Robust Regression Analysis of GCMS Data Reveals Differential Rewiring of Metabolic Networks in Hepatitis B and C Patients
Abstract
:1. Introduction
2. Results
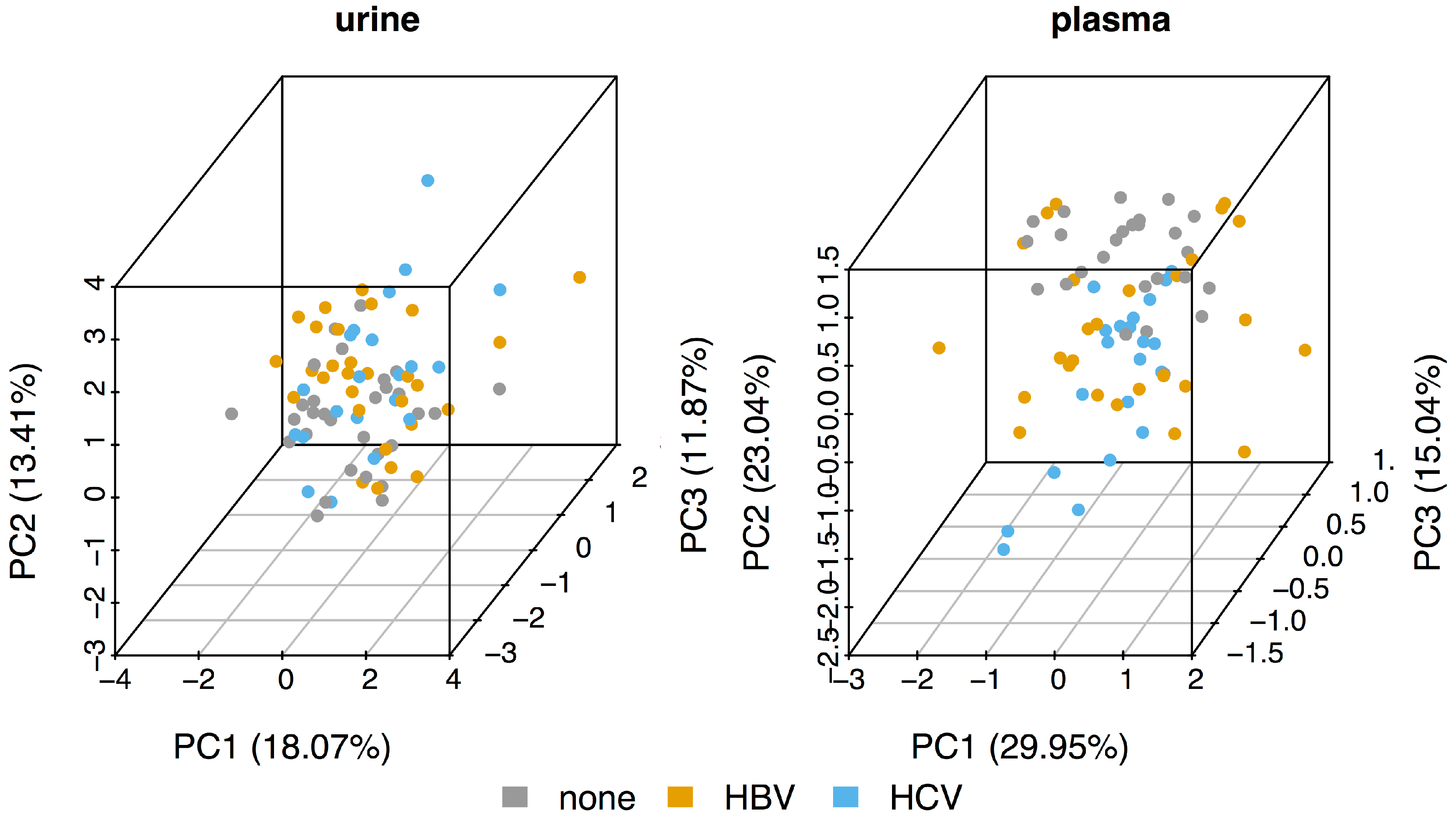
2.1. Direct Comparison of Metabolite Intensities Shows Very Little Difference between HBV and HCV Patients
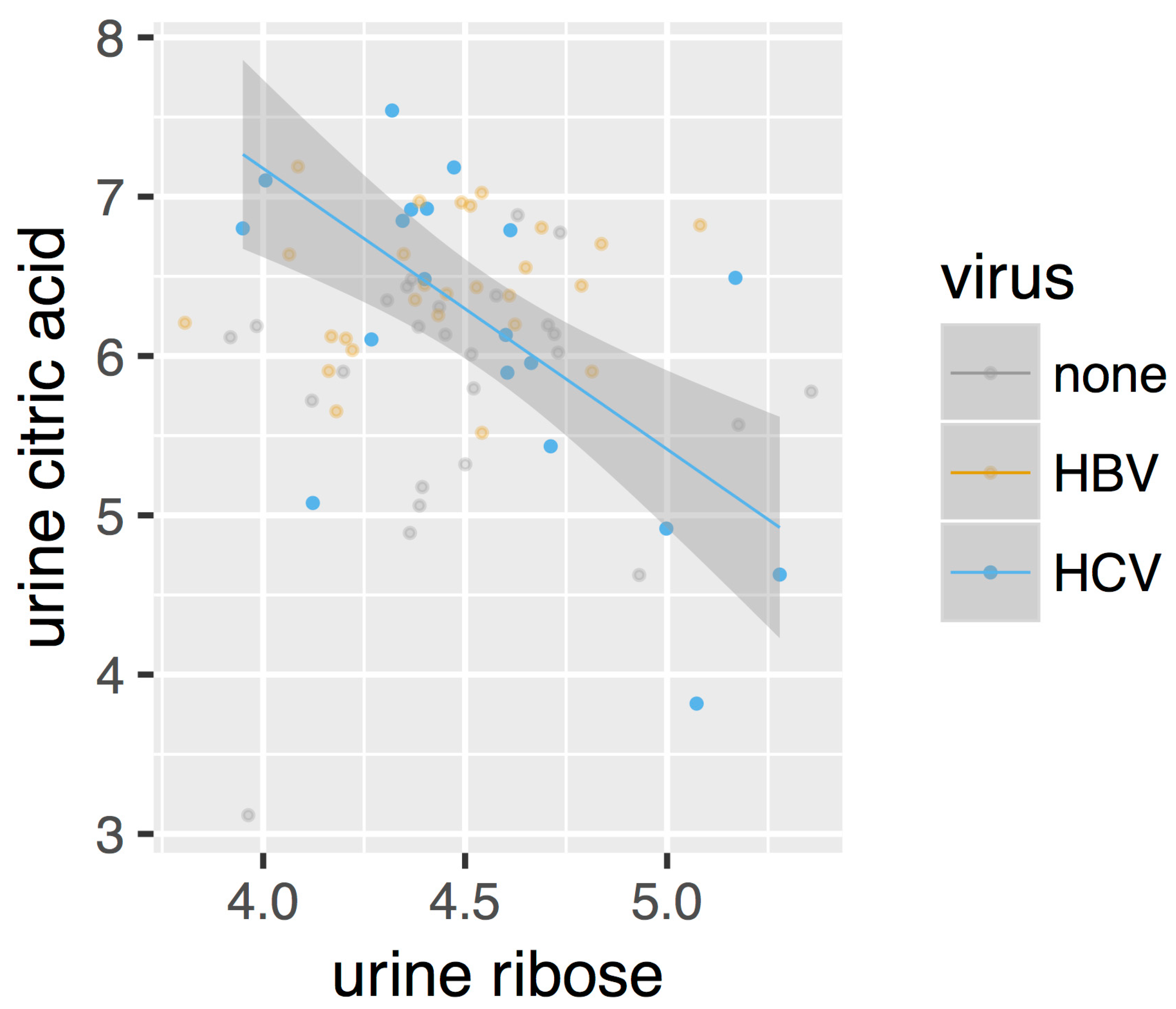
2.2. Regression Analysis Reveals Different Metabolite Correlation Patterns between HBV and HCV Patients
