Microbial Transformation of Bioactive Compounds and Production of ortho-Dihydroxyisoflavones and Glycitein from Natural Fermented Soybean Paste
Abstract
:1. Introduction
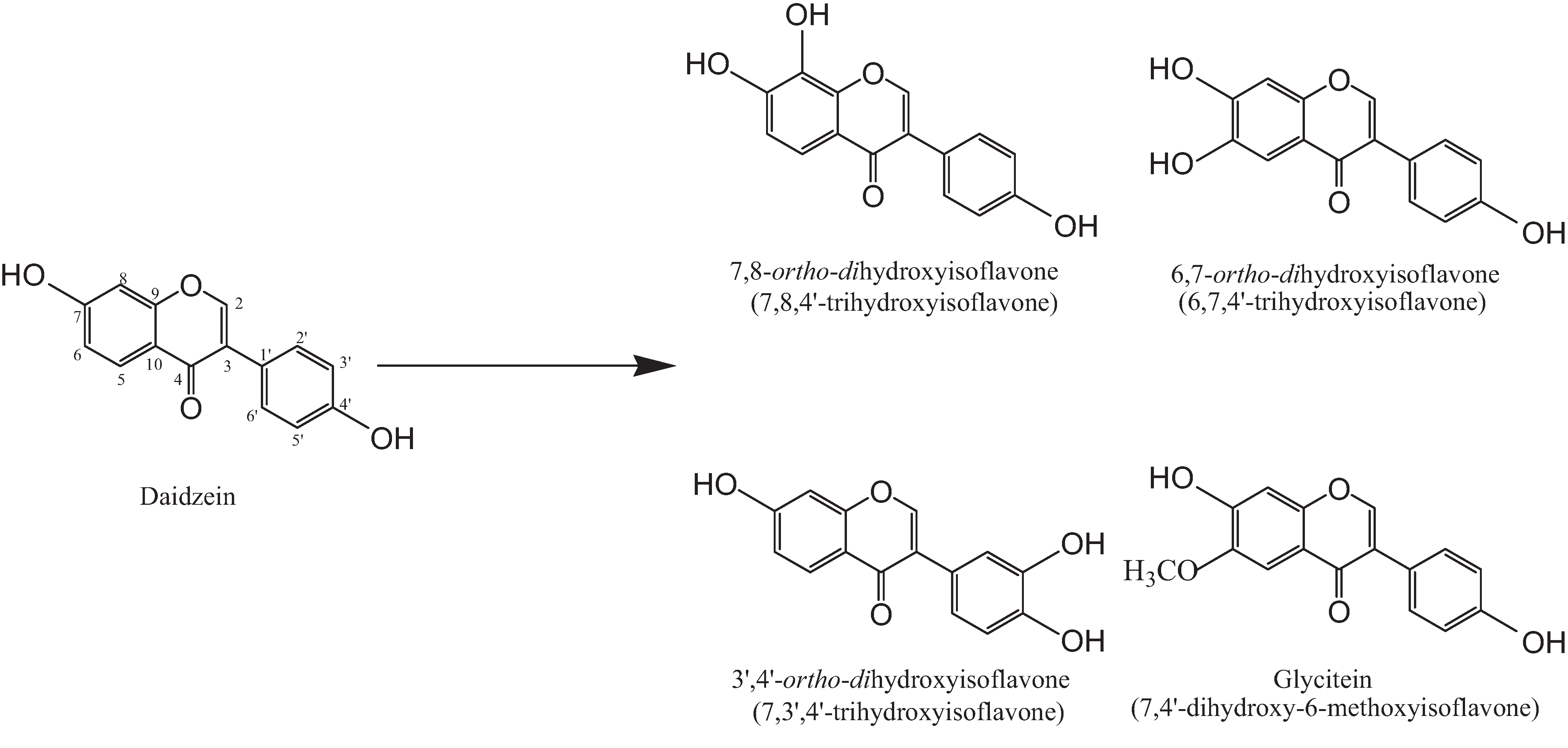
2. Results and Discussion
2.1. Soybean Doenjang and Amounts of ortho-Dihydroxyisoflavones Due to Fermentation Period
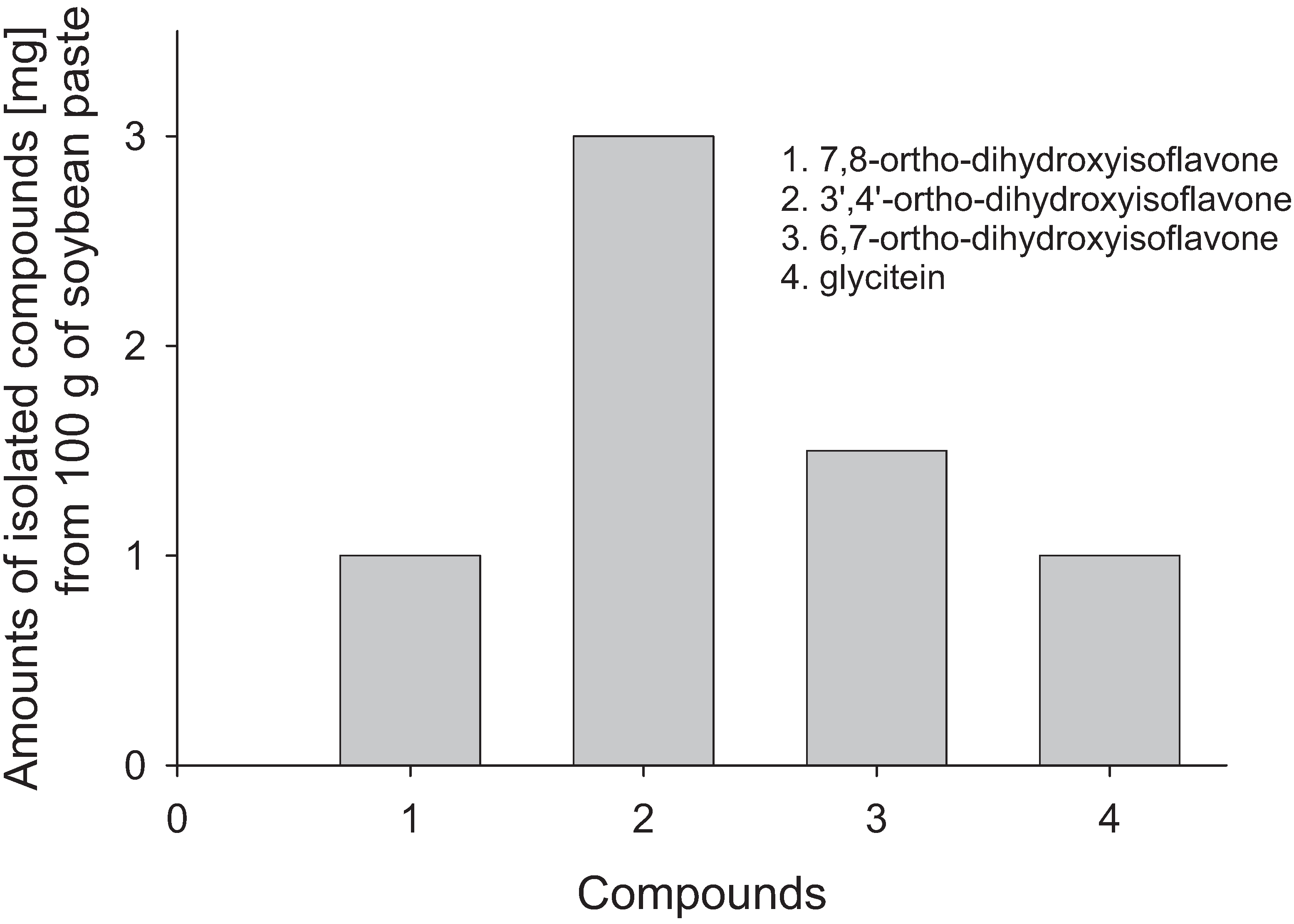
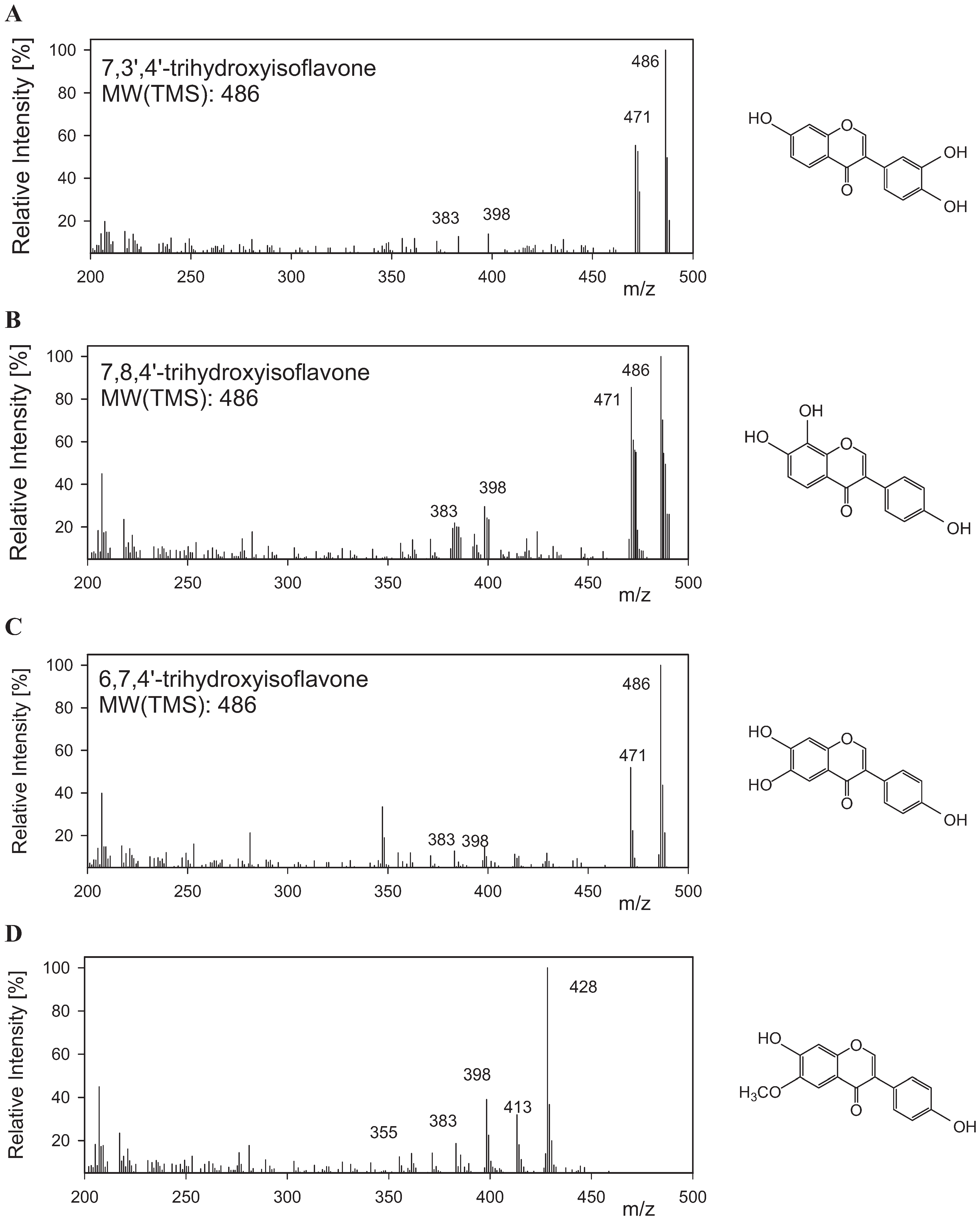
2.2. Isolation and Analysis of Isoflavone Using Microorganism Screened from Soybean Doenjang
| Compound | RT in GC (min) | M+, GC/MS TMS Derivatives | TMS Substitution Pattern |
|---|---|---|---|
| Daidzein | 19.5 | 398 | OH-group in position C7 (A-ring) and C4' (B-ring) |
| 3',4'-ortho-dihydroxyisoflavone | 24.1 | 486 | OH-group in position C7 (A-ring), C3' and C4' (B-ring) |
| 7,8-ortho-dihydroxyisoflavone | 25.1 | 486 | OH-group in position C7, C8 (A-ring) and C4' (B-ring) |
| 6,7-ortho-dihydroxyisoflavone | 26.7 | 486 | OH-group in position C6, C7 (A-ring) and C4' (B-ring) |
| Glycitein | 24.7 | 428 | OH-group in position C7 (A-ring) and C4' (B-ring) |

2.3. Strain Screening Strategy from Soybean Doenjang
2.4. Biotransformation of Isoflavone Using Microorganism Screened Soybean Doenjang and Identification of Reaction Products
3. Experimental
3.1. Chemicals
3.2. HPLC, NMR, GC-MS Analysis for Isolation
3.3. Plate Assay Strategy
3.4. Cultivation and Isolation of Product from Reaction
