polymer nanoparticles. Mol. Pharm. 2011, 8, 774–787. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Su, H.H.; Yang, Y.; Hu, Y.; Zhang, L.; Blancafort, P.; Huang, L. Systemic Delivery of Modified mRNA Encoding Herpes Simplex Virus 1 Thymidine Kinase for Targeted Cancer Gene Therapy. Mol. Ther. 2013, 21, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Devoldere, J.; Dewitte, H.; De Smedt, S.C.; Remaut, K. Evading innate immunity in nonviral mRNA delivery: Don’t shoot the messenger. Drug Discov. Today 2016, 21, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Pulloor, N.K.; Nair, S.; Kostic, A.D.; Bist, P.; Weaver, J.D.; Riley, A.M.; Tyagi, R.; Uchil, P.D.; York, J.D.; Snyder, S.H.; et al. Human genome-wide RNAi screen identifies an essential role for inositol pyrophosphates in Type-I interferon response. PLoS Pathog. 2014, 10, e1003981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahin, U.; Kariko, K.; Tureci, O. mRNA-based therapeutics—Developing a new class of drugs. Nat. Rev. Drug Discov. 2014, 13, 759–780. [Google Scholar] [CrossRef] [PubMed]
- Awe, J.P.; Crespo, A.V.; Li, Y.; Kiledjian, M.; Byrne, J.A. BAY11 enhances OCT4 synthetic mRNA expression in adult human skin cells. Stem Cell Res. Ther. 2013, 4, 15. [Google Scholar] [CrossRef] [PubMed]
- Abu-Ghefreh, A.A.; Canatan, H.; Ezeamuzie, C.I. In vitro and in vivo anti-inflammatory effects of andrographolide. Int. Immunopharmacol. 2009, 9, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Delebinski, C.I.; Georgi, S.; Kleinsimon, S.; Twardziok, M.; Kopp, B.; Melzig, M.F.; Seifert, G. Analysis of proliferation and apoptotic induction by 20 steroid glycosides in 143B osteosarcoma cells in vitro. Cell Prolif. 2015, 48, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.Z.; Smith, K.; Hsieh, P.N.; Mburu, Y.K.; Chattopadhyay, S.; Sen, G.C.; Sarkar, S.N. High-Throughput Screening for TLR3-IFN Regulatory Factor 3 Signaling Pathway Modulators Identifies Several Antipsychotic Drugs as TLR Inhibitors. J. Immunol. 2010, 184, 5768–5776. [Google Scholar] [CrossRef] [PubMed]
- Artlett, C.M.; Sassi-Gaha, S.; Rieger, J.L.; Boesteanu, A.C.; Feghali-Bostwick, C.A.; Katsikis, P.D. The inflammasome activating caspase 1 mediates fibrosis and myofibroblast differentiation in systemic sclerosis. Arthritis Rheum. 2011, 63, 3563–3574. [Google Scholar] [CrossRef] [PubMed]
- Hett, E.C.; Slater, L.H.; Mark, K.G.; Kawate, T.; Monks, B.G.; Stutz, A.; Latz, E.; Hung, D.T. Chemical genetics reveals a kinase-independent role for protein kinase R in pyroptosis. Nat. Chem. Biol. 2013, 9, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.R.; Muramatsu, H.; Nallagatla, S.R.; Bevilacqua, P.C.; Sansing, L.H.; Weissman, D.; Karikó, K. Incorporation of pseudouridine into mRNA enhances translation by diminishing PKR activation. Nucleic Acids Res. 2010, 38, 5884–5892. [Google Scholar] [CrossRef] [PubMed]
- UnitedStates (US) Food and Drug Administration. Drugs (FDA): FDA Approved Drug Products. Available online: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm (accessed on 1 December 2016).
