Untargeted Metabolomics Analysis of Eggplant (Solanum melongena L.) Fruit and Its Correlation to Fruit Morphologies
Abstract
:1. Introduction
2. Results
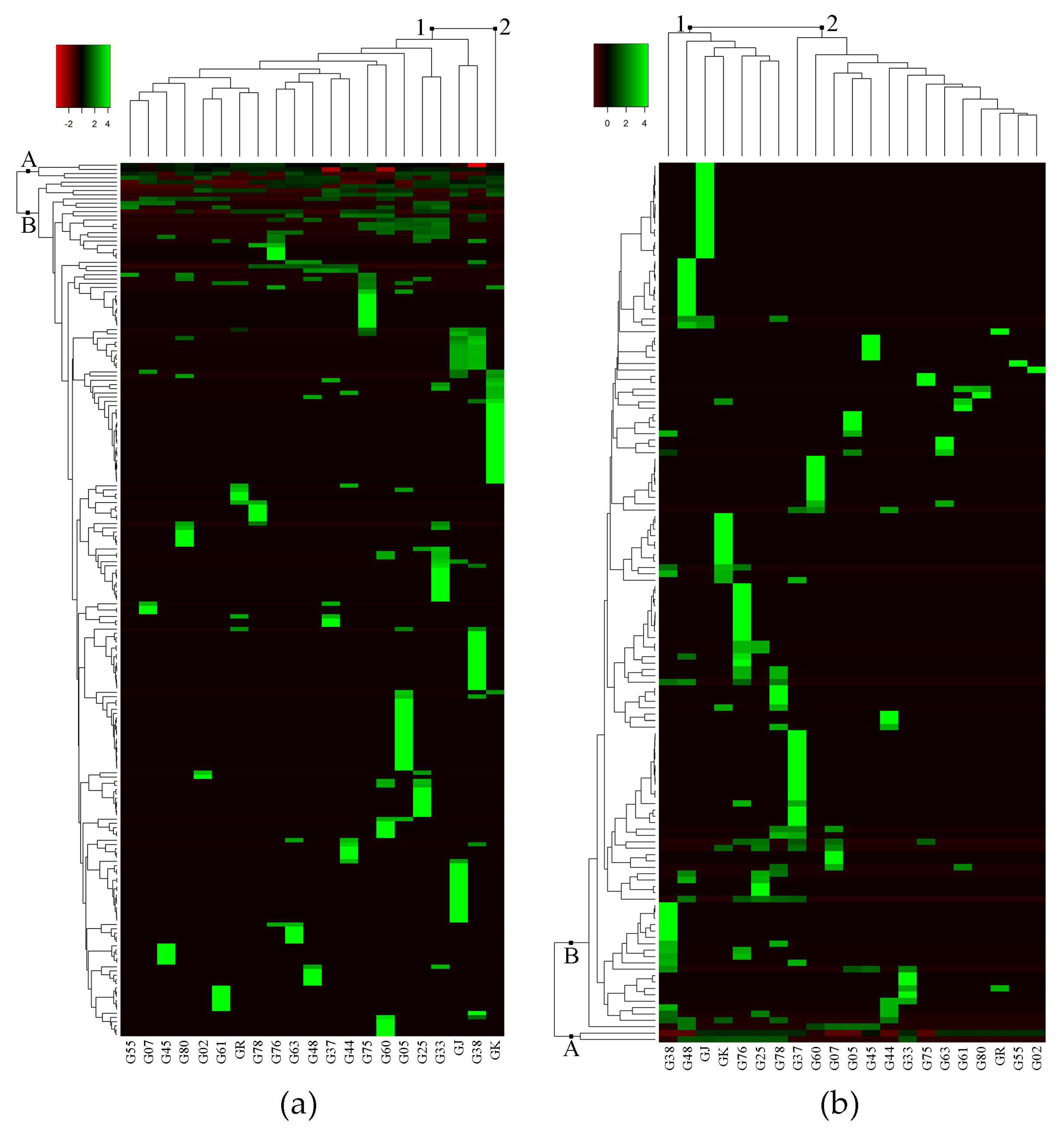
2.1. Untargeted Metabolomics Analysis
2.2. Correlation Analysis of Fruit Metabolites with Fruit Morphology
3. Discussion
3.1. Untargeted Metabolomics Analysis Revealed Unique Metabolites Present in Different Accessions
3.2. Several Metabolites Are Accession-Specific
3.3. Correlation Analysis Shows Relationship between Fruit Morphologies and Metabolites
4. Materials and Methods
4.1. Plant Materials
4.2. Extraction and Sample Preparation
4.3. LC-MS Analysis
4.4. GC-MS Analysis
4.5. Putative Identification of Metabolites
4.6. Fruit Morphology Evaluation
4.7. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Weese, T.L.; Bohs, L. Eggplant origins: Out of Africa, into the Orient. Taxon 2010, 59, 49–56. [Google Scholar] [CrossRef]
- Knapp, S.; Vorontsova, M.S.; Prohens, J. Wild Relatives of the Eggplant (Solanum melongena L.: Solanaceae): New Understanding of Species Names in a Complex Group. PLoS ONE 2013, 8, e57039. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 8 January 2018).