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Lee, S.S. Meju fermentation for a raw material of korean traditional soy products. Korea J. Mycol. 1995, 23, 161–175. [Google Scholar]
- Kim, D.H.; Kim, S.H.; Choi, N.S.; Bai, S.; Chun, S.B. Biochemical characteristics of whole soybean cereals fermented with Aspergillus strains. Korea J. Appl. Microbiol. Biotechnol. 1998, 26, 551–557. [Google Scholar]
- Chung, D.H.; Shim, S.K. Soybean Fermentation Food; Ponds of intelligence Co.: Seoul, Korea, 1994. [Google Scholar]
- Kwon, T.W.; Song, Y.S.; Kim, J.S.; Moon, G.S.; Kim, J.I.; Hong, J.H. Current research on the bioactive functions of soyfoods in Korea. Korea Soybean Digest. 1998, 15, 1–12. [Google Scholar]
- Pratt, D.E.; Birac, P.M. Sources of antioxidant activity of soybeans and soy products. J. Food Sci. 1979, 44, 1720–1725. [Google Scholar] [CrossRef]
- Coward, L.; Barnes, N.C.; Setchell, K.D.R.; Barnes, S. Genistein, daidzein, and their beta-glycoside conjugates: Antitumor isoflavones in soybean foods form American and Asian diets. J. Agric. Food Chem. 1993, 41, 1961–1967. [Google Scholar] [CrossRef]
- Wang, H.; Murphy, P. Isoflavone content in commercial soybean foods. J. Agri. Food Chem. 1994, 42, 1666–1671. [Google Scholar] [CrossRef]
- Eldridge, A.C.; Kwolek, W.F. Soybean isoflavones: Effect of environment and variety on composition. J. Agri. Food Chem. 1983, 31, 394–401. [Google Scholar] [CrossRef]
