Metabolomics of Therapy Response in Preclinical Glioblastoma: A Multi-Slice MRSI-Based Volumetric Analysis for Noninvasive Assessment of Temozolomide Treatment
Abstract
:1. Introduction
2. Results
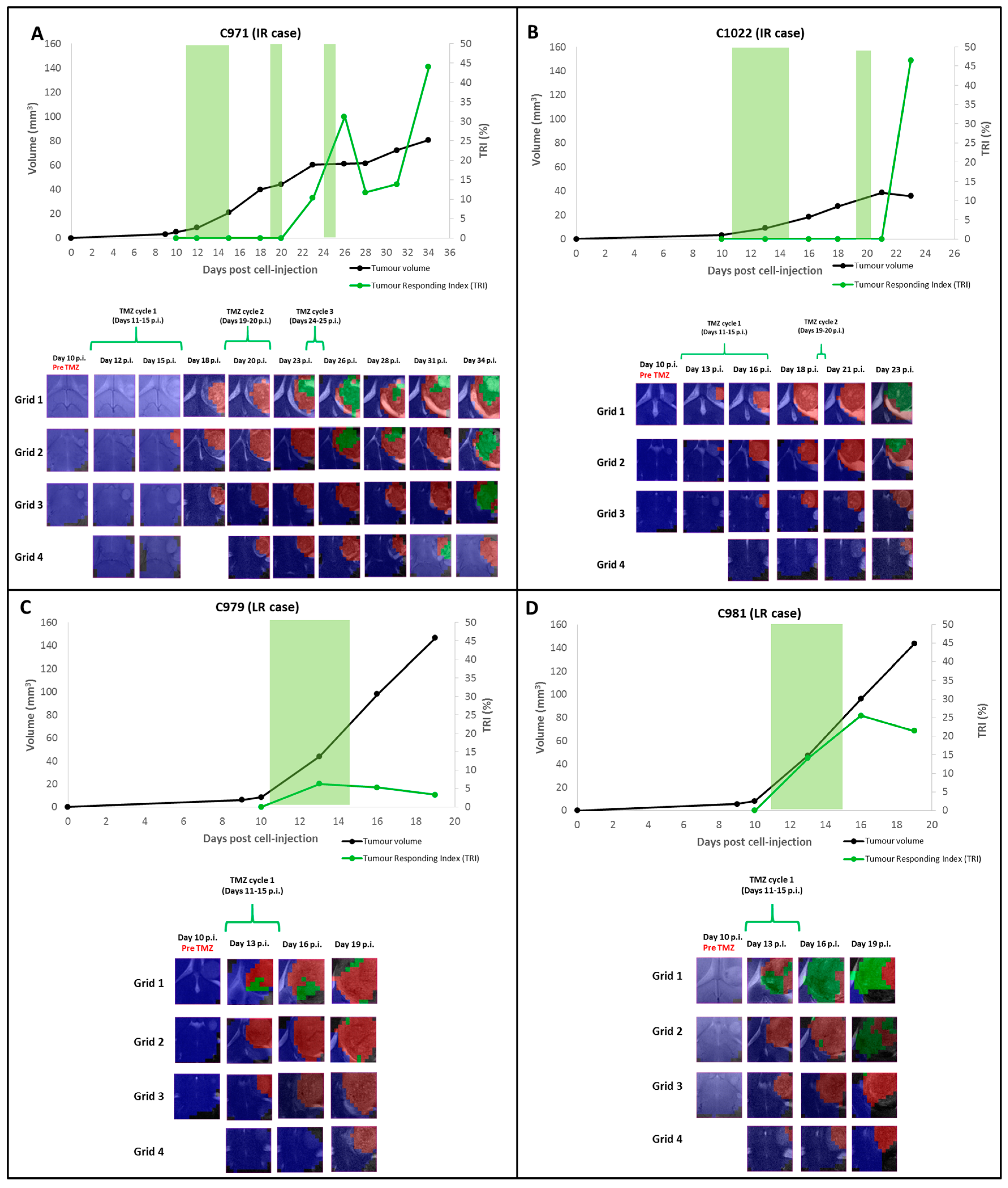
2.1. Group A: Cases Starting Therapy at Day 11 p.i.: Inclusion into Different Groups of Response Level
2.1.1. TRI and Nosological Images Evolution vs. Tumor Volume Evolution
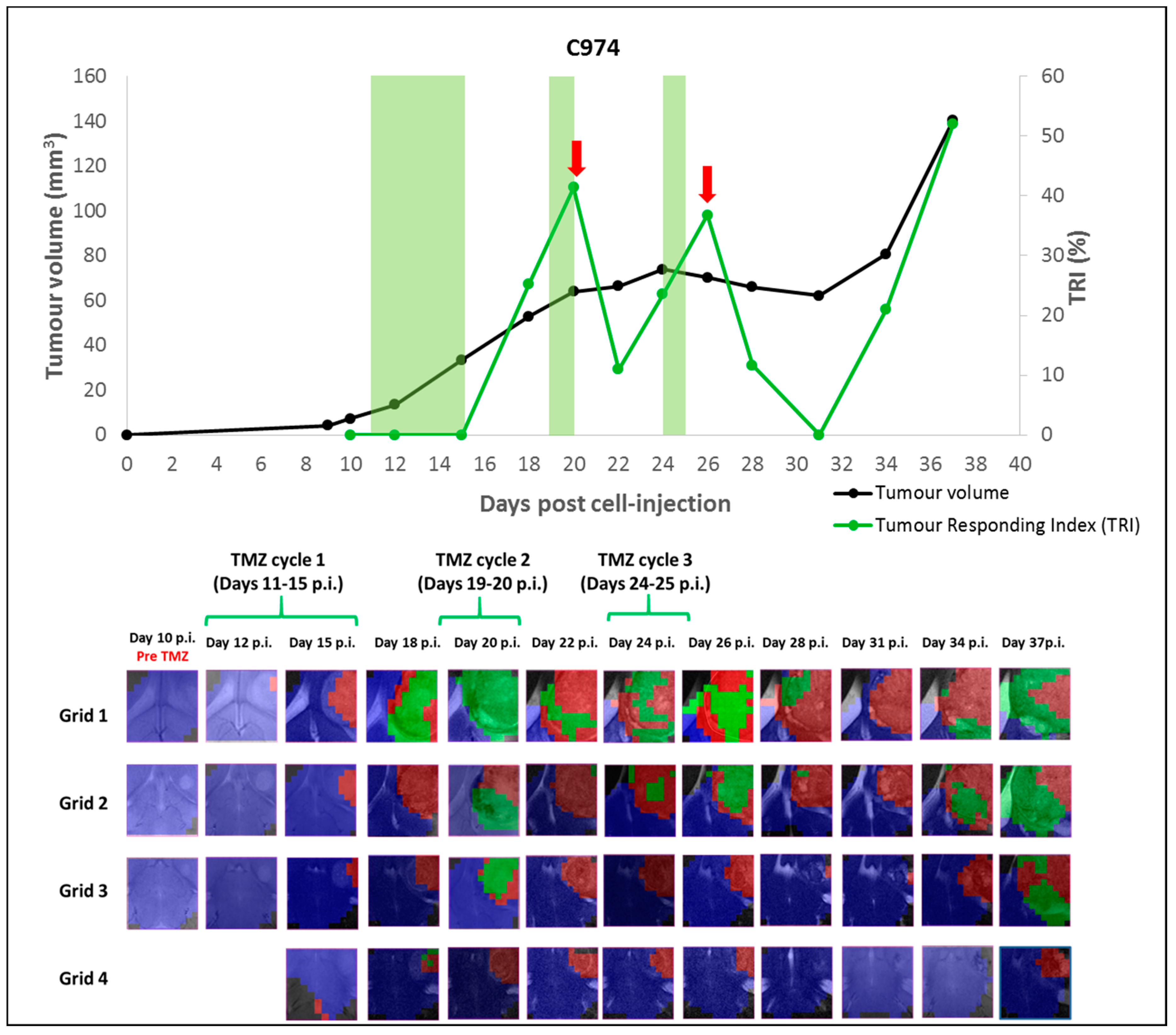
2.1.2. Case C974: Tracking the Evolution of an IR Case along Time
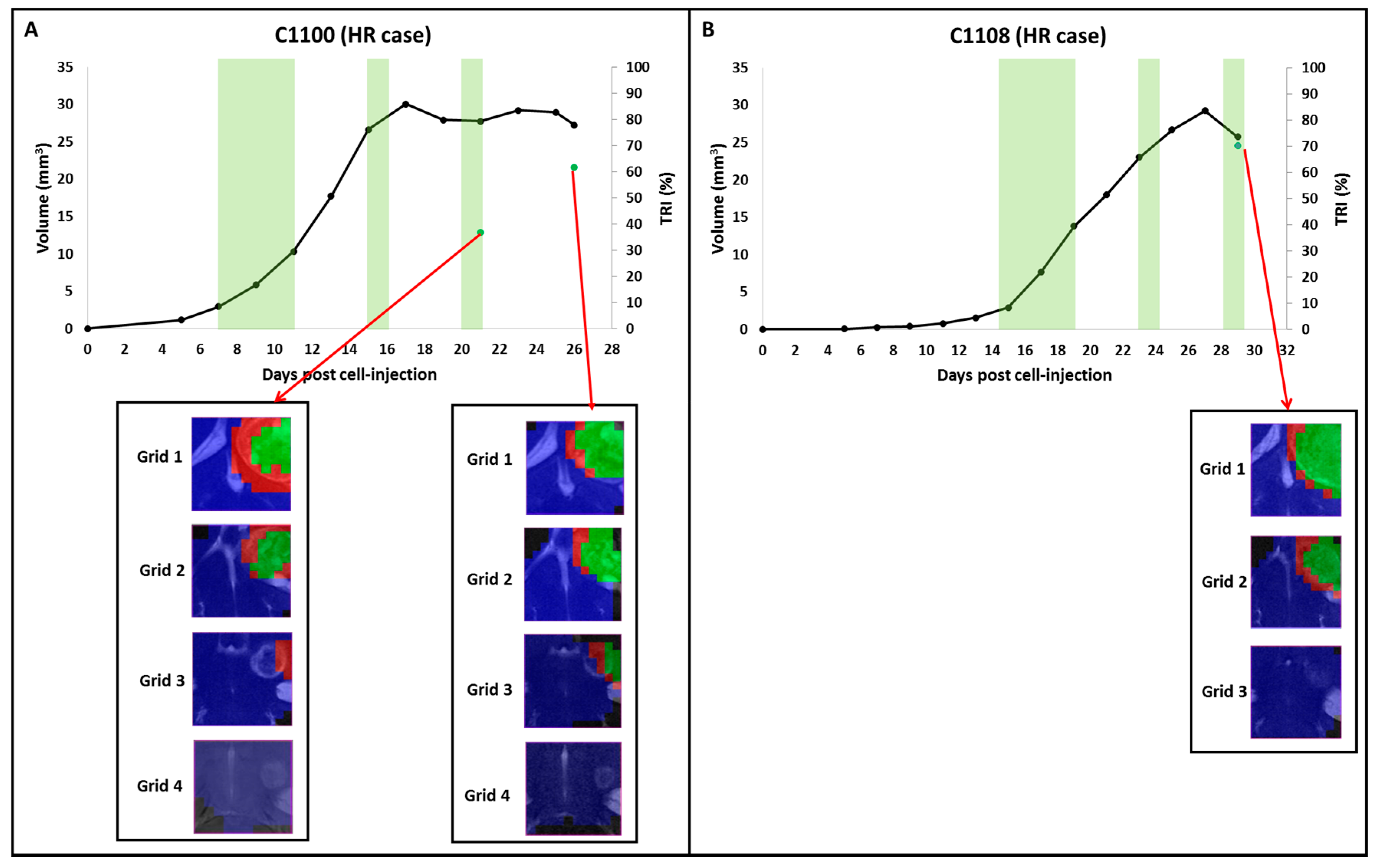
2.2. Group B: Therapy Response in Cases Starting Therapy with Tumor Volume 3–5 mm3: Finding High Response (HR) Cases
2.3. Metabolic Pattern Contributing to Responding and Non-Responding (Red and Green) Areas Detected in MRSI Studies of the Investigated Mice
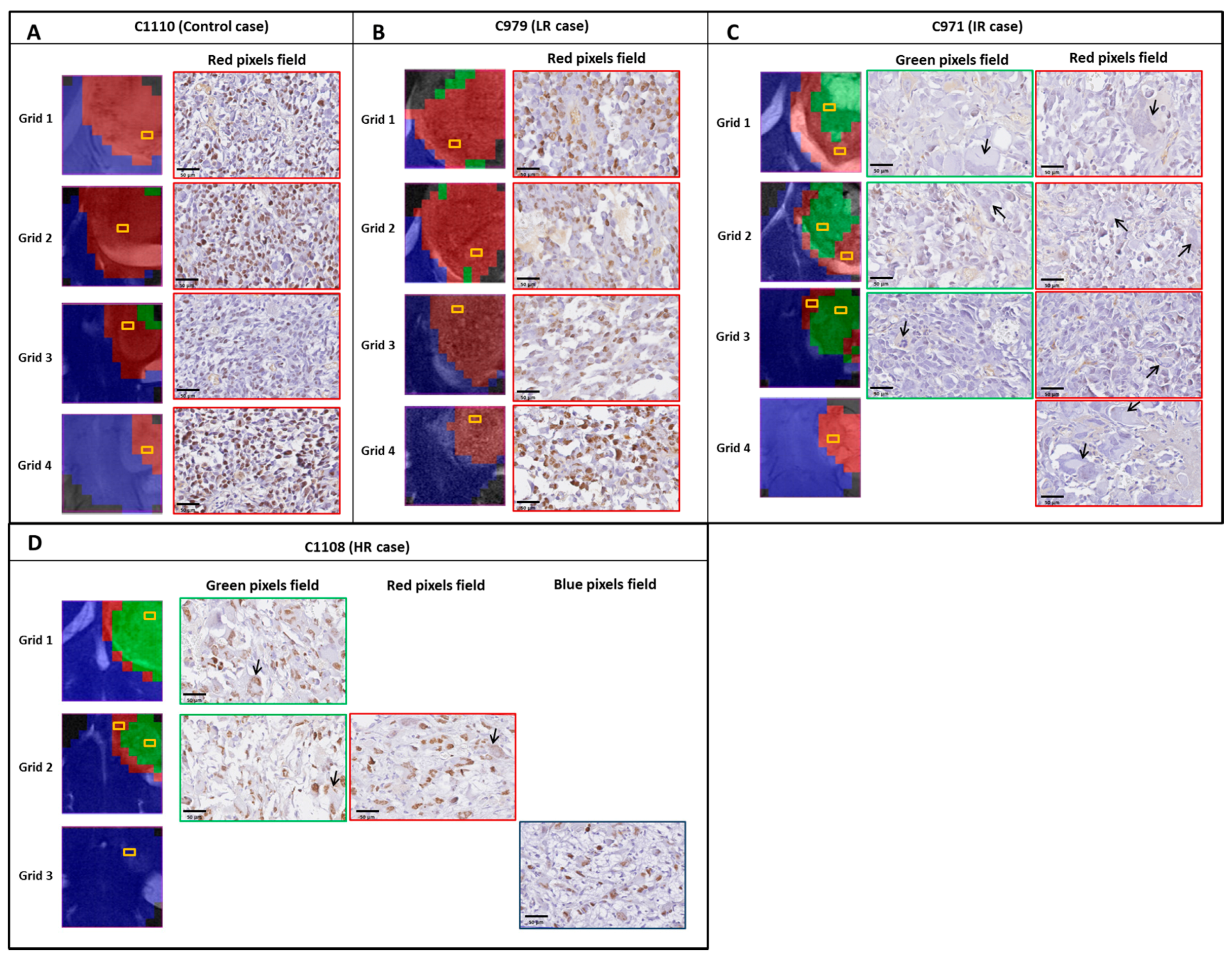
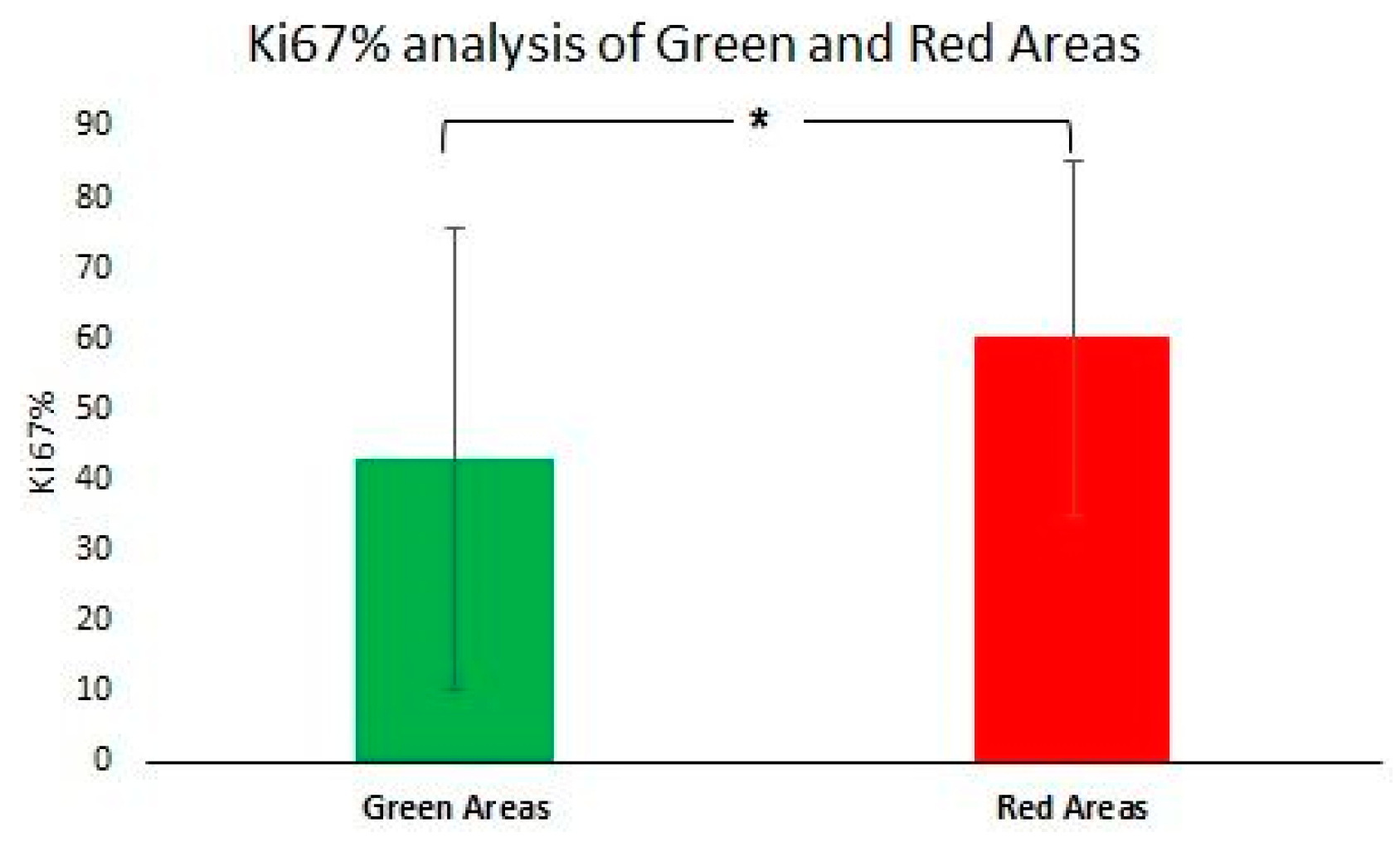
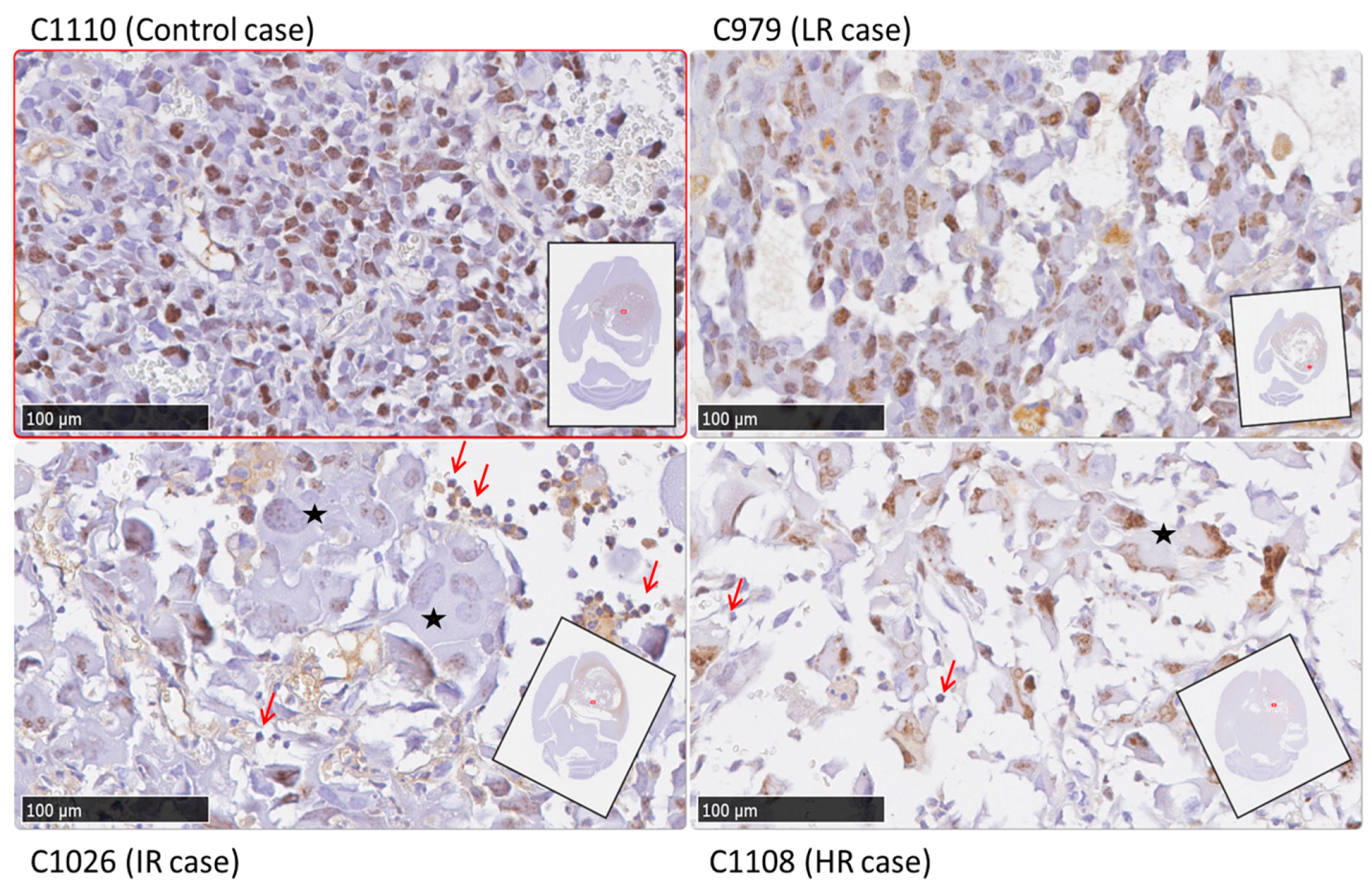
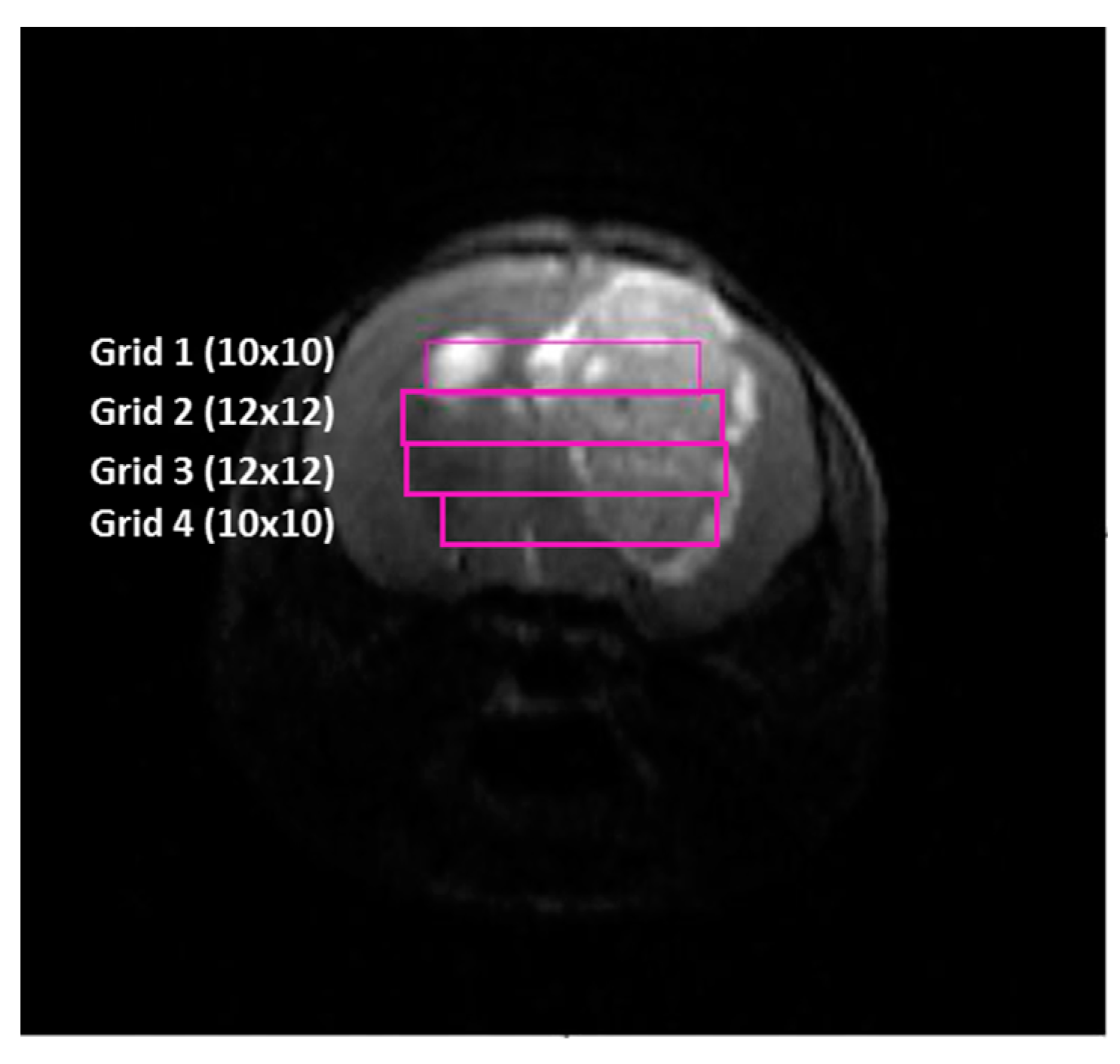
2.4. Histopathology Validation
3. Discussion
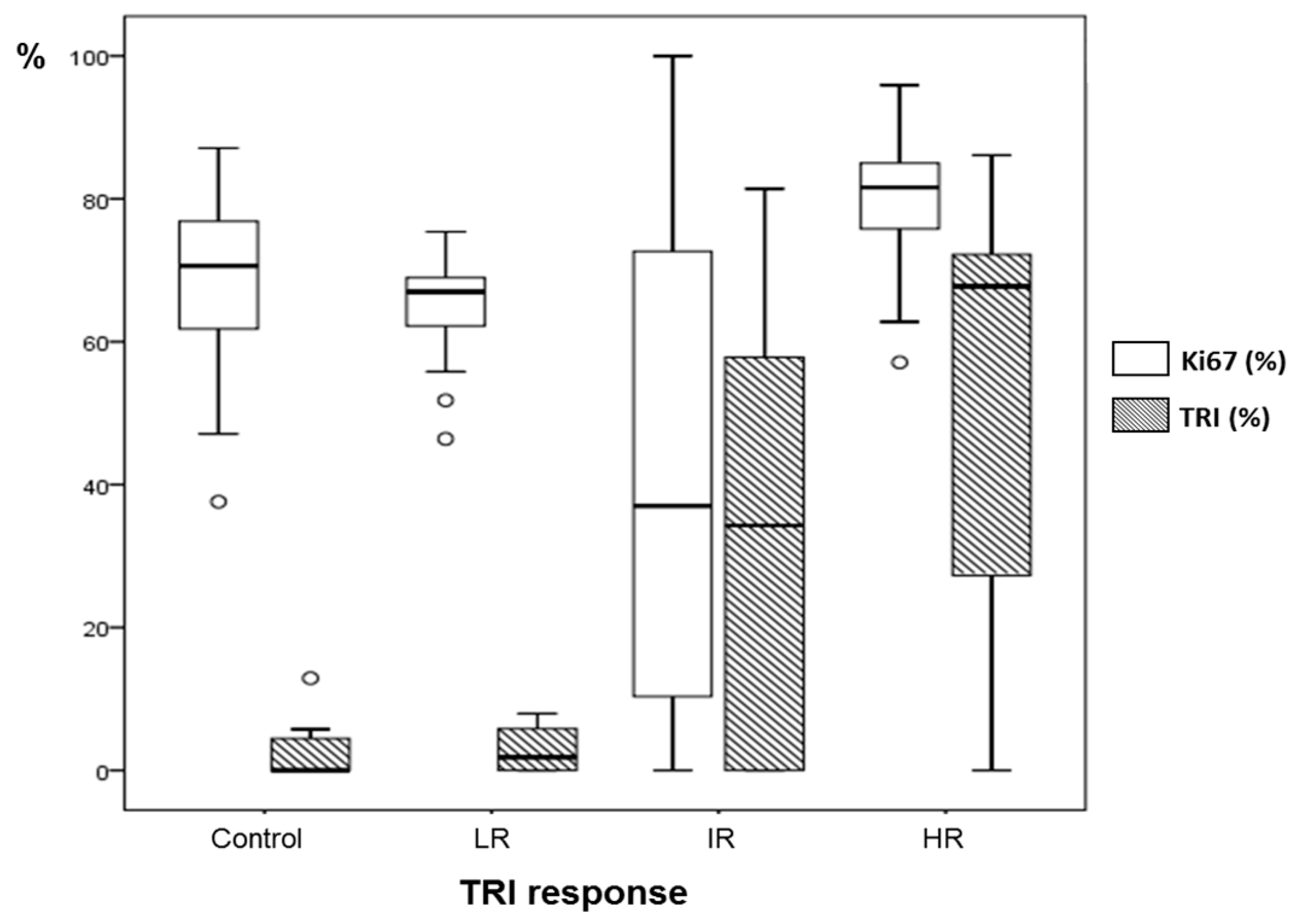
3.1. Multi-Slice MRSI and TRI for Therapy Response Level Evaluation
3.2. Classification of TMZ-Treated Mice after TRI Calculation and Evolution of TRI Values
3.3. Histopathology Results
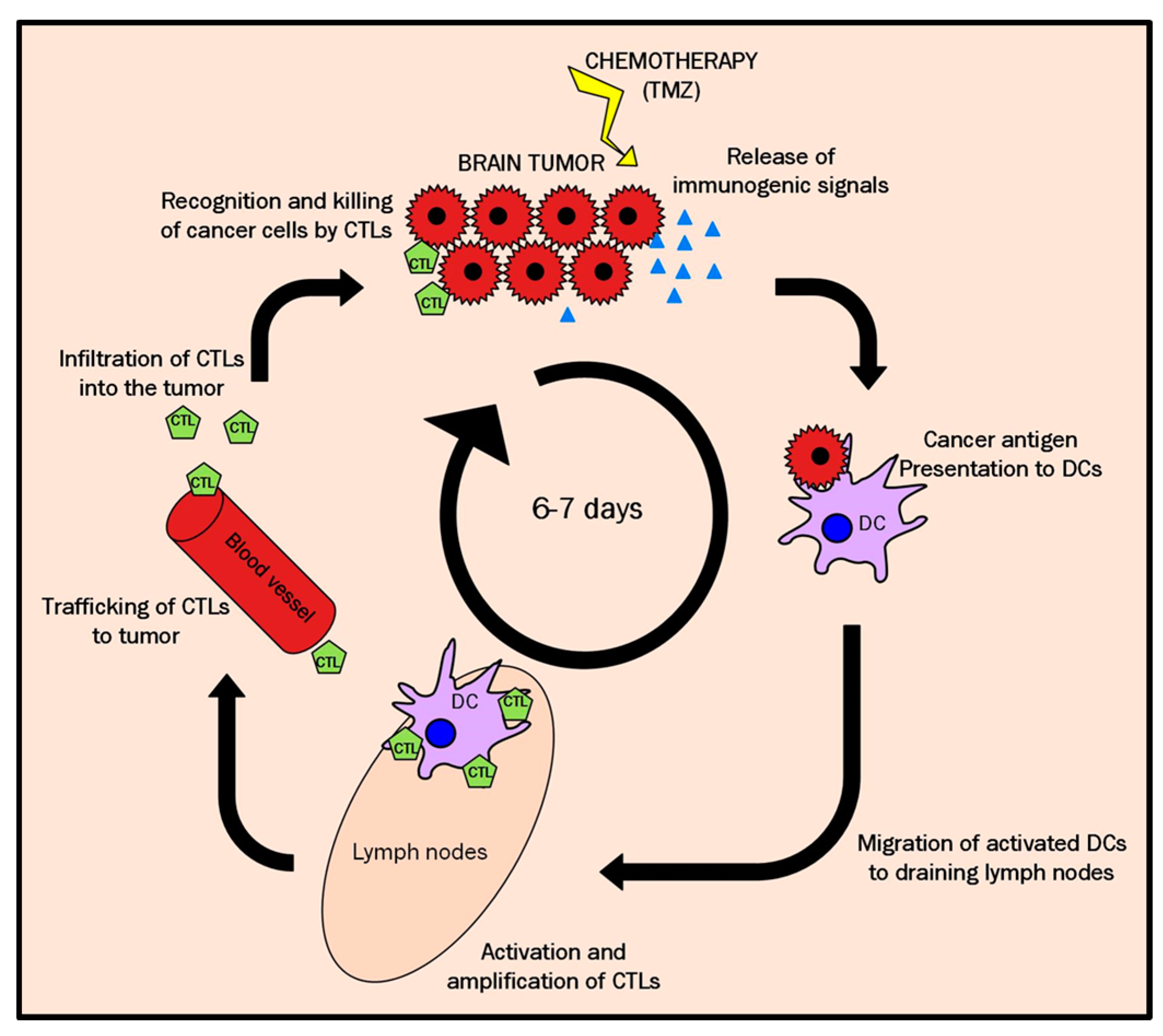
3.4. A Possible Explanation for the Cyclical TRI Behavior: TMZ Therapy Triggering Immune Response in Host
4. Materials and Methods
4.1. GL261 Cells
4.2. Preclinical Glioblastoma Model for in vivo Studies
4.3. Animal Treatment with Temozolomide
4.4. In vivo MRI and MRSI Studies
4.4.1. Data Acquisition
4.4.2. MRI and MRSI Processing and Post Processing
4.5. Tumor Responding Index (TRI) Calculations
Animal Euthanasia and Sample Storage
4.6. Histopathology Studies
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Ala | Alanine |
| Cho | Choline |
| Cre | Creatine |
| CTLs | Cytotoxic T-lymphocytes |
| DMPM | Dynamic MRSI processing Module |
| DWI | Diffusion Weighted Imaging |
| ETL | Echo train length |
| FOV | Field of view |
| GABRMN | Grup d’Aplicacions Biomèdiques de la RMN |
| GBM | Glioblastoma |
| GIC | Glioma-initiating cells |
| Glx | Glutamine + glutamate |
| HE | Hematoxylin-eosin |
| HR | High response |
| IQR | Interquartile range |
| Ins + Gly | Myo-inositol + glycine |
| IR | Intermediate response |
| Lac | Lactate |
| LR | Low response |
| ML | Mobile lipids |
| MM | Macromolecules |
| MTX | Matrix size |
| NA | Number of averages |
| NAA | N-acetyl-aspartate |
| NAc | N-acetyl group containing compounds |