- Zhang, Y.; Hu, Z.; Chu, G.; Huang, C.; Tian, S.; Zhao, Z.; Chen, G. Anthocyanin Accumulation and Molecular Analysis of Anthocyanin Biosynthesis-Associated Genes in Eggplant (Solanum melongena L.). J. Agric. Food Chem. 2014, 62, 2906–2912. [Google Scholar] [CrossRef] [PubMed]
- Hall, R.D. Plant metabolomics: From holistic hope, to hype, to hot topic. New Phytol. 2006, 169, 453–468. [Google Scholar] [CrossRef] [PubMed]
- De Vos, R.C.; Moco, S.; Lommen, A.; Keurentjes, J.J.; Bino, R.J.; Hall, R.D. Untargeted large-scale plant metabolomics using liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2007, 2, 778–791. [Google Scholar] [CrossRef] [PubMed]
- Arbona, V.; Iglesias, D.J.; Talón, M.; Gómez-Cadenas, A. Plant Phenotype Demarcation Using Nontargeted LC-MS and GC-MS Metabolite Profiling. J. Agric. Food Chem. 2009, 57, 7338–7347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tikunov, Y. A Novel Approach for Nontargeted Data Analysis for Metabolomics. Large-Scale Profiling of Tomato Fruit Volatiles. Plant Physiol. 2005, 139, 1125–1137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farag, M.A.; Mohsen, M.; Heinke, R.; Wessjohann, L.A. Metabolomic fingerprints of 21 date palm fruit varieties from Egypt using UPLC/PDA/ESI–qTOF-MS and GC–MS analyzed by chemometrics. Food Res. Int. 2014, 64, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Gad, H.A.; Heiss, A.G.; Wessjohann, L.A. Metabolomics driven analysis of six Nigella species seeds via UPLC-qTOF-MS and GC–MS coupled to chemometrics. Food Chem. 2014, 151, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.-Y.; Choi, W.; Park, J.H.; Lim, J.; Kwon, S.W. Determination of coffee origins by integrated metabolomic approach of combining multiple analytical data. Food Chem. 2010, 121, 1260–1268. [Google Scholar] [CrossRef]
- Jumhawan, U.; Putri, S.P.; Yusianto; Marwani, E.; Bamba, T.; Fukusaki, E. Selection of Discriminant Markers for Authentication of Asian Palm Civet Coffee (Kopi Luwak): A Metabolomics Approach. J. Agric. Food Chem. 2013, 61, 7994–8001. [Google Scholar] [CrossRef] [PubMed]
- Foxman, B.; Srinivasan, U.; Wen, A.; Zhang, L.; Marrs, C.F.; Goldberg, D.; Weyant, R.; McNeil, D.; Crout, R.; Marazita, M. Exploring the effect of dentition, dental decay and familiality on oral health using metabolomics. Infect. Genet. Evol. 2014, 22, 201–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Souza, D.P.; Saunders, E.C.; McConville, M.J.; Likic, V.A. Progressive peak clustering in GC-MS Metabolomic experiments applied to Leishmania parasites. Bioinformatics 2006, 22, 1391–1396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MassBank|High Quality Mass Spectral Database. Available online: http://www.massbank.jp/?lang=en (accessed on 9 January 2018).
- Plant Metabolic Pathway Databases|Plant Metabolic Network. Available online: https://www.plantcyc.org/ (accessed on 9 January 2018).
- Verhoeven, H.A.; de Vos, C.H.R.; Bino, R.J.; Hall, R.D. Plant Metabolomics Strategies Based upon Quadrupole Time of Flight Mass Spectrometry (QTOF-MS). In Plant Metabolomics; Biotechnology in Agriculture and Forestry; Springer: Berlin/Heidelberg, Germany, 2006; pp. 33–48. ISBN 978-3-540-29781-9. [Google Scholar]
- 2,6,10-TRIMETHYL-2,6,10-PENTADECATRIEN-14-ONE. WHO|JECFA. Available online: http://apps.who.int/food-additives-contaminants-jecfa-database/chemical.aspx?chemID=4689 (accessed on 8 January 2018).
- CITRONELLYL FORMATE. WHO|JECFA. Available online: http://apps.who.int/food-additives-contaminants-jecfa-database/chemical.aspx?chemID=4012 (accessed on 8 January 2018).
- FURFURYL ALCOHOL. WHO|JECFA. Available online: http://apps.who.int/food-additives-contaminants-jecfa-database/chemical.aspx?chemID=1781 (accessed on 8 January 2018).