- Dixon, R.A. Phytoestrogens. Annu. Rev. Plant Biol. 2004, 55, 225–261. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Kwon, T.W.; Kim, J.S. Isoflavone contents in some varieties of soybean. Foods Biotech. 1996, 5, 167–169. [Google Scholar]
- Foti, P.; Erba, D.; Riso, P.; Spadafranca, A.; Criscuoli, F.; Testolin, G. Comparison between daidzein and genistein antioxidant activity in primary and cancer lymphocytes. Arch. Biochem. Biophy. 2005, 33, 421–427. [Google Scholar] [CrossRef]
- Kim, H.W.; Ko, E.J.; Ha, S.D.; Song, K.B.; Park, S.K.; Chung, D.H.; Youn, K.S.; Bae, D.H. Physical, mechanical, and antimicrobial properties of edible film produced from defatted soybean meal fermented by Bacillus subtilis. J. Microbiol. Biotechnol. 2005, 15, 815–822. [Google Scholar]
- Lim, J.S.; Jang, C.H.; Lee, I.A.; Kim, H.J.; Lee, C.H.; Kim, J.H.; Park, C.S.; Kwon, D.Y.; Lim, J.; Hwang, Y.H.; et al. Biotransformation of free isoflavones by Bacillus species isolated from traditional cheonggukjang. Food Sci. Biotech. 2009, 18, 1046–1050. [Google Scholar]
- 1Klus, K.; Barz, W. Formation of polyhydroxylated isoflavones from the soybean seed isoflavones daidzein and glycitein by bacteria isolated from tempe. Arch. Microbiol. 1995, 164, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Kulling, S.E.; Honig, D.M.; Metzler, M. Oxidative metabolism of the soy isoflavones daidzein and genistein in humans in vitro and in vivo. J. Agric. Food Chem. 2001, 49, 3024–3033. [Google Scholar] [CrossRef]