| NMF | Non-negative matrix factorization |
| NS | Number of slices |
| PBS | Phosphate-buffered saline |
| p.i. | Post-inoculation |
| ppm | parts per million |
| PR | Pattern recognition |
| PUFA | Polyunsaturated fatty acids |
| RANO | Response assessment in neuro-oncology |
| RARE | Rapid acquisition with relaxation enhancement |
| ST | Slice thickness |
| TAT | Total acquisition time |
| TEeff | Effective echo time |
| TMZ | Temozolomide |
| TR | Repetition time |
| TRI | Tumor responding index |
| VOI | Volume of interest |
| 3DiCSI | 3D interactive chemical shift imaging |
References
- Buckner, J.C. Factors influencing survival in high-grade gliomas. Semin. Oncol. 2003, 30, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Horska, A.; Barker, P.B. Imaging of brain tumors: MR spectroscopy and metabolic imaging. Neuroimaging Clin. N. Am. 2010, 20, 293–310. [Google Scholar] [CrossRef] [PubMed]
- Vogelbaum, M.A.; Jost, S.; Aghi, M.K.; Heimberger, A.B.; Sampson, J.H.; Wen, P.Y.; MacDonald, D.R.; Van Den Bent, M.J.; Chang, S.M. Application of novel response/progression measures for surgically delivered therapies for gliomas: Response Assessment in Neuro-Oncology (RANO) working group. Neurosurgery 2012, 70, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Hygino Da Cruz, L.C.; Rodriguez, I.; Domingues, R.C.; Gasparetto, E.L.; Sorensen, A.G. Pseudoprogression and pseudoresponse: Imaging challenges in the assessment of posttreatment glioma. Am. J. Neuroradiol. 2011, 32, 1978–1985. [Google Scholar] [CrossRef] [PubMed]
- Hattingen, E.; Jurcoane, A.; Bähr, O.; Rieger, J.; Magerkurth, J.; Anti, S.; Steinbach, J.P.; Pilatus, U. Bevacizumab impairs oxidative energy metabolism and shows antitumoral effects in recurrent glioblastomas: A 31P/1H MRSI and quantitative magnetic resonance imaging study. Neuro Oncol. 2011, 13, 1349–1363. [Google Scholar] [CrossRef] [PubMed]
- Nelson, S.J. Assessment of therapeutic response and treatment planning for brain tumors using metabolic and physiological MRI. NMR Biomed. 2011, 24, 734–749. [Google Scholar] [CrossRef] [PubMed]
- Segebarth, C.M.; Balériaux, D.F.; Luyten, P.R.; den Hollander, J.A. Detection of metabolic heterogeneity of human intracranial tumors in vivo by 1H-NMR spectroscopic imaging. Magn. Reson. Med. 1990, 13, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Martorell, S.; Julià-Sapé, M.; Lisboa, P.; Arús, C. Pattern Recognition Analysis of MR Spectra. In eMagRes; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; pp. 945–958. [Google Scholar]