- Gunstone, F.D.; Harwood, J.L.; Dijkstra, A.J. The Lipid Handbook with CD-ROM, 3rd ed.; Taylor & Francis: Oxford, UK, 2007; ISBN 978-0-8493-9688-5. [Google Scholar]
- Letawe, C.; Boone, M.; Piérard, G.E. Digital image analysis of the effect of topically applied linoleic acid on acne microcomedones. Clin. Exp. Dermatol. 1998, 23, 56–58. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, H.M.; New, S.A.; Golden, M.H.; Campbell, M.K.; Reid, D.M. Nutritional associations with bone loss during the menopausal transition: Evidence of a beneficial effect of calcium, alcohol, and fruit and vegetable nutrients and of a detrimental effect of fatty acids. Am. J. Clin. Nutr. 2004, 79, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Bonaventure, G. Disruption of the FATB Gene in Arabidopsis Demonstrates an Essential Role of Saturated Fatty Acids in Plant Growth. Plant Cell Online 2003, 15, 1020–1033. [Google Scholar] [CrossRef] [Green Version]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant Capacity of Tea and Common Vegetables. J. Agric. Food Chem. 1996, 44, 3426–3431. [Google Scholar] [CrossRef]
- Stojanovic, G.; Palic, R.; Alagic, S.; Zeković, Z. Chemical composition and antimicrobial activity of the essential oil and CO2 extracts of semi-oriental tobacco, Otlja. Flavour Fragr. J. 2000, 15, 335–338. [Google Scholar] [CrossRef]
- Dixon, R.A. Natural products and plant disease resistance. Nature 2001, 411, 843. [Google Scholar] [CrossRef] [PubMed]
- 2-(METHYLTHIOMETHYL)-3-PHENYLPROPENAL. WHO|JECFA. Available online: http://apps.who.int/food-additives-contaminants-jecfa-database/chemical.aspx?chemID=1145 (accessed on 8 January 2018).
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; He, S.; Shoemaker, B.A.; et al. PubChem Substance and Compound databases. Nucleic Acids Res. 2016, 44, D1202–D1213. [Google Scholar] [CrossRef] [PubMed]
- ChemSpider|Search and Share Chemistry. Available online: http://www.chemspider.com/ (accessed on 27 August 2018).
- Gould, H.J.; Mayor, J.G. Alternative Seed Treatments to Dieldrin for the Control of Bean Seed Fly (Delia spp.). Plant Pathol. 1975, 24, 245–246. [Google Scholar] [CrossRef]
- Yan, N.; Liu, Y.; Gong, D.; Du, Y.; Zhang, H.; Zhang, Z. Solanesol: A review of its resources, derivatives, bioactivities, medicinal applications, and biosynthesis. Phytochem. Rev. 2015, 14, 403–417. [Google Scholar] [CrossRef]
- Keurentjes, J.J.B.; Fu, J.; de Vos, C.H.R.; Lommen, A.; Hall, R.D.; Bino, R.J.; van der Plas, L.H.W.; Jansen, R.C.; Vreugdenhil, D.; Koornneef, M. The genetics of plant metabolism. Nat. Genet. 2006, 38, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Fiehn, O. Metabolomics—The link between genotypes and phenotypes. Plant Mol. Biol. 2002, 48, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Wahyuni, Y.; Ballester, A.-R.; Sudarmonowati, E.; Bino, R.J.; Bovy, A.G. Metabolite biodiversity in pepper (Capsicum) fruits of thirty-two diverse accessions: Variation in health-related compounds and implications for breeding. Phytochemistry 2011, 72, 1358–1370. [Google Scholar] [CrossRef] [PubMed]
