Staphylococcus aureus Infection Reduces Nutrition Uptake and Nucleotide Biosynthesis in a Human Airway Epithelial Cell Line
Abstract
:1. Introduction
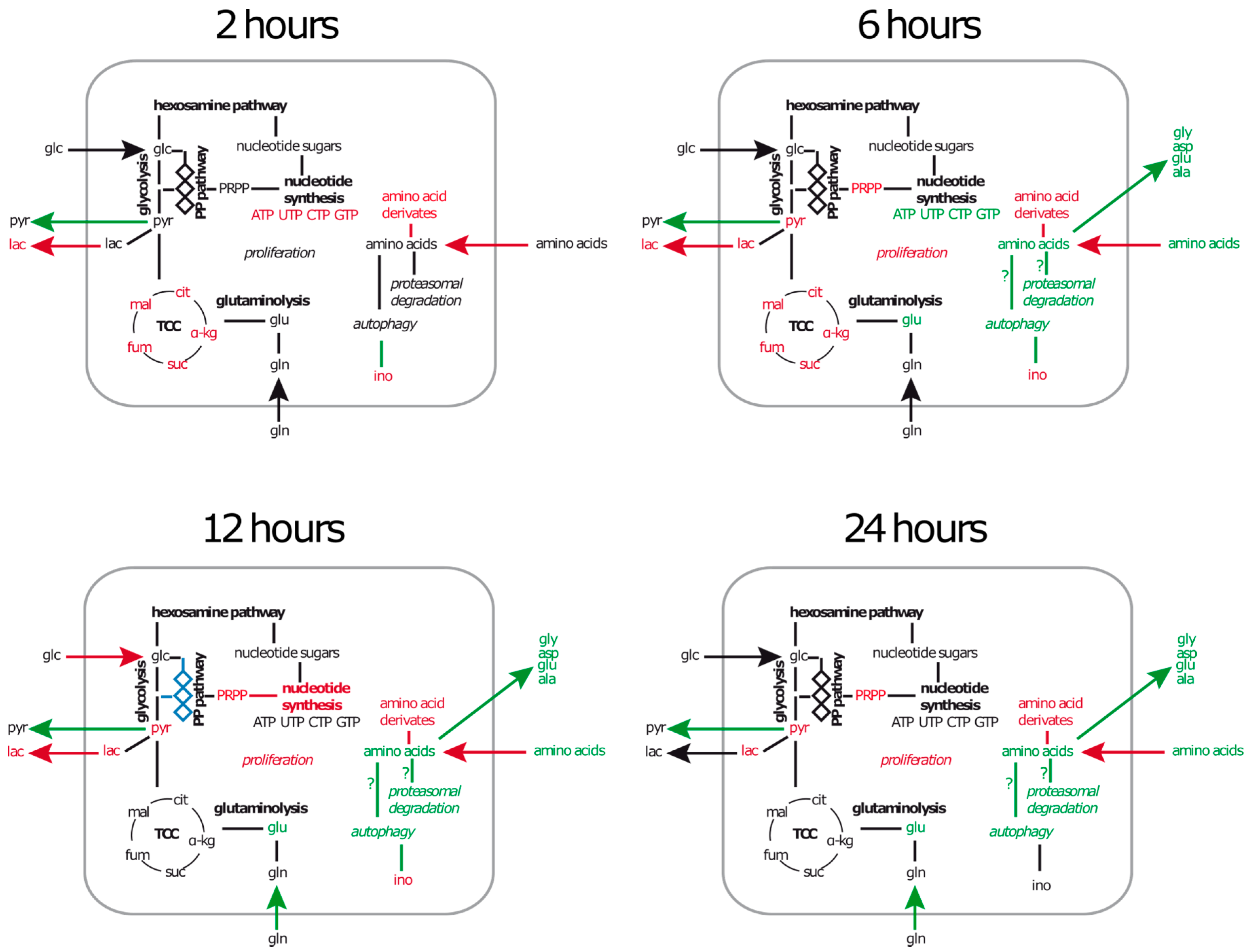
2. Results
2.1. A549 Cells Enter Growth Arrest after Exposure to S. aureus
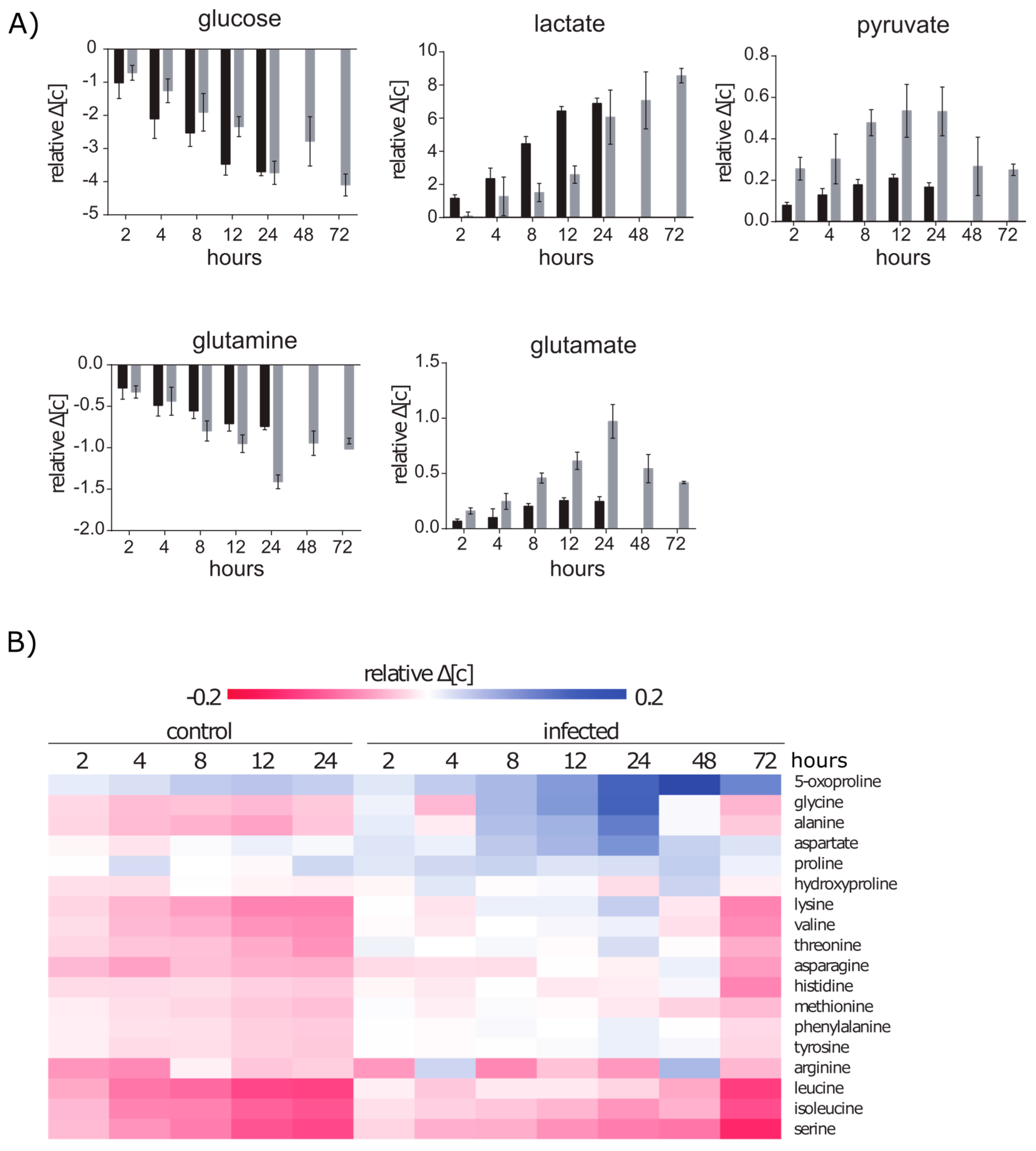
2.2. Extracellular Metabolic Profiles of A549 Cells after Infection with S. aureus
2.2.1. Glucose and Glutamine Are Consumed by A549 under Control and Infection Conditions
2.2.2. Amino Acid Uptake Is Reduced in the Infected A549 Cell Culture but the Secretion of Amino Acids Is Enhanced
2.3. Amino Acids, Amino Acid Derivatives and Nucleotides Show Major Differences after Infection
2.3.1. Intracellular Amino Acids Depleted in Growing Cells, but Accumulated in Infected Cells
2.3.2. Metabolites of Central Carbon Metabolism Show Only Minor Changes under Infection Conditions
2.3.3. During Infection the Concentration of Secondary Metabolites Declines
2.3.4. The Nucleotide Profile Showed Elevated CTP and GTP Levels in Infected A549 Cells
2.4. Metabolic Profiles of Infected A549 Cells in the Presence of Glutamine Analogues Indicate a Different Enzyme Activity after Infection
2.4.1. Inhibition of Purine and Pyrimidine Synthesis by DON and Azaserine
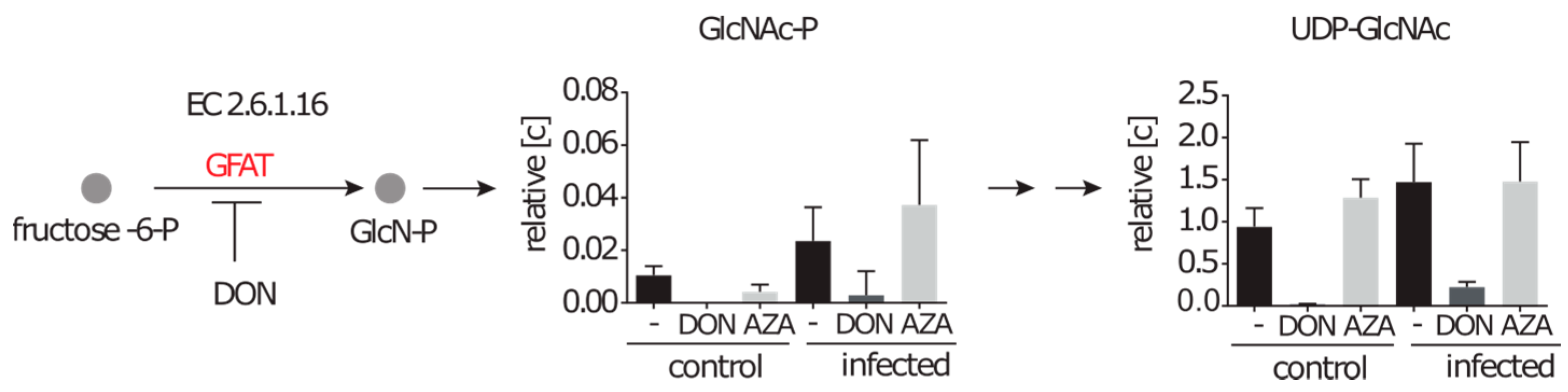
2.4.2. Inhibition of the Hexosamine Pathway by DON under Control and Infection Conditions
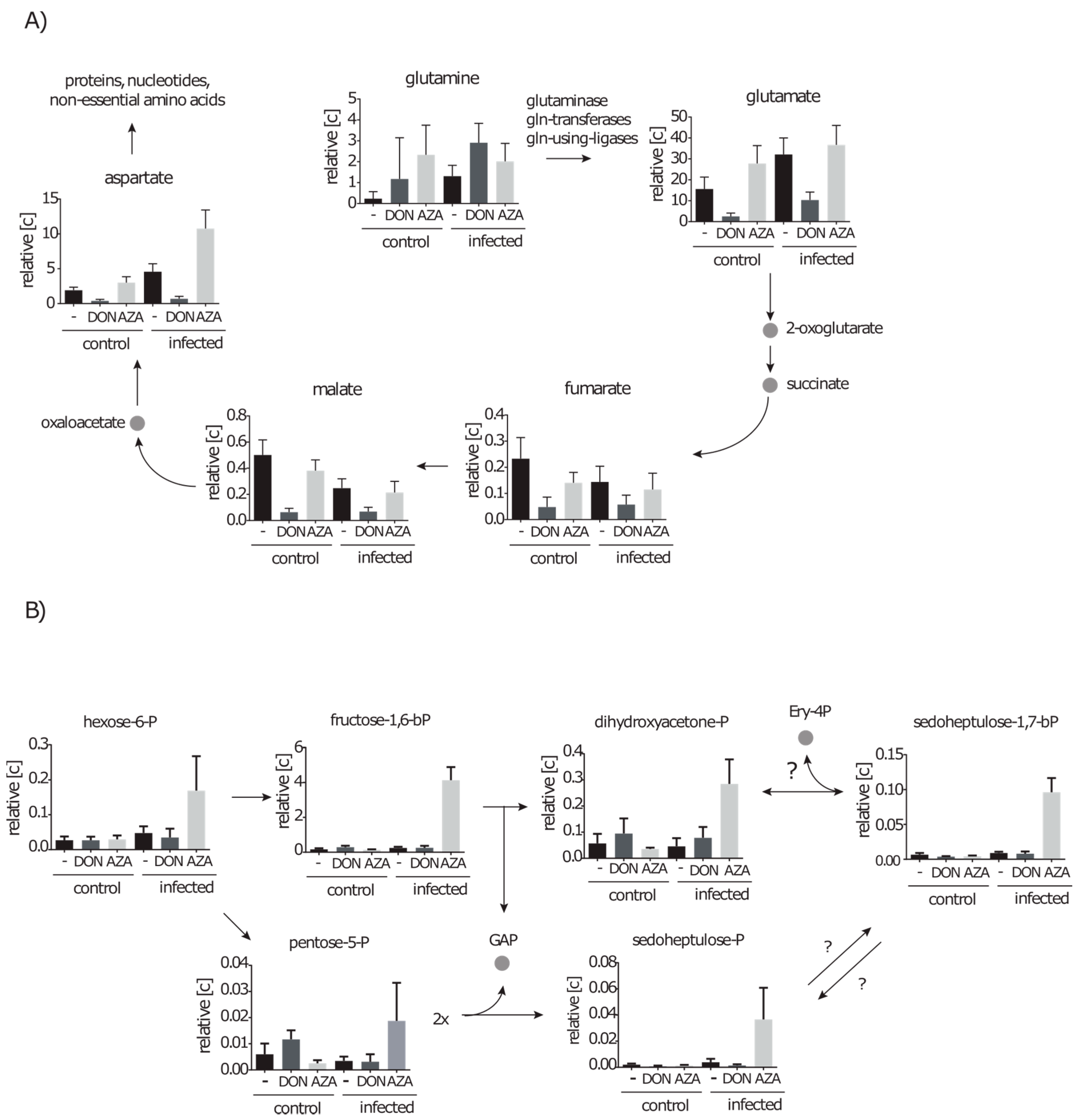
2.4.3. DON Affects Glutaminolysis and Azaserine the Central Carbon Metabolism in Infected Cells
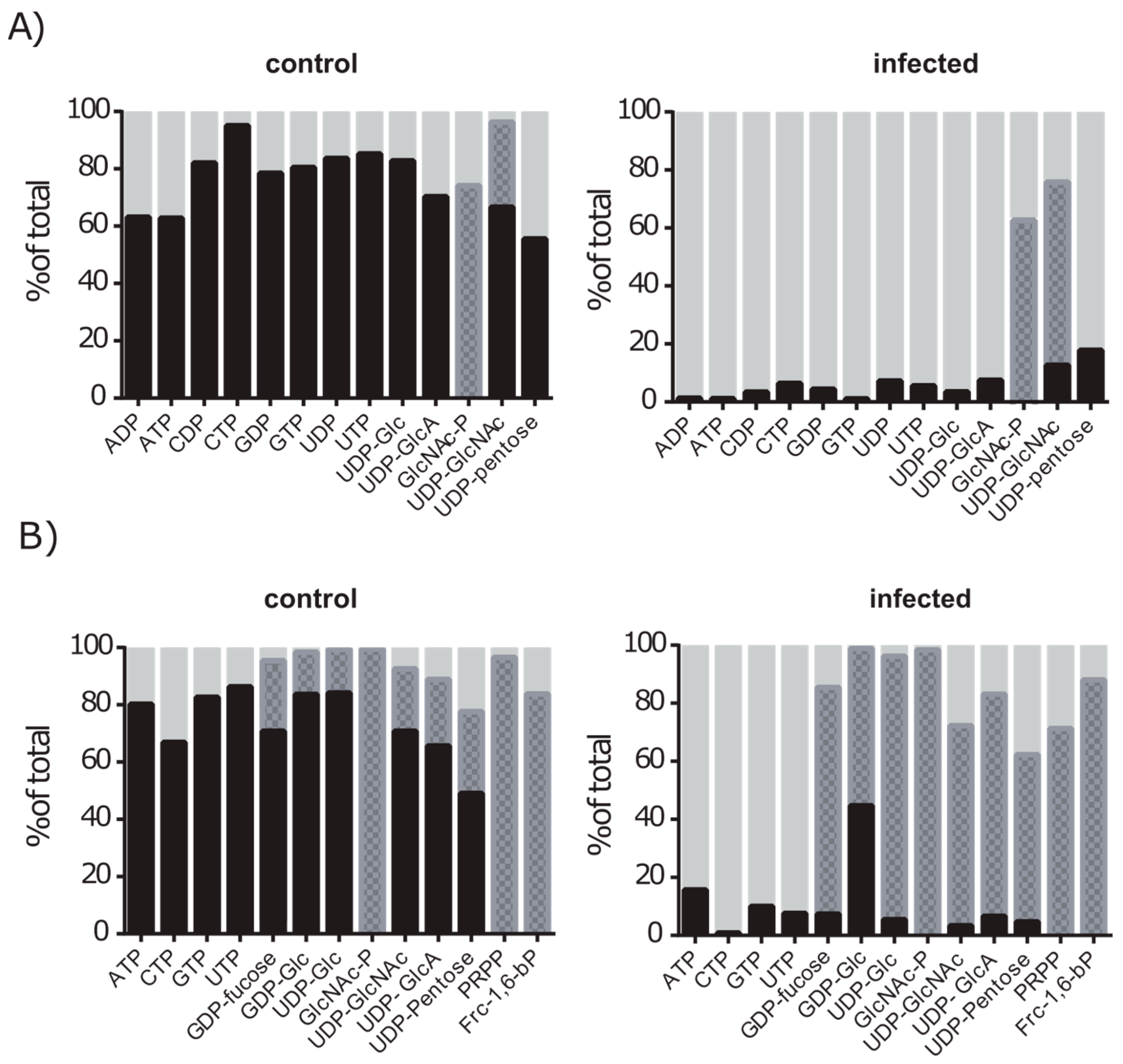
2.5. De novo Purine and Pyrimidine Synthesis during Infection Is Strongly Reduced
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Infection
4.2. Metabolic Inhibitors and Labelled Precursors
4.3. Extracellular and Intracellular Metabolome Samples
4.4. 1H-NMR Spectroscopic Analysis and Data Analysis