- Laudadio, T.; Martínez-Bisbal, M.C.; Celda, B.; Van Huffel, S. Fast nosological imaging using canonical correlation analysis of brain data obtained by two-dimensional turbo spectroscopic imaging. NMR Biomed. 2008, 21, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Simões, R.V.; Ortega-Martorell, S.; Delgado-Goñi, T.; Le Fur, Y.; Pumarola, M.; Candiota, A.P.; Martín, J.; Stoyanova, R.; Cozzone, P.J.; Julià-Sapé, M.; et al. Improving the classification of brain tumors in mice with perturbation enhanced (PE)-MRSI. Integr. Biol. 2012, 4, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Goñi, T.; Ortega-Martorell, S.; Ciezka, M.; Olier, I.; Candiota, A.P.; Julià-Sapé, M.; Fernández, F.; Pumarola, M.; Lisboa, P.J.; Arús, C. MRSI-based molecular imaging of therapy response to temozolomide in preclinical glioblastoma using source analysis. NMR Biomed. 2016, 29, 732–743. [Google Scholar] [CrossRef] [PubMed]
- Marrero, L.; Wyczechowska, D.; Musto, A.E.; Wilk, A.; Vashistha, H.; Zapata, A.; Walker, C.; Velasco-Gonzalez, C.; Parsons, C.; Wieland, S.; et al. Therapeutic Efficacy of Aldoxorubicin in an Intracranial Xenograft Mouse Model of Human Glioblastoma. Neoplasia 2014, 16, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Burton, E.C.; Lamborn, K.R.; Forsyth, P.; Scott, J.; O’Campo, J.; Uyehara-Lock, J.; Prados, M.; Berger, M.; Passe, S.; Uhm, J.; et al. Aberrant p53, mdm2, and proliferation differ in glioblastomas from long-term compared with typical survivors. Clin. Cancer Res. 2002, 8, 180–187. [Google Scholar] [PubMed]
- Ausman, J.I.; Shapiro, W.R.; Rall, D.P. Studies on the chemotherapy of experimental brain tumors: development of an experimental model. Cancer Res. 1970, 30, 2394–2400. [Google Scholar] [PubMed]
- Szatmári, T.; Lumniczky, K.; Désaknai, S.; Trajcevski, S.; Hídvégi, E.J.; Hamada, H.; Sáfrány, G. Detailed characterization of the mouse glioma 261 tumor model for experimental glioblastoma therapy. Cancer Sci. 2006, 97, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Goñi, T.; Julià-Sapé, M.; Candiota, A.P.; Pumarola, M.; Arús, C. Molecular imaging coupled to pattern recognition distinguishes response to temozolomide in preclinical glioblastoma. NMR Biomed. 2014, 27, 1333–1345. [Google Scholar] [CrossRef] [PubMed]
- Soeda, A.; Hara, A.; Kunisada, T.; Yoshimura, S.; Iwama, T.; Park, D.M. The Evidence of Glioblastoma Heterogeneity. Sci. Rep. 2015, 5, 7979. [Google Scholar] [CrossRef] [PubMed]