- Uri, C.; Juhász, Z.; Polgár, Z.; Bánfalvi, Z. A GC–MS-based metabolomics study on the tubers of commercial potato cultivars upon storage. Food Chem. 2014, 159, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Shi, C.; Gao, P.; Yuan, K.; Yang, D.; Lu, X.; Xu, G. Phenotype differentiation of three E. coli strains by GC-FID and GC–MS based metabolomics. J. Chromatogr. B 2008, 871, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-X.; Gao, T.-G.; Knapp, S. Ancient Chinese Literature Reveals Pathways of Eggplant Domestication. Ann. Bot. 2008, 102, 891–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cericola, F.; Portis, E.; Toppino, L.; Barchi, L.; Acciarri, N.; Ciriaci, T.; Sala, T.; Rotino, G.L.; Lanteri, S. The Population Structure and Diversity of Eggplant from Asia and the Mediterranean Basin. PLoS ONE 2013, 8, e73702. [Google Scholar] [CrossRef] [PubMed]
- Doganlar, S.; Frary, A.; Daunay, M.-C.; Lester, R.N.; Tanksley, S.D. Conservation of gene function in the solanaceae as revealed by comparative mapping of domestication traits in eggplant. Genetics 2002, 161, 1713–1726. [Google Scholar] [PubMed]
- Shen, G.; Kiem, P.V.; Cai, X.-F.; Li, G.; Dat, N.T.; Choi, Y.A.; Lee, Y.M.; Park, Y.K.; Kim, Y.H. Solanoflavone, a new biflavonol glycoside from Solanum melongena: Seeking for anti-inflammatory components. Arch. Pharm. Res. 2005, 28, 657–659. [Google Scholar] [CrossRef] [PubMed]
- Akanitapichat, P.; Phraibung, K.; Nuchklang, K.; Prompitakkul, S. Antioxidant and hepatoprotective activities of five eggplant varieties. Food Chem. Toxicol. 2010, 48, 3017–3021. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Heiling, S.; Baldwin, I.T.; Gaquerel, E. Illuminating a plant’s tissue-specific metabolic diversity using computational metabolomics and information theory. Proc. Natl. Acad. Sci. USA 2016, 113, E7610–E7618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Putri, S.P.; Fukusaki, E. Mass Spectrometry-Based Metabolomics: A Practical Guide; CRC Press: Boca Raton, FL, USA, 2016; ISBN 978-1-4822-2377-4. [Google Scholar]
- Hurtado, C.; Parastar, H.; Matamoros, V.; Piña, B.; Tauler, R.; Bayona, J.M. Linking the morphological and metabolomic response of Lactuca sativa L exposed to emerging contaminants using GC × GC-MS and chemometric tools. Sci. Rep. 2017, 7, 6546. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.; Wetzstein, H.Y. Effects of Drying and Extraction Conditions on the Biochemical Activity of Selected Herbs. HortScience 2011, 46, 70–73. [Google Scholar]
- MZmine 2. Available online: http://mzmine.github.io/ (accessed on 9 January 2018).
- The International Union for the Protection of New Varieties of Plants (UPOV). Guidelines for the Conduct of Tests for Distinctness, Uniformity and Stability; UPOV Press: Geneva, Switzerland, 2002. [Google Scholar]
- R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 9 January 2018).
- Alysha, M.D.L.; Jairus, B.B. Metabolomics: Analysis of Metabolomics Data. R Package Version 0.1.4. 2014. Available online: http://cran.r-project.org/package=metabolomics/index.html (accessed on 9 January 2018).
- Wei, T.; Simko, V.; Levy, M.; Xie, Y.; Jin, Y.; Zemla, J. Corrplot: Visualization of a Correlation Matrix. R Package Version 0.84. 2017. Available online: https://cran.r-project.org/web/packages/corrplot/index.html (accessed on 9 January 2018).

| Name | Formula | Class | Accessions |
|---|---|---|---|
| Linoleic acid | C18H32O2 | Saturated Fatty Acid | G02, G05, G07, G25, G33, G37, G44, G45, G48, G55, G60, G61, G63, G75, G76, G78, G80, GJ, GK, GR |
| Palmitic acid | C16H32O2 | Saturated Fatty Acid | G02, G05, G07, G25, G33, G38, G44, G45, G48, G55, G61, G63, G75, G76, G78, G80, GJ, GK, GR |
| α-Tocopherol (Vitamin E) | C29H50O2 | Vitamin | G07, G25, G33, G37, G38, G45, G48, G60, G63, G76, G78, G80, GJ, GK |
| Neophytadiene | C20H38 | Diterpenoid | G02, G05, G07, G33, G37, G38, G48, G55, G63, GK |