- Rufer, C.E.; Kulling, S.E. Antioxidant activity of isoflavones and their major metabolites using different in vitro assays. J. Agric. Food Chem. 2006, 54, 2926–2931. [Google Scholar] [CrossRef]
- Fujita, T.; Funako, T.; Hayashi, H. 8-hydroxydaidzein, an aldose reductase inhibitor from Okara fermented with Aspergillus sp. HK-388. Biosci. Biotechnol. Biochem. 2004, 68, 1588–1590. [Google Scholar] [CrossRef]
- Chen, Y.C.; Sugiyama, Y.; Abe, N.; Kuruto-Niwa, R.; Nozawa, R.; Hirota, A. DPPH radical-scavenging compounds from dou-chi, a soybean fermented food. Biosci. Biotechnol. Biochem. 2005, 69, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the all compounds used in this work are available from the author.
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roh, C. Microbial Transformation of Bioactive Compounds and Production of ortho-Dihydroxyisoflavones and Glycitein from Natural Fermented Soybean Paste. Biomolecules 2014, 4, 1093-1101. https://doi.org/10.3390/biom4041093
Roh C. Microbial Transformation of Bioactive Compounds and Production of ortho-Dihydroxyisoflavones and Glycitein from Natural Fermented Soybean Paste. Biomolecules. 2014; 4(4):1093-1101. https://doi.org/10.3390/biom4041093
Chicago/Turabian StyleRoh, Changhyun. 2014. "Microbial Transformation of Bioactive Compounds and Production of ortho-Dihydroxyisoflavones and Glycitein from Natural Fermented Soybean Paste" Biomolecules 4, no. 4: 1093-1101. https://doi.org/10.3390/biom4041093