- Eder, K.; Kalman, B. Molecular Heterogeneity of Glioblastoma and its Clinical Relevance. Pathol. Oncol. Res. 2014, 20, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Stieber, D.; Golebiewska, A.; Evers, L.; Lenkiewicz, E.; Brons, N.H.C.; Nicot, N.; Oudin, A.; Bougnaud, S.; Hertel, F.; Bjerkvig, R.; et al. Glioblastomas are composed of genetically divergent clones with distinct tumourigenic potential and variable stem cell-associated phenotypes. Acta Neuropathol. 2014, 127, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Remy, C.; Arus, C.; Ziegler, A.; Lai, E.S.; Moreno, A.; Le Fur, Y.; Decorps, M. In vivo, ex vivo and in vitro one- and two-dimensional nuclear magnetic resonance spectroscopy of an intracerebral glioma in rat brain: Assignment of resonances. J. Neurochem. 1994, 62, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Barba, I.; Cabanas, M.E.; Arus, C. The relationship between nuclear magnetic resonance-visible lipids, lipid droplets, and cell proliferation in cultured C6 cells. Cancer Res. 1999, 59, 1861–1868. [Google Scholar] [PubMed]
- Barba, I.; Mann, P.; Cabanas, M.E.; Arus, C.; Gasparovic, C. Mobile lipid production after confluence and pH stress in perfused C6 cells. NMR Biomed. 2001, 14, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Valverde, D.; Quintero, M.R.; Candiota, A.P.; Badiella, L.; Cabanas, M.E.; Arus, C.; Cabañas, M.E.; Arús, C. Analysis of the changes in the 1H-NMR spectral pattern of perchloric acid extracts of C6 cells with growth. NMR Biomed. 2006, 19, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Simões, R.V.; García-Martín, M.L.; Cerdán, S.; Arús, C. Perturbation of mouse glioma MRS pattern by induced acute hyperglycemia. NMR Biomed. 2008, 21, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Simoes, R.V.; Delgado-Goñi, T.; Lope-Piedrafita, S.; Arus, C. 1H-MRSI pattern perturbation in a mouse glioma: The effects of acute hyperglycemia and moderate hypothermia. NMR Biomed. 2010, 23, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Davila, M.; Candiota, A.P.; Pumarola, M.; Arus, C. Minimization of spectral pattern changes during HRMAS experiments at 37 degrees celsius by prior focused microwave irradiation. Magn. Reson. Mater. Phys. 2012, 25, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Hulsey, K.M.; Mashimo, T.; Banerjee, A.; Soesbe, T.C.; Spence, J.S.; Vemireddy, V.; Maher, E.A.; Bachoo, R.M.; Choi, C. 1H-MRS characterization of neurochemical profiles in orthotopic mouse models of human brain tumors. NMR Biomed. 2015, 28, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Hakumäki, J.M.; Poptani, H.; Sandmair, A.M.; Ylä-Herttuala, S.; Kauppinen, R.A. 1H-MRS detects polyunsaturated fatty acid accumulation during gene therapy of glioma: implications for the in vivo detection of apoptosis. Nat. Med. 1999, 5, 1323–1327. [Google Scholar] [CrossRef] [PubMed]