4.5. LC-MS Setup and Analysis
4.6. GC-MS Setup and Analysis
4.7. Statistics and Data Visualisation
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kluytmans, J.; van Belkum, A.; Verbrugh, H. Nasal carriage of Staphylococcus aureus: Epidemiology, underlying mechanisms, and associated risks. Clin. Microbiol. Rev. 1997, 10, 505–520. [Google Scholar] [PubMed]
- Lowy, F.D. Staphylococcus aureus infections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Wertheim, H.F.; Melles, D.C.; Vos, M.C.; van Leeuwen, W.; van Belkum, A.; Verbrugh, H.A.; Nouwen, J.L. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect. Dis. 2005, 5, 751–762. [Google Scholar] [CrossRef]
- Lowy, F.D. Is Staphylococcus aureus an intracellular pathogen? Trends Microbiol. 2000, 8, 341–343. [Google Scholar] [CrossRef]
- Fraunholz, M.; Sinha, B. Intracellular Staphylococcus aureus: Live-in and let die. Front. Cell. Infect. Microbiol. 2012, 2, 43. [Google Scholar] [CrossRef] [PubMed]
- Garzoni, C.; Kelley, W.L. Staphylococcus aureus: New evidence for intracellular persistence. Trends Microbiol. 2009, 17, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Garzoni, C.; Francois, P.; Huyghe, A.; Couzinet, S.; Tapparel, C.; Charbonnier, Y.; Renzoni, A.; Lucchini, S.; Lew, D.P.; Vaudaux, P.; et al. A global view of Staphylococcus aureus whole genome expression upon internalization in human epithelial cells. BMC Genom. 2007, 8, 171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Surmann, K.; Michalik, S.; Hildebrandt, P.; Gierok, P.; Depke, M.; Brinkmann, L.; Bernhardt, J.; Salazar, M.G.; Sun, Z.; Shteynberg, D.; et al. Comparative proteome analysis reveals conserved and specific adaptation patterns of Staphylococcus aureus after internalization by different types of human non-professional phagocytic host cells. Front. Microbiol. 2014, 5, 392. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.A.; Garrod, D.R. Molecular aspects of the epithelial phenotype. Bioessays 1997, 19, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, B.; Skipp, P.; Mould, R.; Rennard, S.; Davies, D.E.; O’Connor, C.D.; Djukanovic, R. Shotgun proteomic analysis of human-induced sputum. Proteomics 2006, 6, 4390–4401. [Google Scholar] [CrossRef] [PubMed]
- Knowles, M.R.; Boucher, R.C. Mucus clearance as a primary innate defense mechanism for mammalian airways. J. Clin. Investig. 2002, 109, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Bien, J.; Sokolova, O.; Bozko, P. Characterization of Virulence Factors of Staphylococcus aureus: Novel Function of Known Virulence Factors That Are Implicated in Activation of Airway Epithelial Proinflammatory Response. J. Pathog. 2011, 2011, 601905. [Google Scholar] [CrossRef] [PubMed]
- Gierok, P.; Harms, M.; Richter, E.; Hildebrandt, J.P.; Lalk, M.; Mostertz, J.; Hochgrafe, F. Staphylococcus aureus alpha-toxin mediates general and cell type-specific changes in metabolite concentrations of immortalized human airway epithelial cells. PLoS ONE 2014, 9, e94818. [Google Scholar] [CrossRef] [PubMed]
- Sinha, B.; Francois, P.P.; Nusse, O.; Foti, M.; Hartford, O.M.; Vaudaux, P.; Foster, T.J.; Lew, D.P.; Herrmann, M.; Krause, K.H. Fibronectin-binding protein acts as Staphylococcus aureus invasin via fibronectin bridging to integrin alpha5beta1. Cell. Microbiol. 1999, 1, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Schroder, A.; Schroder, B.; Roppenser, B.; Linder, S.; Sinha, B.; Fassler, R.; Aepfelbacher, M. Staphylococcus aureus fibronectin binding protein-A induces motile attachment sites and complex actin remodeling in living endothelial cells. Mol. Biol. Cell 2006, 17, 5198–5210. [Google Scholar] [CrossRef] [PubMed]