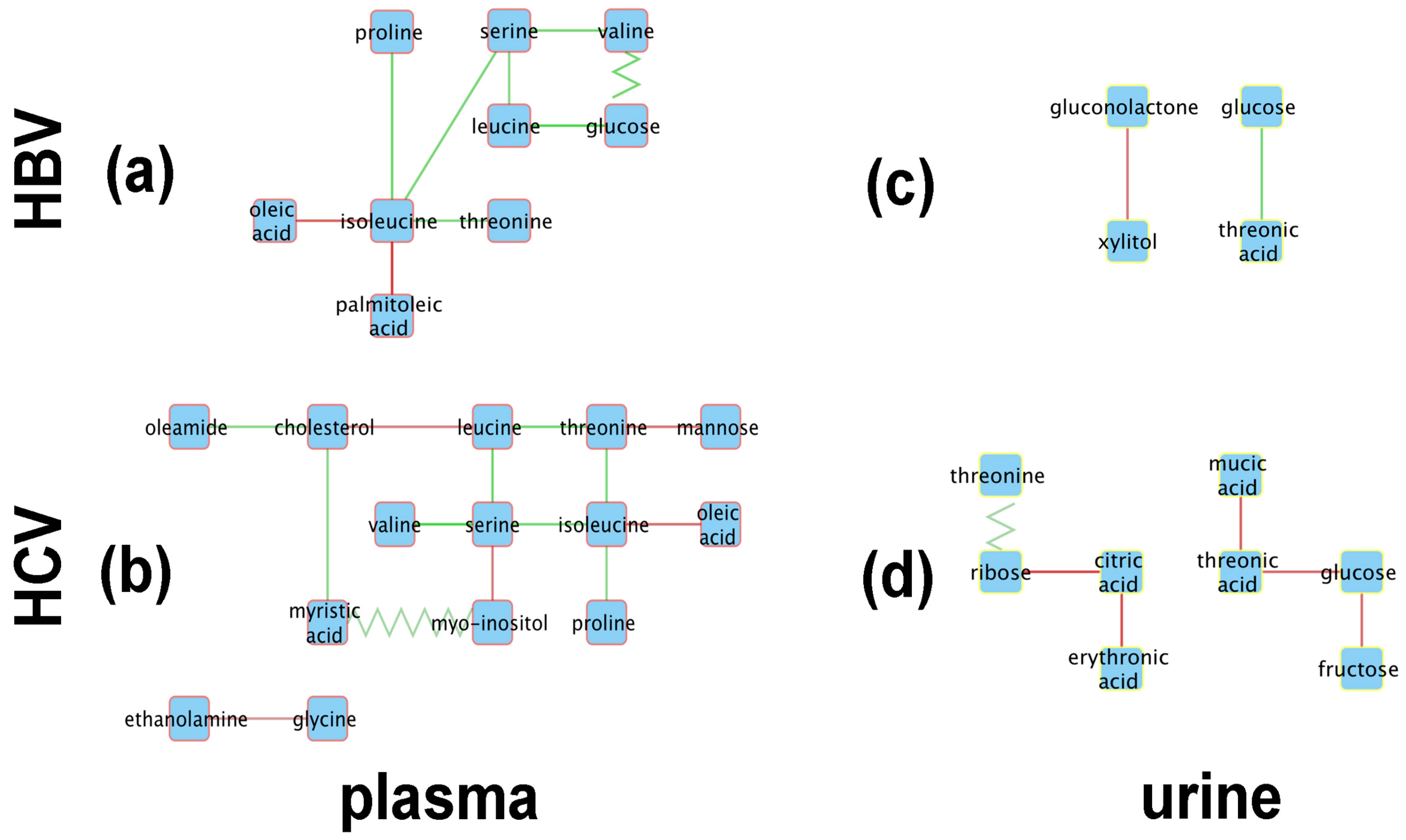
2.3. Mechanistic Interpretations of the Metabolic Perturbation Networks
2.3.1. General Considerations
2.3.2. Analysis of the HBV and HCV Metabolic Perturbation Networks
- The initial PCA analysis of plasma and urine datasets suggested that there were few differences between the HBV+, HCV+, and control metabolomes.