- Candiota, A.P.; Majos, C.; Bassols, A.; Cabanas, M.E.; Acebes, J.J.; Quintero, M.R.; Arus, C. Assignment of the 2.03 ppm resonance in in vivo 1H-MRS of human brain tumour cystic fluid: Contribution of macromolecules. Magn. Reson. Mater. Phys. 2004, 17, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Govindaraju, V.; Young, K.; Maudsley, A.A. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000, 13, 129–153. [Google Scholar] [CrossRef]
- McKnight, T.R.; Noworolski, S.M.; Vigneron, D.B.; Nelson, S.J. An automated technique for the quantitative assessment of 3D-MRSI data from patients with glioma. J. Magn. Reson. Imaging 2001, 13, 167–177. [Google Scholar] [CrossRef]
- Ratai, E.-M.; Zhang, Z.; Snyder, B.S.; Boxerman, J.L.; Safriel, Y.; McKinstry, R.C.; Bokstein, F.; Gilbert, M.R.; Sorensen, A.G.; Barboriak, D.P. Magnetic resonance spectroscopy as an early indicator of response to anti-angiogenic therapy in patients with recurrent glioblastoma: RTOG 0625/ACRIN 6677. Neuro Oncol. 2013, 15, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Provencher, S.W. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001, 14, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Segerman, A.; Niklasson, M.; Haglund, C.; Bergström, T.; Jarvius, M.; Xie, Y.; Westermark, A.; Sönmez, D.; Hermansson, A.; Kastemar, M.; et al. Clonal Variation in Drug and Radiation Response among Glioma-Initiating Cells Is Linked to Proneural-Mesenchymal Transition. Cell Rep. 2016, 17, 2994–3009. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.E.; Moffat, B.A.; Stojanovska, J.; Johnson, T.D.; Li, Z.; Hamstra, D.A.; Rehemtulla, A.; Chenevert, T.L.; Carter, J.; Pietronigro, D.; et al. Therapeutic efficacy of DTI-015 using diffusion magnetic resonance imaging as an early surrogate marker. Clin. Cancer Res. 2004, 10, 7852–7859. [Google Scholar] [CrossRef] [PubMed]
- Oborski, M.J.; Laymon, C.M.; Qian, Y.; Lieberman, F.S.; Nelson, A.D.; Mountz, J.M. Challenges and Approaches to Quantitative Therapy Response Assessment in Glioblastoma Multiforme Using the Novel Apoptosis Positron Emission Tomography Tracer F-18 ML-10. Transl. Oncol. 2014, 7, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, K.; Mizoguchi, M.; Hata, N.; Murata, H.; Hatae, R.; Amano, T.; Nakamizo, A.; Sasaki, T. Complex DNA repair pathways as possible therapeutic targets to overcome temozolomide resistance in glioblastoma. Front. Oncol. 2012, 2, 186. [Google Scholar] [CrossRef] [PubMed]