- Mosca, E.; Barcella, M.; Alfieri, R.; Bevilacqua, A.; Canti, G.; Milanesi, L. Systems biology of the metabolic network regulated by the Akt pathway. Biotechnol. Adv. 2012, 30, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.T.; Lee, C.W.; Tung, W.H.; Wang, S.W.; Lin, C.C.; Shu, J.C.; Yang, C.M. Cooperation of TLR2 with MyD88, PI3K, and Rac1 in lipoteichoic acid-induced cPLA2/COX-2-dependent airway inflammatory responses. Am. J. Pathol. 2010, 176, 1671–1684. [Google Scholar] [CrossRef] [PubMed]
- Oviedo-Boyso, J.; Cortes-Vieyra, R.; Huante-Mendoza, A.; Yu, H.B.; Valdez-Alarcon, J.J.; Bravo-Patino, A.; Cajero-Juarez, M.; Finlay, B.B.; Baizabal-Aguirre, V.M. The phosphoinositide-3-kinase-Akt signaling pathway is important for Staphylococcus aureus internalization by endothelial cells. Infect. Immun. 2011, 79, 4569–4577. [Google Scholar] [CrossRef] [PubMed]
- Mestre, M.B.; Colombo, M.I. Staphylococcus aureus promotes autophagy by decreasing intracellular cAMP levels. Autophagy 2012, 8, 1865–1867. [Google Scholar] [CrossRef] [PubMed]
- Kahl, B.C.; Goulian, M.; van Wamel, W.; Herrmann, M.; Simon, S.M.; Kaplan, G.; Peters, G.; Cheung, A.L. Staphylococcus aureus RN6390 replicates and induces apoptosis in a pulmonary epithelial cell line. Infect. Immun. 2000, 68, 5385–5392. [Google Scholar] [CrossRef] [PubMed]
- Rohmer, L.; Hocquet, D.; Miller, S.I. Are pathogenic bacteria just looking for food? Metabolism and microbial pathogenesis. Trends Microbiol. 2011, 19, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Goetz, M.; Bubert, A.; Wang, G.; Chico-Calero, I.; Vazquez-Boland, J.A.; Beck, M.; Slaghuis, J.; Szalay, A.A.; Goebel, W. Microinjection and growth of bacteria in the cytosol of mammalian host cells. Proc. Natl. Acad. Sci. USA 2001, 98, 12221–12226. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- DeBerardinis, R.J.; Mancuso, A.; Daikhin, E.; Nissim, I.; Yudkoff, M.; Wehrli, S.; Thompson, C.B. Beyond aerobic glycolysis: Transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl. Acad. Sci. USA 2007, 104, 19345–19350. [Google Scholar] [CrossRef] [PubMed]
