Trm5 and TrmD: Two Enzymes from Distinct Origins Catalyze the Identical tRNA Modification, m1G37
Abstract
:1. Introduction
2. Trm5
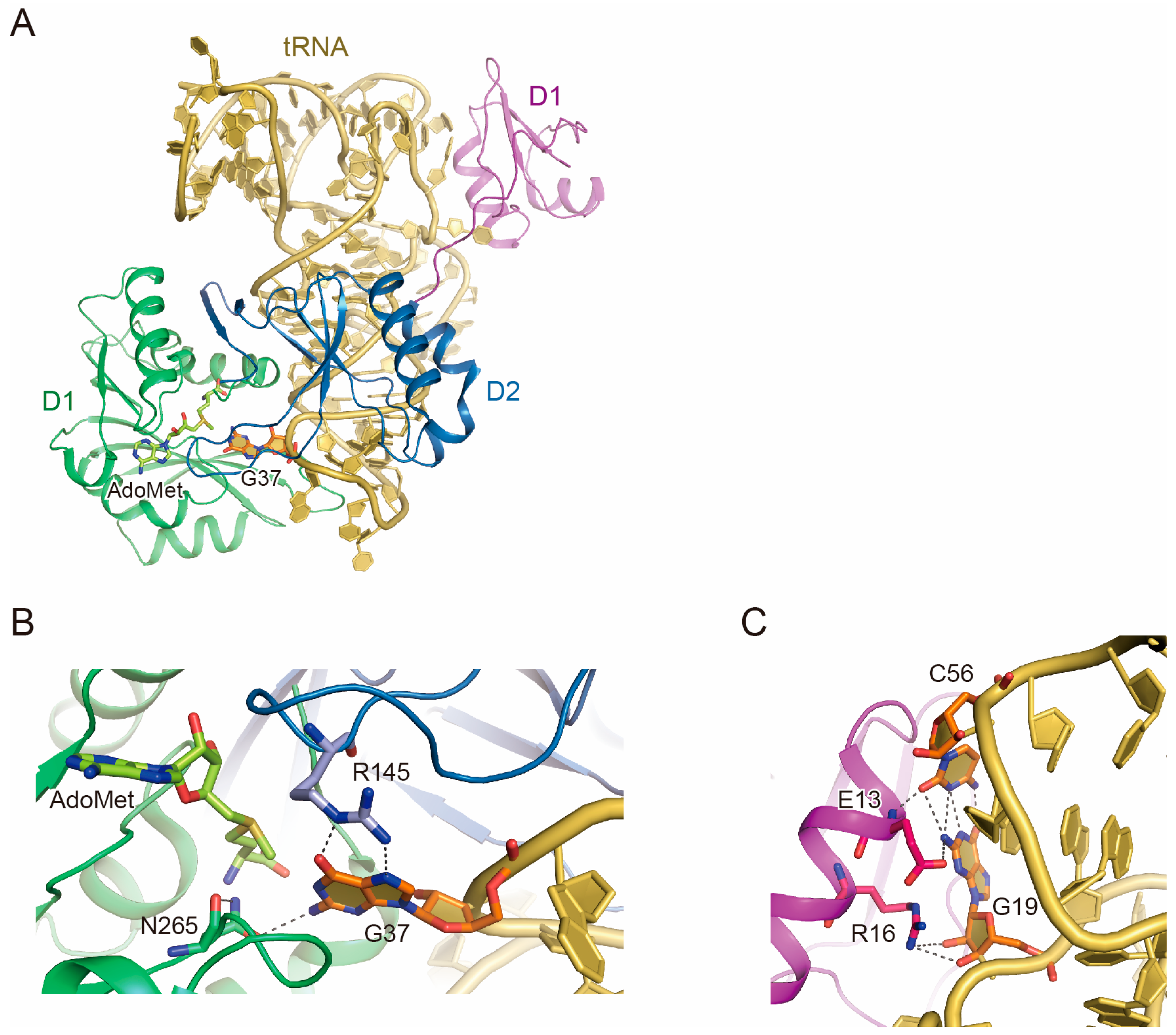
2.1. Trm5 Structure
2.2. tRNA Recognition by Trm5
2.3. Function of D1
2.4. tRNA Maturation Check by Trm5
3. TrmD
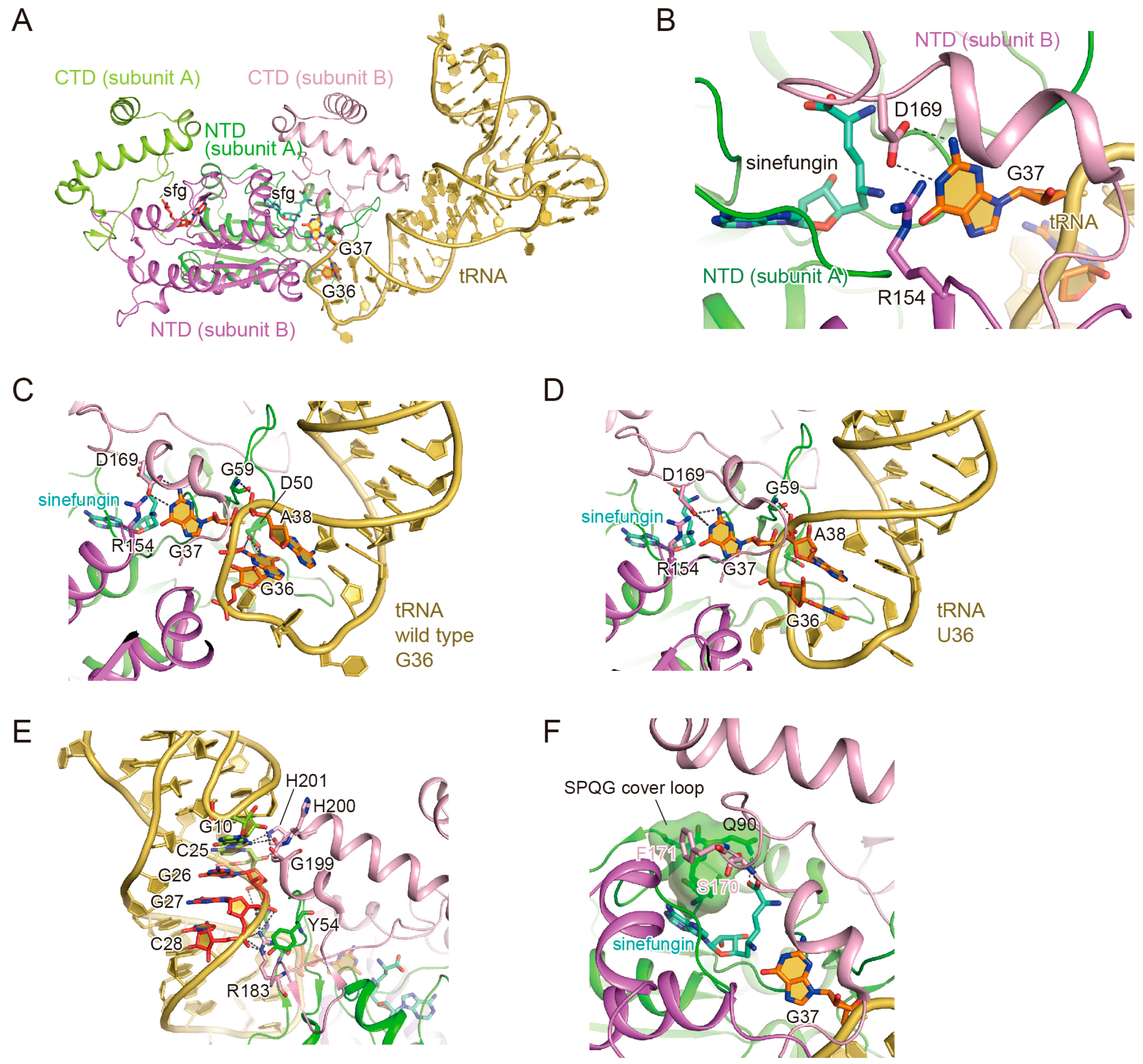
3.1. TrmD Structure
3.2. The Binding of the TrmD Dimer to a Substrate tRNA
3.3. The Interaction with G37
3.4. The Interaction with G36
3.5. Anticodon-Branch Recognition and Detection of Position 37
3.6. Structural Changes of TrmD upon AdoMet Accommodation
3.7. Enzymatic Cycle of TrmD
- AdoMet binding: In apo TrmD, the closed and open conformations of the cover loop form the “tight-knot” and “loose-knot” states, respectively, and AdoMet can be accommodated only in the “loose-knot” state. Subsequently, the bound AdoMet is stabilized by Phe171, which also allows the CTD to assume the tRNA binding form.