- El Meskini, R.; Iacovelli, A.J.; Kulaga, A.; Gumprecht, M.; Martin, P.L.; Baran, M.; Householder, D.B.; Van Dyke, T.; Weaver Ohler, Z. A preclinical orthotopic model for glioblastoma recapitulates key features of human tumors and demonstrates sensitivity to a combination of MEK and PI3K pathway inhibitors. Dis. Model. Mech. 2015, 8, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; McFarland, B.C.; Drygin, D.; Yu, H.; Bellis, S.L.; Kim, H.; Bredel, M.; Benveniste, E.N. Targeting protein kinase CK2 suppresses prosurvival signaling pathways and growth of glioblastoma. Clin. Cancer Res. 2013, 19, 6484–6494. [Google Scholar] [CrossRef] [PubMed]
- Mastronardi, L.; Guiducci, A.; Puzzilli, F.; Ruggeri, A. Relationship between Ki-67 labeling index and survival in high-grade glioma patients treated after surgery with tamoxifen. J. Neurosurg. Sci. 1999, 43, 263–270. [Google Scholar] [PubMed]
- Skjulsvik, A.J.; Mørk, J.N.; Torp, M.O.; Torp, S.H. Ki-67/MIB-1 immunostaining in a cohort of human gliomas. Int. J. Clin. Exp. Pathol. 2014, 7, 8905–8910. [Google Scholar] [PubMed]
- Perry, A.; Schmidt, R.E. Cancer therapy-associated CNS neuropathology: An update and review of the literature. Acta Neuropathol. 2006, 111, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Lucas, D.R.; Kshirsagar, M.P.; Biermann, J.S.; Hamre, M.R.; Thomas, D.G.; Schuetze, S.M.; Baker, L.H. Histologic Alterations from Neoadjuvant Chemotherapy in High-Grade Extremity Soft Tissue Sarcoma: Clinicopathological Correlation. Oncologist 2008, 13, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Font, L.; Villamañan, L.; Arias-Ramos, N.; Vilardell, J.; Plana, M.; Ruzzene, M.; Pinna, L.; Itarte, E.; Arús, C.; Candiota, A.P. Targeting Protein Kinase CK2: Evaluating CX-4945 Potential for GL261 Glioblastoma Therapy in Immunocompetent Mice. Pharmaceuticals 2017, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Liikanen, I.; Ahtiainen, L.; Hirvinen, M.L.M.; Bramante, S.; Cerullo, V.; Nokisalmi, P.; Hemminki, O.; Diaconu, I.; Pesonen, S.; Koski, A.; et al. Oncolytic adenovirus with temozolomide induces autophagy and antitumor immune responses in cancer patients. Mol. Ther. 2013, 21, 1212–1223. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Jordan, M.; Waxman, D.J. Metronomic cyclophosphamide activation of anti-tumor immunity: Tumor model, mouse host, and drug schedule dependence of gene responses and their upstream regulators. BMC Cancer 2016, 16, 623. [Google Scholar] [CrossRef] [PubMed]