- Wise, D.R.; Thompson, C.B. Glutamine addiction: A new therapeutic target in cancer. Trends Biochem. Sci. 2010, 35, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Lyons, S.D.; Sant, M.E.; Christopherson, R.I. Cytotoxic mechanisms of glutamine antagonists in mouse L1210 leukemia. J. Biol. Chem. 1990, 265, 11377–11381. [Google Scholar] [PubMed]
- Ardalan, B.; Arakawa, M.; Villacorte, D.; Jayaram, H.; Cooney, D.A. Effect of L-glutamine antagonists on 5-phosphoribosyl 1-pyrophosphate levels in P388 leukemia and in murine colon adenocarcinomas in vivo. Biochem. Pharmacol. 1982, 31, 1509–1513. [Google Scholar] [CrossRef]
- Alekseeva, L.; Rault, L.; Almeida, S.; Legembre, P.; Edmond, V.; Azevedo, V.; Miyoshi, A.; Even, S.; Taieb, F.; Arlot-Bonnemains, Y.; et al. Staphylococcus aureus-induced G2/M phase transition delay in host epithelial cells increases bacterial infective efficiency. PLoS ONE 2013, 8, e63279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deplanche, M.; Filho, R.A.; Alekseeva, L.; Ladier, E.; Jardin, J.; Henry, G.; Azevedo, V.; Miyoshi, A.; Beraud, L.; Laurent, F.; et al. Phenol-soluble modulin alpha induces G2/M phase transition delay in eukaryotic HeLa cells. FASEB J. 2015, 29, 1950–1959. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Braughton, K.R.; Kretschmer, D.; Bach, T.H.; Queck, S.Y.; Li, M.; Kennedy, A.D.; Dorward, D.W.; Klebanoff, S.J.; Peschel, A.; et al. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat. Med. 2007, 13, 1510–1514. [Google Scholar] [CrossRef] [PubMed]