| Farnesyl acetone | C18H30O | Terpenoid | G02 |
| N,N-Dimethylethylamine | C14H11N | Amines | G05 |
| Hexanal | C6H12O | Alkaloid | G05 |
| 2-Hydroxyethylphosphine | C2H7OP | Flavonoid | G05 |
| (Dimethylamino)acetone | C5H11NO | Alkaloid | G05 |
| 4-Oxononanedioic acid | C9H14O5 | Terpen | G05 |
| Clionasterol | C29H50O | Triterpenoid | G05 |
| 7,11-Hexadecadienal | C16H28O | Pheromon | G05 |
| Ethyl iso-allocholate | C26H44O5 | Steroid | G07 |
| 1,1-Dibutylhydrazine | C8H20N2 | Skeletal formula | G25 |
| Citronellyl formate | C11H20O | Terpenoid | G25 |
| 13-Tetradecenal | C14H26O | Terpenoid | G25 |
| Ammonium oxalate, monohydrate | C2H8N2O4 | Terpenoid | G33 |
| 2-Propoxyethylamine | C5H13NO | Terpenoid | G33 |
| 2-Furanmethanol | C5H6O2 | Terpenoid | G33 |
| 4-Methyl-1,3-dioxane | C5H10O2 | Benzene and Subtituent Derivatives | G33 |
| Ethyl tetradecanoate | C16H32O2 | Unsaturated Fatty Acid | G33 |
| Methyl (9E,12Z)-9,12-octadecadienoate | C19H34O2 | Diterpenoid | G37 |
| Solanesol | C45H74O | Polyterpen | G37 |
| Butanoic acid, 3-hydroxy- | C4H8O3 | Hydroxy Acid and Derivatives | G38 |
| Methoxyethylamine | C3H9NO | Alkaloid | G38 |
| 1,4-Cyclohexanediol, trans- | C6H12O2 | Flavonoid | G38 |
| 3-Butenoic acid | C4H6O2 | Fatty Acids and Conjugate | G38 |
| Pentanal | C5H10O | Saturated Fatty Acid | G38 |
| E-9-Tetradecenal | C14H26O | Unsaturated Fatty Acid | G38 |
| Methyl palmitoleate | C17H32O2 | Fatty Acid Methyl Ester | G38 |
| Octadecyltrichlorosilane | C18H37Cl3Si | Organochlorosilane | G38 |
| 2,4-Dihydroxy-2,5-dimethyl-3(2H)-furan-3-one | C6H8O4 | Flavonoid (Ketone bodies) | G44 |
| Hentriacontane | C31H64 | Alkanes | G45 |
| Diisooctyl phthalate | C24H38O4 | Diterpenoid | G48 |
| 9,10-Dibromopentacosane | C25H50Br2 | Recolcinolic lipid | G48 |
| Hydroperoxide, 1-methylhexyl | C7H16O2 | Terpenoid | G60 |
| Eicosanoic acid | C20H40O2 | Saturated Fatty Acid | G60 |
| Octadecanoic acid | C18H36O2 | Saturated Fatty Acid | G60 |
| Ethyl 9-hexadecenoate | C18H34O2 | Unsaturated Fatty Acid | G60 |
| 2,2-Dideuteropropane | C3H8 | Hydrocarbon | G61 |
| Tetradecanal | C14H28O | Saturated Fatty Acid | G61 |
| Ethyl 9-heptadecenoate | C19H38O2 | Saturated Fatty Acid | G63 |
| Propanamide, N,N-dimethyl- | C5H9NO2 | Steroid | G75 |
| Cycloeicosane | C20H40 | Terpen | G75 |
| trans-Chrysanthemal | C10H16O | Natural pesticide | G75 |
| Z,E-3,13-Octadecadien-1-ol | C18H34O | Unsaturated Fatty Acid | G76 |
| 1-Propoxyoctane | C11H24O | Steroid | G78 |
| Cyclododecanone | C12H22O | Flavonoid (Ketone bodies) | G78 |
| Ketopinic Acid | C10H14O3 | Flavonoid | G80 |
| Propanedioic acid | C3H4O4 | Terpenoid | GJ |
| 3-Amino-2-oxazolidinone | C3H6N2O2 | Alkaloid | GJ |
| 1,3-Cyclopentenedione | C5H6O2 | Alkaloid | GJ |
| Trichloroacetic acid, undec-10-enyl ester | C13H21Cl3O2 | Trichloroacetic Acid | GJ |
| Cyclohexadecanone | C16H30O | Organohalogen compound | GJ |
| Oleyl alcohol, heptafluorobutyrate | C22H35F7O2 | Alkanes | GJ |
| Ethyl octadecanoate | C20H40O2 | Unsaturated Fatty Acid | GK |
| 10-HeptyL-10-Octylicosane | C35H72 | Terpen | GK |
| Pentacosane | C25H52 | Alkanes | GK |
| 1-Chloroheptacosane | C27H55Cl | Alkanes | GK |
| Triacontane | C30H62 | Alkanes | GK |
| 9-Hexacosene | C26H52 | Terpen | GK |
| Nonadecane | C19H40 | Terpen | GK |
| (9E,12E)-9,12-Octadecadienoyl chloride | C18H31ClO | Skeletal formula | GR |
| Name | Formula | Class | Accessions |
|---|---|---|---|