- Ye, J.; Chen, S.; Maniatis, T. Cardiac glycosides are potent inhibitors of interferon-beta gene expression. Nat. Chem. Biol. 2011, 7, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Prassas, I.; Karagiannis, G.S.; Batruch, I.; Dimitromanolakis, A.; Datti, A.; Diamandis, E.P. Digitoxin-induced cytotoxicity in cancer cells is mediated through distinct kinase and interferon signaling networks. Mol. Cancer Ther. 2011, 10, 2083–2093. [Google Scholar] [CrossRef] [PubMed]
- Denicolai, E.; Baeza-Kallee, N.; Tchoghandjian, A.; Carre, M.; Colin, C.; Jiglaire, C.J.; Mercurio, S.; Beclin, C.; Figarella-Branger, D. Proscillaridin A is cytotoxic for glioblastoma cell lines and controls tumor xenograft growth in vivo. Oncotarget 2014, 5, 10934–10948. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.F.; Ye, B.Q.; Li, Y.D.; Wang, J.G.; He, X.J.; Lin, X.; Yao, X.; Ma, D.; Slungaard, A.; Hebbel, R.P.; et al. Andrographolide attenuates inflammation by inhibition of NF-kappa B activation through covalent modification of reduced cysteine 62 of p50. J. Immunol. 2004, 173, 4207–4217. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, Y.; Komohara, Y.; Ikeda, T.; Takeya, M. Corosolic acid inhibits glioblastoma cell proliferation by suppressing the activation of signal transducer and activator of transcription-3 and nuclear factor-kappa B in tumor cells and tumor-associated macrophages. Cancer Sci. 2011, 102, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Kang, O.H.; An, H.J.; Kim, S.B.; Mun, S.H.; Seo, Y.S.; Joung, D.K.; Choi, J.G.; Shin, D.W.; Kwon, D.Y. Tetrandrine suppresses pro-inflammatory mediators in PMA plus A23187-induced HMC-1 cells. Int. J. Mol. Med. 2014, 33, 1335–1340. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Shin, H.J.; Youn, H.S. Parthenolide inhibits TRIF-dependent signaling pathway of Toll-like receptors in RAW264.7 macrophages. Mol. Cells 2011, 31, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Arduini, A.; Baccaro, B.; Furuhashi, M.; Hotamisligil, G.S. Small-molecule inhibitors of PKR improve glucose homeostasis in obese diabetic mice. Diabetes 2014, 63, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Phua, K.K.; Leong, K.W.; Nair, S.K. Transfection efficiency and transgene expression kinetics of mRNA delivered in naked and nanoparticle format. J. Control. Release Off. J. Control. Release Soc. 2013, 166, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Phua, K.K.; Staats, H.F.; Leong, K.W.; Nair, S.K. Intranasal mRNA nanoparticle vaccination induces prophylactic and therapeutic anti-tumor immunity. Sci. Rep. 2014, 4, 5128. [Google Scholar] [CrossRef] [PubMed]
| Group | Name | Mechanism | Cell Type | FDA Approval [19] | Reference |
|---|---|---|---|---|---|
| Cardiac Glycosides | Ouabain | Direct inhibition of sodium-potassium ATPase pump of RIG-I, indirect inhibition of IRF3 and NFĸB | HEK 293T | No | [20] |
| Bufalin | No | ||||
| Gitoxigenin | Yes | ||||
| Digoxin | BxPC3 pancreatic cancer cells | No | [21] | ||
| Proscillaridin | Glioblastoma cells (GBM6, GBM9, U87-MG, U251-MG) | No | [22] | ||
| Natural Compounds | Andrographolide | Inhibition of NFĸB | HEK 293 | No | [23] |
| Rosolic acid | Inhibition of STAT3 and NFĸB | Glioblastoma cells (U737, T98G) | No | [24] | |
| Tetrandrine | Direct inhibition of ERK1/2, JNK1/2; indirect inhibition of NFĸB, IĸB | Human mast cells (HMC-1) | No | [25] | |
| Parthenolide | Inhibits TRIF and MYD88 dependent pathways | RAW264.7 murine macrophages | No | [26] | |
| Pathway Inhibitors | Sertraline | Inhibition of TLR3 | HEK293 | Yes | [15] |
| Fluphenazine | Yes | ||||
| Trifluoperazine | Yes | ||||
| Amlodipine besylate | Yes | ||||
| PKR inhibitor (C16) | Inhibition of PKR | Murine embryonic fibroblasts | No | [27] | |
| 7-Desacetoxy-6,7-dehydrogedunin (7DG) | Inhibition of PKR | J774 murine macrophages | No | [17] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Krishnan, M.N.; Phua, K.K.L. Suppression of mRNA Nanoparticle Transfection in Human Fibroblasts by Selected Interferon Inhibiting Small Molecule Compounds. Biomolecules 2017, 7, 56. https://doi.org/10.3390/biom7030056
Liu Y, Krishnan MN, Phua KKL. Suppression of mRNA Nanoparticle Transfection in Human Fibroblasts by Selected Interferon Inhibiting Small Molecule Compounds. Biomolecules. 2017; 7(3):56. https://doi.org/10.3390/biom7030056
Chicago/Turabian StyleLiu, Yang, Manoj N. Krishnan, and Kyle K.L. Phua. 2017. "Suppression of mRNA Nanoparticle Transfection in Human Fibroblasts by Selected Interferon Inhibiting Small Molecule Compounds" Biomolecules 7, no. 3: 56. https://doi.org/10.3390/biom7030056