- Univariate statistics gave 28 statistically significant differences for a subset of 20 metabolites; 15 in plasma and 13 in urine.
- Robust regression analysis revealed networks of correlations between pairs of metabolites that mainly appear in HBV+ and HCV+ plasma and urine but are not observed in healthy controls.
- The positive and negative correlations provided novel insights into the HBV+ liver compared with the HCV+ liver.
3. Discussion
4. Materials and Methods
4.1. Patient Selection
4.2. Gas Chromatography-Mass Spectrometry (GCMS) Analysis of Plasma and Urine
4.3. Data Processing, Batch Correction, and Integration
- A matrix was constructed in which the columns represent the samples and the rows the measured metabolites
- A floor value, , was calculated as for all elements of , where
- All elements of , where , are assigned a value of
- The entire matrix is now transformed such that
- A vector, , is calculated as the column means of , as well as a vector, , as the column standard deviations. A vector, , is defined as
- The vector, , is subtracted from each row of
- Each row of is divided by
- Finally, all values of are offset by a value such that the lowest value of is zero.
- The result of these operations is a matrix in which all columns have the same mean and standard deviation.
4.4. Detection of Differentially Abundant Metabolites
4.5. Robust Regression Analysis
- A null model, with a single intercept, representing the case that is independent of both and .
- An intercept-only model, with a different intercept per subject group, modelling the case that only varies with .
- A single intercept, single slope model, for the case in which only correlates with and is independent of
- A multiple intercept, single slope model, representing the case in which depends on both and but in which the slope does not vary between the different patient groups.
- An interaction model, where depends on both and , as well as the interaction between them, meaning that the slope changes between the patient groups.
- When the interaction coefficient for the virus was zero, the edge was not included in the network.
- When the general slope was zero but the interaction coefficient was different from zero, the edge type was called ‘appear’
- When the general slope was different from zero but the interaction coefficient was zero, the edge type was called ‘disappear’
- When the both the general slope and the interaction coefficient were different from zero and the slope of the HBV or HCV patient group had a different sign than the general slope, the edge type was called ‘flip’. Note that the slope of a patient group is calculated as the sum of the general slope and the interaction term.
- When both the general slope and the interaction term were different from zero, the edge type was called ‘change’.
4.6. Integration with Metabolic Networks
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A


References
- Xie, Q.; Fan, F.; Wei, W.; Liu, Y.; Xu, Z.; Zhai, L.; Qi, Y.; Ye, B.; Zhang, Y.; Basu, S.; et al. Multi-omics analyses reveal metabolic alterations regulated by hepatitis B virus core protein in hepatocellular carcinoma cells. Sci. Rep. 2017, 7, 41089. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Cheng, M.; Chang, S.; Tang, H.; Chiu, C.; Yeh, C.; Shiao, M. Recovery of pan-genotypic and genotype-specific amino acid alterations in chronic hepatitis C after viral clearance: transition at the crossroad of metabolism and immunity. Amino acids 2017, 49, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Elsemman, I.E.; Mardinoglu, A.; Shoaie, S.; Soliman, T.H.; Nielsen, J. Systems biology analysis of hepatitis C virus infection reveals the role of copy number increases in regions of chromosome 1q in hepatocellular carcinoma metabolism. Mol. Biosyst. 2016, 12, 1496–1506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.-T.; Jen, C.-L.; Yang, H.-I.; Lee, M.-H.; Su, J.; Lu, S.-N.; Iloeje, U.H.; Chen, C.-J. Lifetime risk and sex difference of hepatocellular carcinoma among patients with chronic hepatitis B and C. J. Clin. Oncol. 2011, 29, 3643–3650. [Google Scholar] [CrossRef] [PubMed]
- Le Naour, F.; Brichory, F.; Misek, D.E.; Bréchot, C.; Hanash, S.M.; Beretta, L. A distinct repertoire of autoantibodies in hepatocellular carcinoma identified by proteomic analysis. Mol. Cell. Proteom. 2002, 1, 197–203. [Google Scholar] [CrossRef]
- Lin, X.; Ma, Z.-M.; Yao, X.; Zhang, Y.-P.; Wen, Y.-M. Replication efficiency and sequence analysis of full-length hepatitis B virus isolates from hepatocellular carcinoma tissues. Int. J. Cancer 2002, 102, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Kato, N. Genome of human hepatitis C virus (HCV): gene organization, sequence diversity, and variation. Microb. Comp. Genom. 2000, 5, 129–151. [Google Scholar] [CrossRef] [PubMed]