- tRNA binding: Following AdoMet binding, TrmD starts searching for position 37 in the substrate tRNA. Considering the steric hindrance, we propose that TrmD first binds to the phosphate groups in the anticodon stem. Then, TrmD and the tRNA anticodon loop mutually change their conformations, and finally, Gly59 captures the phosphate group at position 38. This Gly59–position 38 interaction flips the base moiety of position 37 into the catalytic pocket. TrmD then binds tightly to the tRNA when position 37 is guanosine. In the anticodon loop with G37 trapped, TrmD further recognizes G36 in a guanosine-specific manner. After the recognition of both G36 and G37 by TrmD, the anticodon loop structure adopts the tight form, which provides space for the TrmD interdomain helix to fold above G37.
- Methyl transfer: At this point, the substrate tRNA is tightly trapped by TrmD, and the arrangements of the AdoMet, G37, and the residues composing the catalytic site are ready for the reaction. The methyl moiety of AdoMet is transferred to the N1-atom of G37, producing the m1G37-modified tRNA and the byproduct, AdoHcy. Upon the collapse of the interdomain helix and the opening of the cover loop, the reaction products are released from TrmD, which makes TrmD ready for the next reaction.
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Czerwoniec, A.; Dunin-Horkawicz, S.; Purta, E.; Kaminska, K.H.; Kasprzak, J.M.; Bujnicki, J.M.; Grosjean, H.; Rother, K. Modomics: A database of RNA modification pathways. 2008 update. Nucleic Acids Res. 2009, 37, D118–D121. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, S.; Nishimura, S. Modified nucleotides and codon recognition. In tRNA: Structure, Biosynthesis, and Function; Söll, D., Rajbhandary, U.L., Eds.; ASM Press: Washington, DC, USA, 1995; pp. 207–223. [Google Scholar]
- Bjork, G.R. Biosynthesis and function of modified nucleotides. In tRNA: Structure, Biosynthesis, and Function; Söll, D., Rajbhandary, U.L., Eds.; ASM Press: Washington, DC, USA, 1995; pp. 165–205. [Google Scholar]
- Helm, M. Post-transcriptional nucleotide modification and alternative folding of RNA. Nucleic Acids Res. 2006, 34, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Bjork, G.R.; Wikstrom, P.M.; Bystrom, A.S. Prevention of translational frameshifting by the modified nucleoside 1-methylguanosine. Science 1989, 244, 986–989. [Google Scholar] [CrossRef] [PubMed]
- Hagervall, T.G.; Tuohy, T.M.; Atkins, J.F.; Bjork, G.R. Deficiency of 1-methylguanosine in tRNA from Salmonella typhimurium induces frameshifting by quadruplet translocation. J. Mol. Biol. 1993, 232, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Gamper, H.B.; Masuda, I.; Frenkel-Morgenstern, M.; Hou, Y.M. Maintenance of protein synthesis reading frame by EF-P and m1G37-tRNA. Nat. Commun. 2015, 6, 7226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.M.; Liu, C.; Slater, S.; Hou, Y.M. Aminoacylation of tRNA with phosphoserine for synthesis of cysteinyl-tRNAcys. Nat. Struct. Mol. Biol. 2008, 15, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Hauenstein, S.I.; Perona, J.J. Redundant synthesis of cysteinyl-tRNAcys in Methanosarcina mazei. J. Biol. Chem. 2008, 283, 22007–22017. [Google Scholar] [CrossRef] [PubMed]