| 2,5-Bis(N-hexylmethylsilyl)thiophene | C18H36SSi2 | Terpen | G07, G25, G37, G76, G78 |
| 2-(Methylthiomethyl)-3-phenyl-2-propenal | C11H12OS | Terpenoid | G05, G33, G38, G45 |
| Boscalid | C18H12Cl2N2O | Alkaloids | G07, G25, G37, GK |
| Dimethisterone | C23H32O2 | Steroid | G25, G48, G78 |
| Glucolepidiin | C17H14Cl2N2O2 | Alkaloids | G48, G78, GJ |
| Methyl-2-alpha-l-fucopyranosyl-beta-d-galactoside | C13H24O10 | Glycoside | G25, G48 |
| L-dopachromate | C9H6NO4 | Terpenoid | G37, G38 |
| L-saccharopine | C11H19N2O6 | Alpha amino acids | G37, GK |
| Coumachlor | C19H15ClO4 | Steroid | G37, G78 |
| 5-Methoxytryptamine | C11H14N2O | Alkaloids | G38, G78 |
| Clopidol | C7H7Cl2NO | Alkaloids | G44, G60 |
| 4,4′-Ditolylthiourea | C15H16N2S | Steroid | G48, GJ |
| Octylbenzene | C14H22 | Terpenoid | G76, G78 |
| Cyclopentolate | C17H25NO3 | Steroid | G76, G78 |
| Trioxilin A3 | C20H33O5 | Steroid | G78, GK |
| Pyrazinemethanethiol | C5H6N2S | Monoterpene | G05 |
| 1-Diethoxyphosphoryl-4-hydroxy-nonan-2-one | C13H27O5P | Terpenoid | G05 |
| 1,2,4-Trithiolane | C2H4S3 | Steroid | G07 |
| 2-Chloro-1,4-naphthoquinone | C10H5ClO2 | Monoterpene | G37 |
| Methyl 6-O-galloyl-beta-d-glucopyranoside | C14H18O10 | Flavonoid | G37 |
| Propericiazine | C21H23N3OS | Alkaloids | G37 |
| N-Phenylacetylglutamic acid | C13H17NO5 | Steroid | G37 |
| Carprofen | C15H12ClNO2 | Steroid | G37 |
| 8-O-Methyloblongine | C20H26NO3 | Steroid | G37 |
| 5,5′-Methylenedi(2-para-tolylperhydropyrrolo(3,4-c)pyrrole-1,3-dione) | C27H28N4O4 | Alkaloids | G38 |
| 1-Deoxy-d-xylulose | C5H10O4 | Flavonoid | G38 |
| Cis-Zeatin | C10H13N5O | Alkaloids | G38 |
| 1-Hydroperoxy-8-carboxyoctyl-3,4-epoxynon-(2E)-enyl-ether | C12H18BNO2 | Alkaloids | G38 |
| Pentobarbital sodium | C11H17N2O3 | Alkaloids | G38 |
| Noladin Ether | C23H40O3 | Steroid | G45 |
| Lariciresinol | C20H24O6 | Monoterpene | G48 |
| 1,2-Bis(4-nitrophenyl)ethane | C14H12N2O4 | Stilbenoid | G48 |
| 1,2,4-Nonadecanetriol | C19H40O3 | Terpen | G55 |
| 1-Piperideine-6-carboxylate | C6H8NO2 | Alkaloids | G60 |
| 9-Chloro-10-hydroxy-octadecanoic acid | C18H35ClO3 | Alkaloids | G60 |
| Diphenidol | C21H27NO | Monoterpene | G61 |
| 1-Tetradecanoyl-glycero-3-phosphoserine | C20H40NO9P | Fatty acid | G63 |
| Ginkgolic acid | C22H34O3 | Terpenoid | G76 |
| Hexythiazox | C17H21ClN2O2S | Alkaloids | G76 |
| Atovaquone | C22H19ClO3 | Steroid | G76 |
| 5-Formiminotetrahydrofolate | C20H24N8O6 | Monoterpene | G76 |
| Butocarboxim | C7H14N2O2S | Alkaloids | G76 |
| Elaeocarpidine | C17H21N3 | Alkaloids | G76 |
| Mecarphon | C7H14NO4PS2 | Organophosphorus | G78 |
| Ethyl 18-bromooctadec-17-en-5,7,15-triynoate | C20H25BrO2 | Monoterpene | GJ |
| 2-Acetoxy-7-bromo-4-isopropyltropone | C12H13BrO3 | Terpen | GJ |
| N-(1-Deoxy-1-fructosyl)histidine | C12H19N3O7 | Steroid | GJ |
| Docosanoic acid | C22H44O2 | Terpen | GK |
| Leflunomide | C12H9F3N2O2 | Steroid | GK |
| Yohimbinic acid | C20H24N2O3 | Alkaloids | GK |
| Methyl aminolevulinate | C6H11NO3 | Alkaloids | GR |
| Code | Morphological Characteristics | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FL | Fruit: length (1) very short (<1 cm), (3) short (~ 2cm), (5) medium (~5 cm), (7) long (~10 cm), (9) very long (>20 cm) | ||||||||||||||||||||
| FD | Fruit: maximum diameter (1) very small (<1 cm), (3) small (~2 cm), (5) medium (~3 cm), (7) large (~5 cm), (9) very large (>10 cm) | ||||||||||||||||||||
| FLD | Fruit: ratio length/maximum diameter (1) very small, (3) small, (5) medium, (7) large, (9) very large | ||||||||||||||||||||