- Godoy, M.M.G.; Lopes, E.P.A.; Silva, R.O.; Hallwass, F.; Koury, L.C.A.; Moura, I.M.; Gonçalves, S.M.C.; Simas, A.M. Hepatitis C virus infection diagnosis using metabonomics. J. Viral. Hepat. 2010, 17, 854–858. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, M.A.; Saghatelian, A.; Yang, P.L. Identification of an overabundant cholesterol precursor in hepatitis B virus replicating cells by untargeted lipid metabolite profiling. J. Am. Chem. Soc. 2009, 131, 5030–5031. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.; Dong, L.; Wu, H.; Liu, T.; Wang, J.; Shen, X. Gas chromatography/mass spectrometry screening of serum metabolomic biomarkers in hepatitis B virus infected cirrhosis patients. Clin. Chem. Lab. Med. 2009, 47, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Coelho, M.; Nunes, P.; Mendes, V.M.; Manadas, B.; Heerschap, A.; Jones, J.G. Effect of global ATGL knockout on murine fasting glucose kinetics. J. Diabetes Res. 2015, 2015, 542029. [Google Scholar] [CrossRef] [PubMed]
- Nagao, H.; Nishizawa, H.; Bamba, T.; Nakayama, Y.; Isozumi, N.; Nagamori, S.; Kanai, Y.; Tanaka, Y.; Kita, S.; Fukuda, S.; et al. Increased dynamics of tricarboxylic acid cycle and glutamate synthesis in obese adipose tissue: in vivo metabolic turnover analysis. J. Biol. Chem. 2017, 292, 4469–4483. [Google Scholar] [CrossRef] [PubMed]
- Crown, S.B.; Marze, N.; Antoniewicz, M.R. Catabolism of branched chain amino acids contributes significantly to synthesis of odd-chain and even-chain fatty acids in 3T3-L1 adipocytes. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Sheikholeslami, Z.; Jolicoeur, M.; Henry, O. Probing the metabolism of an inducible mammalian expression system using extracellular isotopomer analysis. J. Biotechnol. 2013, 164, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Chang, H.H.; Wang, C.; Jiang, J.; Wang, X.; Niu, J. Multi-TGDR, a multi-class regularization method, identifies the metabolic profiles of hepatocellular carcinoma and cirrhosis infected with hepatitis B or hepatitis C virus. BMC Bioinform. 2014, 15, 97. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Sun, H.; Han, Y.; Yan, G.; Wang, X. Urinary metabolic biomarker and pathway study of hepatitis B virus infected patients based on UPLC-MS system. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Embade, N.; Mariño, Z.; Diercks, T.; Cano, A.; Lens, S.; Cabrera, D.; Navasa, M.; Falcón-Pérez, J.M.; Caballería, J.; Castro, A.; et al. Metabolic characterization of advanced liver fibrosis in hcv patients as studied by serum 1H-NMR spectroscopy. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Yano, Y.; Hirano, H.; Momose, K.; Yoshida, M.; Azuma, T. Serum NX-DCP as a New Noninvasive Model to Predict Significant Liver Fibrosis in Chronic Hepatitis C. Hepat. Mon. 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- Sarfaraz, M.O.; Myers, R.P.; Coffin, C.S.; Gao, Z.-H.; Shaheen, A.A.M.; Crotty, P.M.; Zhang, P.; Vogel, H.J.; Weljie, A.M. A quantitative metabolomics profiling approach for the noninvasive assessment of liver histology in patients with chronic hepatitis C. Clin. Transl. Med. 2016, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, R.; Kang, H.; Fan, Z.; Du, Z. Human liver tissue metabolic profiling research on hepatitis B virus-related hepatocellular carcinoma. World J. Gastroenterol. 2013, 19, 3423–3432. [Google Scholar] [CrossRef] [PubMed]
- Baniasadi, H.; Gowda, G.A.N.; Gu, H.; Zeng, A.; Zhuang, S.; Skill, N.; Maluccio, M.; Raftery, D. Targeted metabolic profiling of hepatocellular carcinoma and hepatitis C using LC-MS/MS. Electrophoresis 2013, 34, 2910–2917. [Google Scholar] [CrossRef] [PubMed]
- Bowers, J.; Hughes, E.; Skill, N.; Maluccio, M.; Raftery, D. Detection of hepatocellular carcinoma in hepatitis C patients: biomarker discovery by LC-MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2014, 966, 154–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitian, A.I.; Nelson, D.R.; Liu, C.; Xu, Y.; Ararat, M.; Cabrera, R. Integrated metabolomic profiling of hepatocellular carcinoma in hepatitis C cirrhosis through GC/MS and UPLC/MS-MS. Liver Int. 2014, 34, 1428–1444. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Cheng, J.; Fan, C.; Shi, X.; Cao, Y.; Sun, B.; Ding, H.; Hu, C.; Dong, F.; Yan, X. Serum Metabolomics to Identify the Liver Disease-Specific Biomarkers for the Progression of Hepatitis to Hepatocellular Carcinoma. Sci. Rep. 2015, 5, 18175. [Google Scholar] [CrossRef] [PubMed]