- Yokota, S.; Okabayashi, T.; Yokosawa, N.; Fujii, N. Growth arrest of epithelial cells during measles virus infection is caused by upregulation of interferon regulatory factor 1. J. Virol. 2004, 78, 4591–4598. [Google Scholar] [CrossRef] [PubMed]
- Liljeroos, M.; Vuolteenaho, R.; Rounioja, S.; Henriques-Normark, B.; Hallman, M.; Ojaniemi, M. Bacterial ligand of TLR2 signals Stat activation via induction of IRF1/2 and interferon-alpha production. Cell. Signal. 2008, 20, 1873–1881. [Google Scholar] [CrossRef] [PubMed]
- Richter, E.; Harms, M.; Ventz, K.; Gierok, P.; Chilukoti, R.K.; Hildebrandt, J.P.; Mostertz, J.; Hochgrafe, F. A multi-omics approach identifies key hubs associated with cell type-specific responses of airway epithelial cells to staphylococcal alpha-toxin. PLoS ONE 2015, 10, e0122089. [Google Scholar] [CrossRef] [PubMed]
- Maurer, K.; Reyes-Robles, T.; Alonzo, F., 3rd; Durbin, J.; Torres, V.J.; Cadwell, K. Autophagy mediates tolerance to Staphylococcus aureus alpha-toxin. Cell Host Microbe 2015, 17, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Rath, S.; Ziesemer, S.; Witte, A.; Konkel, A.; Muller, C.; Hildebrandt, P.; Volker, U.; Hildebrandt, J.P. S. aureus hemolysin A-induced IL-8 and IL-6 release from human airway epithelial cells is mediated by activation of p38- and Erk-MAP kinases and additional, cell-type specific signalling mechanisms. Cell. Microbiol. 2013, 15, 1253–1265. [Google Scholar] [CrossRef] [PubMed]
- Zielinska, A.K.; Beenken, K.E.; Joo, H.S.; Mrak, L.N.; Griffin, L.M.; Luong, T.T.; Lee, C.Y.; Otto, M.; Shaw, L.N.; Smeltzer, M.S. Defining the Strain-Dependent Impact of the Staphylococcal Accessory Regulator (sarA) on the Alpha-Toxin Phenotype of Staphylococcus aureus. J. Bacteriol. 2011, 193, 2948–2958. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.D.; Malachowa, N.; Whitney, A.R.; Braughton, K.R.; Gardner, D.J.; Long, D.; Bubeck Wardenburg, J.; Schneewind, O.; Otto, M.; Deleo, F.R. Comparative analysis of USA300 virulence determinants in a rabbit model of skin and soft tissue infection. J. Infect. Dis. 2011, 204, 937–941. [Google Scholar] [CrossRef] [PubMed]