- Perret, V.; Garcia, A.; Grosjean, H.; Ebel, J.P.; Florentz, C.; Giege, R. Relaxation of a transfer RNA specificity by removal of modified nucleotides. Nature 1990, 344, 787–789. [Google Scholar] [CrossRef] [PubMed]
- Bystrom, A.S.; Bjork, G.R. Chromosomal location and cloning of the gene (trmD) responsible for the synthesis of tRNA m1G methyltransferase in Escherichia coli k-12. Mol. Gen. Genet. MGG 1982, 188, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Urbonavicius, J.; Qian, Q.; Durand, J.M.; Hagervall, T.G.; Bjork, G.R. Improvement of reading frame maintenance is a common function for several tRNA modifications. EMBO J. 2001, 20, 4863–4873. [Google Scholar] [CrossRef] [PubMed]
- Persson, B.C.; Bylund, G.O.; Berg, D.E.; Wikstrom, P.M. Functional analysis of the ffh-trmD region of the Escherichia coli chromosome by using reverse genetics. J. Bacteriol. 1995, 177, 5554–5560. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Esberg, B.; Curran, J.F.; Bjork, G.R. Three modified nucleosides present in the anticodon stem and loop influence the in vivo aa-tRNA selection in a tRNA-dependent manner. J. Mol. Biol. 1997, 271, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Brule, H.; Elliott, M.; Redlak, M.; Zehner, Z.E.; Holmes, W.M. Isolation and characterization of the human tRNA-(N1G37) methyltransferase (TRM5) and comparison to the Escherichia coli TrmD protein. Biochemistry 2004, 43, 9243–9255. [Google Scholar] [CrossRef] [PubMed]
- Christian, T.; Hou, Y.M. Distinct determinants of tRNA recognition by the TrmD and Trm5 methyl transferases. J. Mol. Biol. 2007, 373, 623–632. [Google Scholar] [CrossRef] [PubMed]
- De Crecy-Lagard, V.; Brochier-Armanet, C.; Urbonavicius, J.; Fernandez, B.; Phillips, G.; Lyons, B.; Noma, A.; Alvarez, S.; Droogmans, L.; Armengaud, J.; et al. Biosynthesis of wyosine derivatives in tRNA: An ancient and highly diverse pathway in archaea. Mol. Biol. Evol. 2010, 27, 2062–2077. [Google Scholar] [CrossRef] [PubMed]
- Goto-Ito, S.; Ito, T.; Ishii, R.; Muto, Y.; Bessho, Y.; Yokoyama, S. Crystal structure of archaeal tRNA(m1G37)methyltransferase aTrm5. Proteins 2008, 72, 1274–1289. [Google Scholar] [CrossRef] [PubMed]
- Umitsu, M.; Nishimasu, H.; Noma, A.; Suzuki, T.; Ishitani, R.; Nureki, O. Structural basis of adomet-dependent aminocarboxypropyl transfer reaction catalyzed by tRNA-wybutosine synthesizing enzyme, TYW2. Proc. Natl. Acad. Sci. USA 2009, 106, 15616–15621. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Jia, Q.; Chen, R.; Wei, Y.; Li, J.; Ma, J.; Xie, W. Crystal structures of the bifunctional tRNA methyltransferase Trm5a. Sci. Rep. 2016, 6, 33553. [Google Scholar] [CrossRef] [PubMed]
- Goto-Ito, S.; Ito, T.; Kuratani, M.; Bessho, Y.; Yokoyama, S. Tertiary structure checkpoint at anticodon loop modification in tRNA functional maturation. Nat. Struct. Mol. Biol. 2009, 16, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Matsuo, M.; Tanaka, S.; Akimoto, H.; Asahi, S.; Nishimura, S.; Katze, J.R.; Hashizume, T.; Crain, P.F.; McCloskey, J.A.; et al. Biosynthesis of archaeosine, a novel derivative of 7-deazaguanosine specific to archaeal tRNA, proceeds via a pathway involving base replacement on the tRNA polynucleotide chain. J. Biol. Chem. 1997, 272, 20146–20151. [Google Scholar] [CrossRef] [PubMed]