- Schoeman, J.C.; Hou, J.; Harms, A.C.; Vreeken, R.J.; Berger, R.; Hankemeier, T.; Boonstra, A. Metabolic characterization of the natural progression of chronic hepatitis B. Genom. Med. 2016, 8, 64. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.-F.; Hsieh, W.-C.; Yang, C.-W.; Su, H.-M.; Tsai, T.-F.; Sung, W.-C.; Huang, W.; Su, I.-J. A biphasic response pattern of lipid metabolomics in the stage progression of hepatitis B virus X tumorigenesis. Mol. Carcinog. 2016, 55, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Automat. Contr. 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Hou, Y.; Zhu, W.; Chen, C.; Wang, Y.; Duan, Z.; Yan, C. Exploration on serum metabolic biomarkers of hepatitis B virus infected patients based on gas chromatography-mass spectrometry. Se Pu 2015, 33, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhu, W.; Zhang, L.; Lei, H.; Wu, X.; Guo, L.; Chen, X.; Wang, Y.; Tang, H. The metabolic responses to hepatitis B virus infection shed new light on pathogenesis and targets for treatment. Sci. Rep. 2015, 5, 8421. [Google Scholar] [CrossRef] [PubMed]
- Yue, D.; Zhang, Y.; Cheng, L.; Ma, J.; Xi, Y.; Yang, L.; Su, C.; Shao, B.; Huang, A.; Xiang, R.; et al. Hepatitis B virus X protein (HBx)-induced abnormalities of nucleic acid metabolism revealed by 1H-NMR-based metabonomics. Sci. Rep. 2016, 6, 24430. [Google Scholar]
- Colpitts, C.C.; El-Saghire, H.; Pochet, N.; Schuster, C.; Baumert, T.F. High-throughput approaches to unravel hepatitis C virus-host interactions. Virus Res. 2016, 218, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Roe, B.; Kensicki, E.; Mohney, R.; Hall, W.W. Metabolomic profile of hepatitis C virus-infected hepatocytes. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.-H.; Sun, H.; Han, Y.; Yan, G.-L.; Yuan, Y.; Song, G.-C.; Yuan, X.-X.; Xie, N.; Wang, X.-J. Ultraperformance liquid chromatography-mass spectrometry based comprehensive metabolomics combined with pattern recognition and network analysis methods for characterization of metabolites and metabolic pathways from biological data sets. Anal. Chem. 2013, 85, 7606–7612. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Sugimoto, M.; Igarashi, K.; Saito, K.; Shao, L.; Katsumi, T.; Tomita, K.; Sato, C.; Okumoto, K.; Nishise, Y.; et al. Dynamics of serum metabolites in patients with chronic hepatitis C receiving pegylated interferon plus ribavirin: a metabolomics analysis. Metabolism 2013, 62, 1577–1586. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-J.; Yan, G.-L.; Zhang, A.-H.; Sun, H.; Piao, C.-Y.; Li, W.-Y.; Sun, C.; Wu, X.-H.; Li, X.-H.; Chen, Y. Metabolomics and proteomics approaches to characterize and assess proteins of bear bile powder for hepatitis C virus. Chin. J. Nat. Med. 2013, 11, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Semmo, N.; Weber, T.; Idle, J.R.; Beyoğlu, D. Metabolomics reveals that aldose reductase activity due to AKR1B10 is upregulated in hepatitis C virus infection. J. Viral. Hepat. 2015, 22, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Yabe-Nishimura, C. Aldose reductase in glucose toxicity: A potential target for the prevention of diabetic complications. Pharmacol. Rev. 1998, 50, 21–33. [Google Scholar] [PubMed]
- Patel, D.P.; Krausz, K.W.; Xie, C.; Beyoglu, D.; Gonzalez, F.J.; Idle, J.R. Profiling by gas chromatography-mass spectrometry of energy metabolism in high-fat diet-fed obese mice. PLoS ONE 2017, 12, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Keogh, A.; Senkardes, S.; Idle, J.R.; Kucukguzel, S.G.; Beyoglu, D. A novel diflunisal hydrazide-hydrazone antihepatitis c virus and antiproliferative agent alters metabolic networks in hepatoma cells. Metabolites 2017, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Shaw, W. Increased urinary excretion of a 3-(3-hydroxyphenyl)-3-hydroxypropionic acid (HPHPA), an abnormal phenylalanine metabolite of Clostridia spp. in the gastrointestinal tract, in urine samples from patients with autism and schizophrenia. Nutr. Neurosci. 2010, 13, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Shaw, W. Elevated urinary glyphosate and clostridia metabolites with altered dopamine metabolism in triplets with autistic spectrum disorder or suspected seizure disorder: A case study. Integr. Med. 2017, 16, 50–57. [Google Scholar]
- Chalmers, R.A.; Lawson, A.M.; Hauschildt, S.; Watts, R.W. The urinary excretion of glycollic acid and threonic acid by xylitol-infused patients and their relationship to the possible role of ‘active glycoladehyde’ in the transketolase reaction in vivo. Biochem. Soc. Trans. 1975, 3, 518–521. [Google Scholar] [CrossRef] [PubMed]
- Hauschildt, S.; Chalmers, R.A.; Lawson, A.M.; Schultis, K.; Watts, R.W. Metabolic investigations after xylitol infusion in human subjects. Am. J. Clin. Nutr. 1976, 29, 258–273. [Google Scholar] [PubMed]