- Karman, J.; Ling, C.; Sandor, M.; Fabry, Z. Initiation of immune responses in brain is promoted by local dendritic cells. J. Immunol. 2004, 173, 2353–2361. [Google Scholar] [CrossRef] [PubMed]
- Tabbekh, M.; Mokrani-Hammani, M.; Bismuth, G.; Mami-Chouaib, F. T-cell modulatory properties of CD5 and its role in antitumor immune responses. Oncoimmunology 2013, 2, e22841. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Mellman, I. Oncology meets immunology: The cancer-immunity cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Vacchelli, E.; Aranda, F.; Eggermont, A.; Galon, J.; Sautès-fridman, C.; Cremer, I.; Zitvogel, L.; Kroemer, G.; Galluzzi, L. Chemotherapy with immunogenic cell death inducers. Oncoimmunology 2014, 3, e27878. [Google Scholar] [CrossRef] [PubMed]
- Balermpas, P.; Michel, Y.; Wagenblast, J.; Seitz, O.; Weiss, C.; Rödel, F.; Rödel, C.; Fokas, E. Tumour-infiltrating lymphocytes predict response to definitive chemoradiotherapy in head and neck cancer. Br. J. Cancer 2014, 110, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Flomenberg, P.; Harshyne, L.; Kenyon, L.; Andrews, D.W. Preclinical Glioblastoma Patients Exhibit Circulating Tumor-Specific CD8+ T Cells. Clin. Cancer Res. 2005, 11, 5292–5299. [Google Scholar] [CrossRef] [PubMed]
- Ehtesham, M.; Samoto, K.; Kabos, P.; Acosta, F.L.; Gutierrez, M.A.; Black, K.L.; Yu, J.S. Treatment of intracranial glioma with in situ interferon-gamma and tumor necrosis factor-alpha gene transfer. Cancer Gene Ther. 2002, 9, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Waxman, D.J. Metronomic cyclophosphamide eradicates large implanted GL261 gliomas by activating antitumor CD8+ T cell responses and immune memory. Oncoimmunology 2015, 4, e1005521. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Martorell, S.; Ruiz, H.; Vellido, A.; Olier, I.; Romero, E.; Julià-Sapé, M.; Martín, J.D.; Jarman, I.H.; Arús, C.; Lisboa, P.J.G. A novel semi-supervised methodology for extracting tumor type-specific MRS sources in human brain data. PLoS ONE 2013, 8, e83773. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Martorell, S.; Lisboa, P.J.G.; Vellido, A.; Simões, R.V.; Pumarola, M.; Julià-Sapé, M.; Arús, C. Convex Non-Negative Matrix Factorization for Brain Tumor Delimitation from MRSI Data. PLoS ONE 2012, 7, e47824. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Li, T.; Jordan, M.I. Convex and semi-nonnegative matrix factorizations. IEEE Trans. Pattern Anal. Mach. Intell. 2010, 32, 45–55. [Google Scholar] [CrossRef] [PubMed]