- Dean, P. Functional domains and motifs of bacterial type III effector proteins and their roles in infection. FEMS Microbiol. Rev. 2011, 35, 1100–1125. [Google Scholar] [CrossRef] [PubMed]
- Werth, N.; Beerlage, C.; Rosenberger, C.; Yazdi, A.S.; Edelmann, M.; Amr, A.; Bernhardt, W.; von Eiff, C.; Becker, K.; Schafer, A.; et al. Activation of hypoxia inducible factor 1 is a general phenomenon in infections with human pathogens. PLoS ONE 2010, 5, e11576. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Yang, J.Y.; Jeon, B.Y.; Yoon, Y.J.; Cho, S.N.; Kang, Y.H.; Ryu do, H.; Hwang, G.S. (1)H NMR-based metabolomic profiling in mice infected with Mycobacterium tuberculosis. J. Proteome Res. 2011, 10, 2238–2247. [Google Scholar] [CrossRef] [PubMed]
- Somashekar, B.S.; Amin, A.G.; Rithner, C.D.; Troudt, J.; Basaraba, R.; Izzo, A.; Crick, D.C.; Chatterjee, D. Metabolic profiling of lung granuloma in Mycobacterium tuberculosis infected guinea pigs: Ex vivo 1H magic angle spinning NMR studies. J. Proteome Res. 2011, 10, 4186–4195. [Google Scholar] [CrossRef] [PubMed]
- Lecuit, M.; Sonnenburg, J.L.; Cossart, P.; Gordon, J.I. Functional genomic studies of the intestinal response to a foodborne enteropathogen in a humanized gnotobiotic mouse model. J. Biol. Chem. 2007, 282, 15065–15072. [Google Scholar] [CrossRef] [PubMed]
- Ojcius, D.M.; Degani, H.; Mispelter, J.; Dautry-Varsat, A. Enhancement of ATP levels and glucose metabolism during an infection by Chlamydia. NMR studies of living cells. J. Biol. Chem. 1998, 273, 7052–7058. [Google Scholar] [CrossRef] [PubMed]