- Hollenbaugh, J.A.; Montero, C.; Schinazi, R.F.; Munger, J.; Kim, B. Metabolic profiling during HIV-1 and HIV-2 infection of primary human monocyte-derived macrophages. Virology 2016, 491, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Engelke, U.F.; Zijlstra, F.S.; Mochel, F.; Valayannopoulos, V.; Rabier, D.; Kluijtmans, L.A.; Perl, A.; Verhoeven-Duif, N.M.; de Lonlay, P.; Wamelink, M.M.; et al. Mitochondrial involvement and erythronic acid as a novel biomarker in transaldolase deficiency. Biochim. Biophys. Acta 2010, 1802, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.G.; Zhao, W.; Zhang, J.; Wu, X.; Hu, J.; Yin, G.C.; Xu, Y.J. Metabolomics and eicosanoid analysis identified serum biomarkers for distinguishing hepatocellular carcinoma from hepatitis B virus-related cirrhosis. Oncotarget 2017. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Huang, Y.; Lian, M.; Fan, Z.; Tian, Y.; Wang, Y.; Kang, H.; Liu, S.; Liu, S.; Li, T.; et al. Metabolic profiling of hepatitis B virus-related hepatocellular carcinoma with diverse differentiation grades. Oncol. Lett. 2017, 13, 1204–1210. [Google Scholar] [PubMed]
- Hao, J.; Yang, T.; Zhou, Y.; Gao, G.Y.; Xing, F.; Peng, Y.; Tao, Y.Y.; Liu, C.H. Serum metabolomics analysis reveals a distinct metabolic profile of patients with primary biliary cholangitis. Sci. Rep. 2017, 7, 784. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Zhang, L.; Liu, S. Application of liquid chromatography-mass spectrometry in the study of metabolic profiling of cirrhosis in different grades. Se Pu 2011, 29, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Calleja-Panero, J.L.; Llop-Herrera, E.; Ruiz-Moraga, M.; de-la-Revilla-Negro, J.; Calvo-Bonacho, E.; Pons-Renedo, F.; Martinez-Porras, J.L.; Vallejo-Gutierrez, D.; Arregui, C.; Abreu-Garcia, L. Prevalence of viral hepatitis (B and C) serological markers in healthy working population. Rev. Esp. Enferm. Dig. 2013, 105, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Owen, O.E.; Kalhan, S.C.; Hanson, R.W. The key role of anaplerosis and cataplerosis for citric acid cycle function. J. Biol. Chem. 2002, 277, 30409–30412. [Google Scholar] [CrossRef] [PubMed]
- Young, G.A.; Hill, G.L. Evaluation of protein-energy malnutrition in surgical patients from plasma valine and other amino acids, proteins, and anthropometric measurements. Am. J. Clin. Nutr. 1981, 34, 166–172. [Google Scholar] [PubMed]
- Dodd, K.M.; Tee, A.R. Leucine and mTORC1: A complex relationship. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E1329–E1342. [Google Scholar] [CrossRef] [PubMed]
- Bagga, S.; Rawat, S.; Ajenjo, M.; Bouchard, M.J. Hepatitis B virus (HBV) X protein-mediated regulation of hepatocyte metabolic pathways affects viral replication. Virology 2016, 498, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.D.; Wu, H.; Huang, S.; Zhang, H.L.; Qin, C.J.; Zhao, L.H.; Fu, G.B.; Zhou, X.; Wang, X.M.; Tang, L.; et al. HBx regulates fatty acid oxidation to promote hepatocellular carcinoma survival during metabolic stress. Oncotarget 2016, 7, 6711–6726. [Google Scholar] [CrossRef] [PubMed]
- Osman, D.; Ali, O.; Obada, M.; El-Mezayen, H.; El-Said, H. Chromatographic determination of some biomarkers of liver cirrhosis and hepatocellular carcinoma in Egyptian patients. Biomed. Chromatogr. 2017, 31. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, J.C. Oxygen-accepting antioxidants which arise during ascorbate oxidation. Anal. Biochem. 1998, 265, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Herbert, R.W.; Hirst, E.L.; Percival, E.G.V.; Reynolds, R.J.W.; Smith, F. The constitution of ascorbic acid. J. Chem. Soc. 1933, 2, 1270–1290. [Google Scholar] [CrossRef]
- Sun, Q.; Weinger, J.G.; Mao, F.; Liu, G. Regulation of structural and functional synapse density by L-threonate through modulation of intraneuronal magnesium concentration. Neuropharmacology 2016, 108, 426–439. [Google Scholar] [CrossRef] [PubMed]
- Trezzi, J.P.; Galozzi, S.; Jaeger, C.; Barkovits, K.; Brockmann, K.; Maetzler, W.; Berg, D.; Marcus, K.; Betsou, F.; Hiller, K.; et al. Distinct metabolomic signature in cerebrospinal fluid in early parkinson’s disease. Mov. Disord. 2017. [Google Scholar] [CrossRef]
- Holecek, M.; Skalska, H.; Mraz, J. Plasma amino acid levels after carbon tetrachloride induced acute liver damage. A dose-response and time-response study in rats. Amino Acids 1999, 16, 1–11. [Google Scholar] [PubMed]