| GS | Fruit: general shape (1) globular, (2) ovoid, (3) obovate, (4) pear shaped, (5) club shaped, (6) ellipsoid, (7) cylindrical | ||||||||||||||||||||
| PTS | Fruit: size of pistil scar (1) very small, (3) small, (5) medium, (7) large, (9) very large | ||||||||||||||||||||
| APX | Fruit: apex (1) indented, (2) flattened, (3) rounded, (4) pointed | ||||||||||||||||||||
| DPS | Fruit: depth of indentation of pistil scar (1) absent or very shallow, (3) shallow, (5) medium, (7) deep, (9) very deep | ||||||||||||||||||||
| CVT | Only for cylindrical types: Fruit: curvature (1) absent or very weak, (3) weak, (5) medium, (7) strong, (9) very strong | ||||||||||||||||||||
| MC | Fruit: main color of skin at harvest maturity (1) white, (2) green, (3) violet, (4) yellow | ||||||||||||||||||||
| ICS | Fruit: intensity of main color of skin (1) very light, (3) light, (5) medium, (7) dark, (9) very dark | ||||||||||||||||||||
| PTC | Fruit: patches (1) absent, (9) present | ||||||||||||||||||||
| STR | Fruit: stripes (1) absent, (9) present | ||||||||||||||||||||
| PST | Fruit: prominence of stripes (3) weak, (5) medium, (7) strong | ||||||||||||||||||||
| DST | Fruit: density of stripes (3) sparse, (5) medium, (7) dense | ||||||||||||||||||||
| GL | Fruit: glossiness at harvest maturity (3) weak, (5) medium, (7) strong | ||||||||||||||||||||
| RBS | Fruit: ribs (1) absent or very weak, (3) weak, (5) medium, (7) strong, (9) very strong | ||||||||||||||||||||
| AUC | Fruit: anthocyanin coloration underneath calyx (1) absent, (9) present | ||||||||||||||||||||
| IUC | Fruit: intensity of anthocyanin coloration underneath calyx (3) weak, (5) medium, (7) strong | ||||||||||||||||||||
| LPD | Fruit: length of peduncle (1) very short, (3) short, (5) medium, (7) long, (9) very long | ||||||||||||||||||||
| CL | Fruit: size of calyx (1) very small, (3) small, (5) medium, (7) large, (9) very large | ||||||||||||||||||||
| ACL | Fruit: anthocyanin coloration of calyx (1) absent, (9) present | ||||||||||||||||||||
| ICL | Fruit: intensity of anthocyanin coloration of calyx - (1) very weak, (3) weak, (5) medium, (7) strong, (9) very strong | ||||||||||||||||||||
| SCL | Fruit: spininess of calyx (1) absent or very weak, (3) weak, (5) medium, (7) strong, (9) very strong | ||||||||||||||||||||
| CCL | Fruit: creasing of calyx (1) very weak, (3) weak, (5) medium, (7) strong, (9) very strong | ||||||||||||||||||||
| CFL | Fruit: color of flesh (1) whitish, (2) greenish | ||||||||||||||||||||
| CPR | Fruit: color of skin at physiological ripeness (1) yellow, (2) orange, (3) ochre, (4) brown | ||||||||||||||||||||
| Code | G02 | G05 | G07 | G25 | G33 | G37 | G38 | G44 | G45 | G48 | G55 | G60 | G61 | G63 | G75 | G76 | G78 | G80 | GJ | GK | GR |
| FL | 5 | 5 | 9 | 5 | 9 | 5 | 9 | 3 | 5 | 7 | 9 | 3 | 9 | 7 | 9 | 3 | 7 | 7 | 9 | 3 | 7 |
| FD | 7 | 7 | 7 | 7 | 7 | 5 | 7 | 3 | 5 | 7 | 5 | 5 | 7 | 7 | 7 | 3 | 7 | 7 | 5 | 5 | 9 |
| FLD | 1 | 3 | 7 | 1 | 7 | 5 | 7 | 3 | 5 | 5 | 9 | 1 | 7 | 7 | 7 | 3 | 7 | 7 | 9 | 1 | 3 |
| GS | 1 | 2 | 5 | 1 | 6 | 3 | 7 | 2 | 2 | 3 | 7 | 2 | 7 | 5 | 5 | 2 | 6 | 5 | 7 | 2 | 1 |
| PTS | 5 | 5 | 5 | 9 | 7 | 5 | 7 | 3 | 5 | 3 | 5 | 3 | 9 | 7 | 9 | 3 | 9 | 5 | 3 | 3 | 7 |
| APX | 3 | 1 | 1 | 1 | 3 | 3 | 4 | 1 | 1 | 1 | 3 | 3 | 3 | 4 | 7 | 1 | 4 | 4 | 4 | 1 | 1 |
| DPS | 1 | 3 | 1 | 3 | 1 | 1 | 1 | 3 | 1 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 5 | 3 |