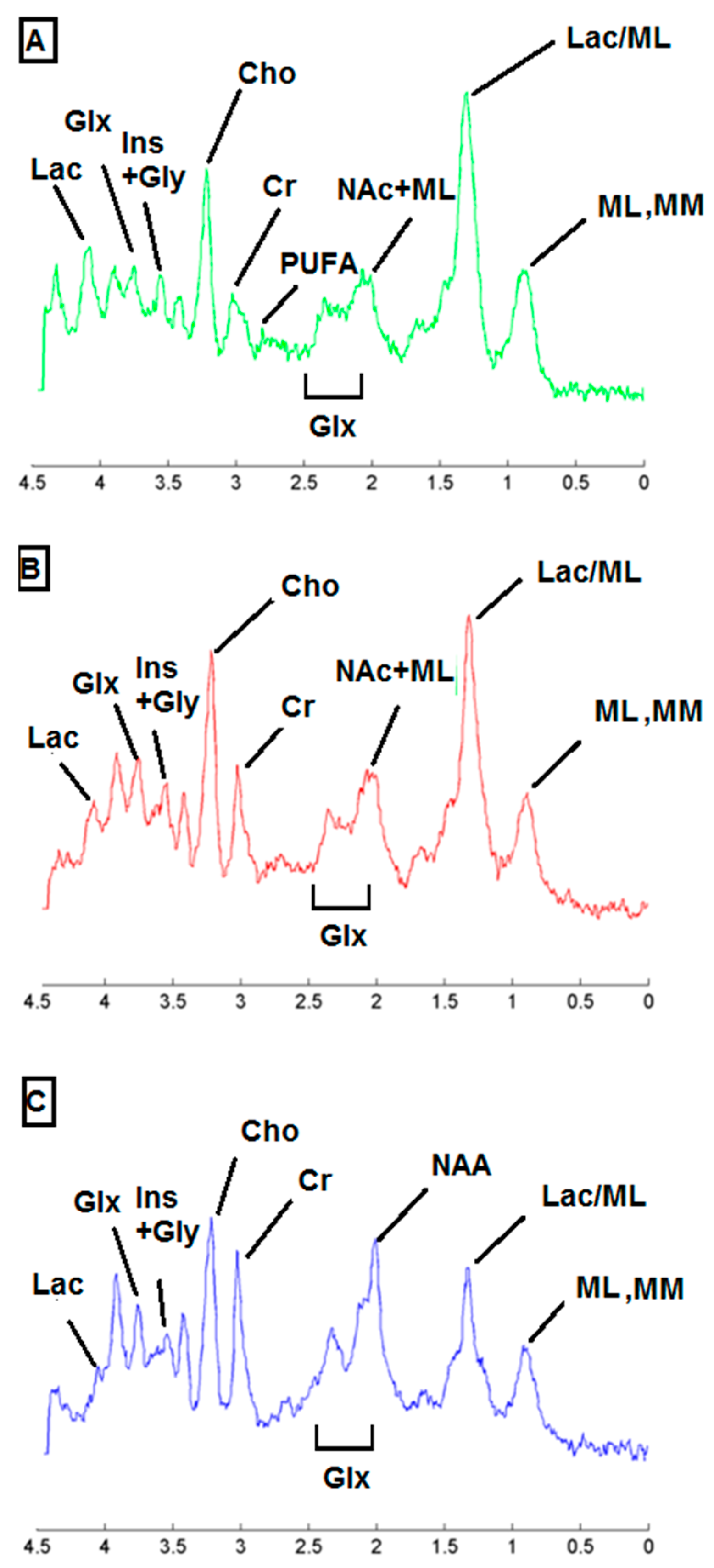

| Metabolite | ppm |
|---|---|
| Mobile Lipids + Macromolecules | 0.90 |
| Mobile Lipids + Lactate | 1.33 |
| Alanine | 1.47 |
| N-acetyl-aspartate + N-acetyl containing compounds + Mobile lipids | 2.02 |
| Glutamate + glutamine | 2.10 − 2.40 + 3.80 |
| PUFA (Mobile Lipids) | 2.80 |
| GABA | 3.00 |
| Total Creatine | 3.03 |
| Choline-containing compounds | 3.21 |
| Scyllo-inositol | 3.34 |
| Taurine | 3.42 |
| Myo-inositol + glycine | 3.55 |
| Lactate | 4.10 |
| Case | Ki67% ± SD (Green Fields) | Ki67% ± SD (Red Fields) | Ki67% ± SD (Global) | TRI% | Classification by TRI Criteria |
|---|---|---|---|---|---|
| C971 | 17.2 ± 16.8 | 25.7 ± 17.0 | 22.0 ± 17.2 | 44.1 | Intermediate |
| C1022 | 19.0 ± 20.9 | 54.9 ± 32.6 * | 42.5 ± 33.5 | 46.5 | Intermediate |
| C1026 | 53.5 ± 30.3 | 73.0 ± 26.8 | 66.0 ± 39.1 | 38.9 | Intermediate |
| C979 | n.a. | 64.8 ± 7.3 | 64.8 ± 7.3 | 3.3 | Low |
| C1100 | 82.9 ± 4.6 | 92.5 ± 0.6 | 82.2 ± 7.7 | 66.3 | High |
| C1108 | 75.1 ± 9.5 | 75.8 ± 0.2 | 79.3 ± 10.1 | 70.3 | High |
| C1110 | n.a. | 63.9 ± 13.0 | 63.9 ± 13.0 | 0 | Control |
| C1111 | n.a. | 73.3 ± 6.4 | 73.3 ± 6.4 | 0 | Control |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arias-Ramos, N.; Ferrer-Font, L.; Lope-Piedrafita, S.; Mocioiu, V.; Julià-Sapé, M.; Pumarola, M.; Arús, C.; Candiota, A.P. Metabolomics of Therapy Response in Preclinical Glioblastoma: A Multi-Slice MRSI-Based Volumetric Analysis for Noninvasive Assessment of Temozolomide Treatment. Metabolites 2017, 7, 20. https://doi.org/10.3390/metabo7020020
Arias-Ramos N, Ferrer-Font L, Lope-Piedrafita S, Mocioiu V, Julià-Sapé M, Pumarola M, Arús C, Candiota AP. Metabolomics of Therapy Response in Preclinical Glioblastoma: A Multi-Slice MRSI-Based Volumetric Analysis for Noninvasive Assessment of Temozolomide Treatment. Metabolites. 2017; 7(2):20. https://doi.org/10.3390/metabo7020020
Chicago/Turabian StyleArias-Ramos, Nuria, Laura Ferrer-Font, Silvia Lope-Piedrafita, Victor Mocioiu, Margarida Julià-Sapé, Martí Pumarola, Carles Arús, and Ana Paula Candiota. 2017. "Metabolomics of Therapy Response in Preclinical Glioblastoma: A Multi-Slice MRSI-Based Volumetric Analysis for Noninvasive Assessment of Temozolomide Treatment" Metabolites 7, no. 2: 20. https://doi.org/10.3390/metabo7020020