- Zhong, F.; Xu, M.; Bruno, R.S.; Ballard, K.D.; Zhu, J. Targeted High Performance Liquid Chromatography Tandem Mass Spectrometry-based Metabolomics differentiates metabolic syndrome from obesity. Exp. Biol. Med. 2017, 242, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Kaimoto, T.; Shibuya, M.; Nishikawa, K.; Maeda, H. High incidence of lipid deposition in the liver of rats fed a diet supplemented with branched-chain amino acids under vitamin B6 deficiency. J. Nutr. Sci. Vitaminol. 2013, 59, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Vance, J.E. Phospholipid synthesis and transport in mammalian cells. Traffic 2015, 16, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.; Farine, L.; Pandey, K.; Menon, A.K.; Butikofer, P. Lipid topogenesis—35years on. Biochim. Biophys. Acta 2016, 1861, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Bayer, K.; Banning, C.; Bruss, V.; Wiltzer-Bach, L.; Schindler, M. Hepatitis C virus is released via a noncanonical secretory route. J. Virol. 2016, 90, 10558–10573. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Ng, D.T. O-mannosylation: The other glycan player of ER quality control. Semin. Cell. Dev. Biol. 2015, 41, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yang, R.; Jiang, X.; Yang, Y.; Peng, F.; Yuan, H. Serum metabolite profiles of postoperative fatigue syndrome in rat following partial hepatectomy. J. Clin. Biochem. Nutr. 2016, 58, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, M.; Huonker, M.; Dimeo, F.; Heinz, N.; Gastmann, U.; Treis, N.; Steinacker, J.M.; Keul, J.; Kajewski, R.; Haussinger, D. Serum amino acid concentrations in nine athletes before and after the 1993 Colmar ultra triathlon. Int. J. Sports Med. 1995, 16, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Kong, J.; Wu, J.; Wang, X.; Lai, M. GC-MS-based metabolomics identifies an amino acid signature of acute ischemic stroke. Neurosci. Lett. 2017, 642, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, M.; Joosen, A.M.; Clarke, M.M.; Mugridge, O.; Frost, G.; Engel, B.; Taillart, K.; Lloyd, A.J.; Draper, J.; Lodge, J.K. Changes in the human plasma and urinary metabolome associated with acute dietary exposure to sucrose and the identification of potential biomarkers of sucrose intake. Mol. Nutr. Food Res. 2016, 60, 444–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sternfeld, L.; Saunders, F. The Fermentation of Mucic Acid by Some Intestinal Bacteria. J. Bacteriol. 1938, 36, 53–56. [Google Scholar] [PubMed]
- Fahrner, R.; Beyoglu, D.; Beldi, G.; Idle, J.R. Metabolomic markers for intestinal ischemia in a mouse model. J. Surg. Res. 2012, 178, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Su, I.J.; Wang, L.H.; Hsieh, W.C.; Wu, H.C.; Teng, C.F.; Tsai, H.W.; Huang, W. The emerging role of hepatitis B virus pre-S2 deletion mutant proteins in HBV tumorigenesis. J. Biomed. Sci. 2014, 21, 98. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.F.; Hsieh, W.C.; Wu, H.C.; Lin, Y.J.; Tsai, H.W.; Huang, W.; Su, I.J. Hepatitis B virus pre-S2 mutant induces aerobic glycolysis through mammalian target of rapamycin signal cascade. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Shoji, I.; Deng, L.; Matsui, C.; Hotta, H. Hepatitis C virus-induced glucose metabolic disorder. Uirusu 2015, 65, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.C.; Chen, I.Y.; Chang, S.C.; Wu, S.C.; Hung, T.M.; Lee, P.H.; Shimotohno, K.; Chang, M.F. Hepatitis C virus NS5A protein enhances gluconeogenesis through upregulation of Akt-/JNK-PEPCK signalling pathways. Liver Int. 2014, 34, 1358–1368. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.C.; He, T.; Yan, Z.; Lindner, J.; Sherry, A.D.; Malloy, C.R.; Browning, J.D.; Magnuson, M.A. Cytosolic phosphoenolpyruvate carboxykinase does not solely control the rate of hepatic gluconeogenesis in the intact mouse liver. Cell. Metab. 2007, 5, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Kasai, D.; Adachi, T.; Deng, L.; Nagano-Fujii, M.; Sada, K.; Ikeda, M.; Kato, N.; Ide, Y.H.; Shoji, I.; Hotta, H. HCV replication suppresses cellular glucose uptake through down-regulation of cell surface expression of glucose transporters. J. Hepatol. 2009, 50, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, T.I.; Adam, T.; Qadri, I. Upregulated hepatic expression of mitochondrial PEPCK triggers initial gluconeogenic reactions in the HCV-3 patients. Asian Pac. J. Trop. Med. 2015, 8, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Sears, D.D.; Hsiao, G.; Hsiao, A.; Yu, J.G.; Courtney, C.H.; Ofrecio, J.M.; Chapman, J.; Subramaniam, S. Mechanisms of human insulin resistance and thiazolidinedione-mediated insulin sensitization. Proc. Natl. Acad. Sci. USA 2009, 106, 18745–18750. [Google Scholar] [CrossRef] [PubMed]
- Allison, M.E.; Wreghitt, T.; Palmer, C.R.; Alexander, G.J. Evidence for a link between hepatitis C virus infection and diabetes mellitus in a cirrhotic population. J. Hepatol. 1994, 21, 1135–1139. [Google Scholar] [CrossRef]