| CVT | 1 | 1 | 5 | 1 | 1 | 1 | 3 | 1 | 1 | 1 | 7 | 1 | 3 | 3 | 1 | 1 | 1 | 1 | 5 | 1 | 1 |
| MC | 2 | 2 | 3 | 3 | 1 | 2 | 2 | 3 | 2 | 2 | 2 | 2 | 3 | 3 | 2 | 3 | 2 | 3 | 3 | 5 | 1 |
| ICS | 5 | 3 | 7 | 7 | 5 | 3 | 3 | 5 | 5 | 5 | 3 | 5 | 7 | 3 | 3 | 1 | 1 | 5 | 9 | 5 | 5 |
| PTC | 9 | 9 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| STR | 9 | 9 | 9 | 9 | 9 | 9 | 1 | 9 | 9 | 9 | 1 | 9 | 9 | 9 | 1 | 9 | 1 | 9 | 1 | 1 | 1 |
| PST | 7 | 7 | 3 | 3 | 3 | 7 | 3 | 3 | 5 | 3 | 3 | 7 | 3 | 3 | 3 | 7 | 3 | 3 | 3 | 3 | 3 |
| DST | 7 | 5 | 3 | 3 | 3 | 5 | 3 | 3 | 5 | 3 | 3 | 7 | 3 | 3 | 3 | 7 | 3 | 3 | 3 | 3 | 3 |
| GL | 3 | 3 | 7 | 5 | 3 | 3 | 3 | 5 | 3 | 3 | 5 | 3 | 5 | 1 | 3 | 3 | 3 | 5 | 7 | 3 | 3 |
| RBS | 5 | 5 | 1 | 5 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 5 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| AUC | 1 | 1 | 1 | 9 | 1 | 9 | 9 | 9 | 1 | 9 | 1 | 1 | 9 | 1 | 1 | 1 | 1 | 1 | 9 | 1 | 1 |
| IUC | 3 | 3 | 3 | 5 | 3 | 3 | 5 | 5 | 3 | 5 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 7 | 3 | 3 |
| LPD | 5 | 5 | 7 | 7 | 9 | 5 | 9 | 5 | 5 | 5 | 5 | 5 | 9 | 9 | 9 | 7 | 9 | 7 | 5 | 5 | 7 |
| CL | 3 | 5 | 3 | 7 | 3 | 3 | 5 | 3 | 3 | 1 | 3 | 5 | 5 | 9 | 1 | 1 | 5 | 3 | 3 | 1 | 3 |
| ACL | 1 | 1 | 1 | 9 | 1 | 9 | 9 | 9 | 1 | 9 | 1 | 1 | 9 | 1 | 1 | 1 | 1 | 1 | 9 | 1 | 1 |
| ICL | 1 | 1 | 1 | 5 | 1 | 3 | 5 | 5 | 1 | 3 | 1 | 1 | 3 | 3 | 1 | 1 | 1 | 1 | 9 | 1 | 1 |
| SCL | 1 | 1 | 1 | 1 | 1 | 1 | 3 | 1 | 1 | 3 | 1 | 1 | 1 | 9 | 1 | 1 | 1 | 1 | 3 | 1 | 3 |
| CCL | 3 | 5 | 3 | 1 | 3 | 3 | 3 | 1 | 3 | 3 | 1 | 1 | 5 | 3 | 5 | 3 | 5 | 3 | 7 | 3 | 1 |
| CFL | 2 | 1 | 2 | 2 | 1 | 2 | 2 | 2 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 |
| CPR | 1 | 1 | 4 | 4 | 1 | 1 | 1 | 3 | 1 | 1 | 2 | 1 | 4 | 4 | 2 | 2 | 1 | 4 | 3 | 1 | 1 |
| Accessions Code | Fruit Morphology | |
|---|---|---|
| Color | Shape | |
| G02 | Green with stripes and patches | Globular |
| G05 | Light green with stripes and patches | Ovoid |
| G07 | Dark purple with stripes | Club shaped |
| G25 | Dark purple with stripes | Globular |
| G33 | White with stripes | Ellipsoid |
| G37 | Light green with stripes | Obovate |
| G38 | Light green | Cylindrical |
| G44 | Purple | Ovoid |
| G45 | Green with stripes | Ovoid |
| G48 | Green with stripes and patches | Obovate |
| G55 | Light green | Cylindrical |
| G60 | Green with stripes | Ovoid |
| G61 | Dark purple | Cylindrical |
| G63 | Light purple with stripes | Club shaped |
| G75 | Light green | Club shaped |
| G76 | Very light purple with green stripes | Ovoid |
| G78 | Very light green | Ellipsoid |
| G80 | Purple with stripes | Club shaped |
| GJ | Very dark purple | Cylindrical |
| GK | Yellow | Ovoid |
| GR | White | Globular |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanifah, A.; Maharijaya, A.; Putri, S.P.; Laviña, W.A.; Sobir. Untargeted Metabolomics Analysis of Eggplant (Solanum melongena L.) Fruit and Its Correlation to Fruit Morphologies. Metabolites 2018, 8, 49. https://doi.org/10.3390/metabo8030049
Hanifah A, Maharijaya A, Putri SP, Laviña WA, Sobir. Untargeted Metabolomics Analysis of Eggplant (Solanum melongena L.) Fruit and Its Correlation to Fruit Morphologies. Metabolites. 2018; 8(3):49. https://doi.org/10.3390/metabo8030049
Chicago/Turabian StyleHanifah, Abu, Awang Maharijaya, Sastia P. Putri, Walter A. Laviña, and Sobir. 2018. "Untargeted Metabolomics Analysis of Eggplant (Solanum melongena L.) Fruit and Its Correlation to Fruit Morphologies" Metabolites 8, no. 3: 49. https://doi.org/10.3390/metabo8030049