- Ishitani, R.; Nureki, O.; Nameki, N.; Okada, N.; Nishimura, S.; Yokoyama, S. Alternative tertiary structure of tRNA for recognition by a posttranscriptional modification enzyme. Cell 2003, 113, 383–394. [Google Scholar] [CrossRef]
- Hall, K.B.; Sampson, J.R.; Uhlenbeck, O.C.; Redfield, A.G. Structure of an unmodified tRNA molecule. Biochemistry 1989, 28, 5794–5801. [Google Scholar] [CrossRef] [PubMed]
- Derrick, W.B.; Horowitz, J. Probing structural differences between native and in vitro transcribed Escherichia coli valine transfer RNA: Evidence for stable base modification-dependent conformers. Nucleic Acids Res. 1993, 21, 4948–4953. [Google Scholar] [CrossRef] [PubMed]
- Perret, V.; Garcia, A.; Puglisi, J.; Grosjean, H.; Ebel, J.P.; Florentz, C.; Giege, R. Conformation in solution of yeast tRNAAsp transcripts deprived of modified nucleotides. Biochimie 1990, 72, 735–743. [Google Scholar] [CrossRef]
- Ahn, H.J.; Kim, H.W.; Yoon, H.J.; Lee, B.I.; Suh, S.W.; Yang, J.K. Crystal structure of tRNA(m1G37)methyltransferase: Insights into tRNA recognition. EMBO J. 2003, 22, 2593–2603. [Google Scholar] [CrossRef] [PubMed]
- Elkins, P.A.; Watts, J.M.; Zalacain, M.; van Thiel, A.; Vitazka, P.R.; Redlak, M.; Andraos-Selim, C.; Rastinejad, F.; Holmes, W.M. Insights into catalysis by a knotted TrmD tRNA methyltransferase. J. Mol. Biol. 2003, 333, 931–949. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Masuda, I.; Yoshida, K.; Goto-Ito, S.; Sekine, S.; Suh, S.W.; Hou, Y.M.; Yokoyama, S. Structural basis for methyl-donor-dependent and sequence-specific binding to tRNA substrates by knotted methyltransferase TrmD. Proc. Natl. Acad. Sci. USA 2015, 112, E4197–E4205. [Google Scholar] [CrossRef] [PubMed]
- Christian, T.; Lahoud, G.; Liu, C.; Hou, Y.M. Control of catalytic cycle by a pair of analogous tRNA modification enzymes. J.Mol. Biol. 2010, 400, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Lahoud, G.; Goto-Ito, S.; Yoshida, K.; Ito, T.; Yokoyama, S.; Hou, Y.M. Differentiating analogous tRNA methyltransferases by fragments of the methyl donor. RNA 2011, 17, 1236–1246. [Google Scholar] [CrossRef] [PubMed]
| Trm5 | TrmD | |
|---|---|---|
| Reaction catalyzed | N1-methylation of tRNA G37 | N1-methylation of tRNA G37 |
| Organisms | Eukaryotes, Archaea | Bacteria |
| MTase class | Class-I | Class-IV |
| Protein fold | Rossmann fold, monomer | Deep-trefoil knot, dimer |
| Cofactor | AdoMet | AdoMet |
| Substrate requirement | L-shaped tRNA with G37 | tRNA anticodon stem loop with G36G37 and D stem |
| Stoichiometry | 1 tRNA/1 Trm5 | 1 tRNA/2 TrmD |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goto-Ito, S.; Ito, T.; Yokoyama, S. Trm5 and TrmD: Two Enzymes from Distinct Origins Catalyze the Identical tRNA Modification, m1G37. Biomolecules 2017, 7, 32. https://doi.org/10.3390/biom7010032
Goto-Ito S, Ito T, Yokoyama S. Trm5 and TrmD: Two Enzymes from Distinct Origins Catalyze the Identical tRNA Modification, m1G37. Biomolecules. 2017; 7(1):32. https://doi.org/10.3390/biom7010032
Chicago/Turabian StyleGoto-Ito, Sakurako, Takuhiro Ito, and Shigeyuki Yokoyama. 2017. "Trm5 and TrmD: Two Enzymes from Distinct Origins Catalyze the Identical tRNA Modification, m1G37" Biomolecules 7, no. 1: 32. https://doi.org/10.3390/biom7010032