- Lerat, H.; Imache, M.R.; Polyte, J.; Gaudin, A.; Mercey, M.; Donati, F.; Baudesson, C.; Higgs, M.R.; Picard, A.; Magnan, C.; et al. Hepatitis C virus induces a prediabetic state by directly impairing hepatic glucose metabolism in mice. J. Biol. Chem. 2017, 292, 12860–12873. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, T.; Nagao, Y.; Abe, K.; Imazeki, F.; Honda, K.; Yamasaki, K.; Miyanishi, K.; Taniguchi, E.; Kakuma, T.; Kato, J.; et al. Effects of branched-chain amino acids and zinc-enriched nutrients on prognosticators in HCV-infected patients: A multicenter randomized controlled trial. Mol. Med. Rep. 2015, 11, 2159–2166. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.S. The Emerging Role of Branched-chain amino acids in insulin resistance and metabolism. Nutrients 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, T.; Taniguchi, E.; Itou, M.; Sumie, S.; Yamagishi, S.; Sata, M. The pathogenesis, complications and therapeutic strategy for hepatitis C virus-associated insulin resistance in the era of anti-viral treatment. Rev. Recent Clin. Trials 2010, 5, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Cassol, E.; Misra, V.; Holman, A.; Kamat, A.; Morgello, S.; Gabuzda, D. Plasma metabolomics identifies lipid abnormalities linked to markers of inflammation, microbial translocation, and hepatic function in HIV patients receiving protease inhibitors. BMC infect. Dis. 2013, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golla, S.; Golla, J.P.; Krausz, K.W.; Manna, S.K.; Simillion, C.; Beyoglu, D.; Idle, J.R.; Gonzalez, F.J. Metabolomic analysis of mice exposed to gamma radiation reveals a systemic understanding of total-body exposure. Radiat. Res. 2017, 187, 612–629. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Keogh, A.; Treves, S.; Idle, J.R.; Beyoglu, D. The metabolomic profile of gamma-irradiated human hepatoma and muscle cells reveals metabolic changes consistent with the Warburg effect. PeerJ 2016, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leek, J.T.; Johnson, W.E.; Parker, H.S.; Jaffe, A.E.; Storey, J.D. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics 2012, 28, 882–883. [Google Scholar] [CrossRef] [PubMed]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S; Springer: Berlin/Heidelberg, Germany, 2002; Available online: http://www.springer.com/gp/book/9780387954578 (accessed on 6 October 2017).

| Metabolite | RT (min) | Plasma | Urine |
|---|---|---|---|
| lactic acid | 13.32 | X | X |
| glycolic acid | 13.80 | - | X |
| p-cresol | 16.36 | - | X |
| valine | 19.28 | X | - |
| urea | 20.20 | X | - |
| ethanolamine | 21.05 | X | - |
| leucine | 21.24 | X | - |
| isoleucine | 21.98 | X | - |
| proline | 22.08 | X | - |
| glycine | 22.42 | X | X |
| serine | 24.30 | X | X |
| threonine | 25.21 | X | X |
| threitol | 28.38 | - | X |
| erythronic acid | 29.73 | - | X |
| threonic acid | 30.79 | - | X |
| ribose * | 30.97 33.29 33.71 | - | X |
| 4-hydroxyphenylacetic acid | 32.10 | - | X |
| xylitol | 34.56 | - | X |
| arabitol | 34.90 | - | X |
| fucose | 35.06 | - | X |
| citric acid | 37.95 | - | X |
| HPHPA ** | 37.96 | - | X |
| myristic acid | 38.11 | X | - |
| gluconolactone | 39.15 | - | X |
| fructose | 39.55 | - | X |
| glucose *** | 39.92 40.25 | X | X |
| mannose | 40.69 | X | - |
| tyrosine | 40.79 | X | - |
| mannitol | 41.02 | - | X |
| gluconic acid | 42.20 | - | X |
| palmitoleic acid | 42.46 | X | - |
| mucic acid | 42.54 | - | X |
| scyllo-inositol | 42.76 | - | X |
| myo-inositol | 44.78 | X | X |
| oleic acid | 46.80 | X | - |
| stearic acid | 47.33 | X | - |
| oleamide | 50.53 | X | - |
| sucrose | 53.72 | - | X |
| cholesterol | 61.47 | X | - |
| Appear | Flip | ||
|---|---|---|---|
| HBV | plasma | 8 | 1 |
| urine | 2 | 0 | |
| HCV | plasma | 13 | 1 |
| urine | 5 | 1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simillion, C.; Semmo, N.; Idle, J.R.; Beyoğlu, D. Robust Regression Analysis of GCMS Data Reveals Differential Rewiring of Metabolic Networks in Hepatitis B and C Patients. Metabolites 2017, 7, 51. https://doi.org/10.3390/metabo7040051
Simillion C, Semmo N, Idle JR, Beyoğlu D. Robust Regression Analysis of GCMS Data Reveals Differential Rewiring of Metabolic Networks in Hepatitis B and C Patients. Metabolites. 2017; 7(4):51. https://doi.org/10.3390/metabo7040051
Chicago/Turabian StyleSimillion, Cedric, Nasser Semmo, Jeffrey R. Idle, and Diren Beyoğlu. 2017. "Robust Regression Analysis of GCMS Data Reveals Differential Rewiring of Metabolic Networks in Hepatitis B and C Patients" Metabolites 7, no. 4: 51. https://doi.org/10.3390/metabo7040051