- Heink, S.; Ludwig, D.; Kloetzel, P.M.; Kruger, E. IFN-gamma-induced immune adaptation of the proteasome system is an accelerated and transient response. Proc. Natl. Acad. Sci. USA 2005, 102, 9241–9246. [Google Scholar] [CrossRef] [PubMed]
- Surmann, K.; Simon, M.; Hildebrandt, P.; Pfortner, H.; Michalik, S.; Stentzel, S.; Steil, L.; Dhople, V.M.; Bernhardt, J.; Schluter, R.; et al. A proteomic perspective of the interplay of Staphylococcus aureus and human alveolar epithelial cells during infection. J. Proteom. 2015, 128, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Steele, S.; Brunton, J.; Ziehr, B.; Taft-Benz, S.; Moorman, N.; Kawula, T. Francisella tularensis harvests nutrients derived via ATG5-independent autophagy to support intracellular growth. PLoS Pathog. 2013, 9, e1003562. [Google Scholar] [CrossRef] [PubMed]
- Price, C.T.; Al-Quadan, T.; Santic, M.; Rosenshine, I.; Abu Kwaik, Y. Host proteasomal degradation generates amino acids essential for intracellular bacterial growth. Science 2011, 334, 1553–1557. [Google Scholar] [CrossRef] [PubMed]
- Schnaith, A.; Kashkar, H.; Leggio, S.A.; Addicks, K.; Kronke, M.; Krut, O. Staphylococcus aureus subvert autophagy for induction of caspase-independent host cell death. J. Biol. Chem. 2007, 282, 2695–2706. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Floto, R.A.; Berger, Z.; Imarisio, S.; Cordenier, A.; Pasco, M.; Cook, L.J.; Rubinsztein, D.C. Lithium induces autophagy by inhibiting inositol monophosphatase. J. Cell Biol. 2005, 170, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Geiger, T.; Francois, P.; Liebeke, M.; Fraunholz, M.; Goerke, C.; Krismer, B.; Schrenzel, J.; Lalk, M.; Wolz, C. The stringent response of Staphylococcus aureus and its impact on survival after phagocytosis through the induction of intracellular PSMs expression. PLoS Pathog. 2012, 8, e1003016. [Google Scholar] [CrossRef] [PubMed]
- Geiger, T.; Kastle, B.; Gratani, F.L.; Goerke, C.; Wolz, C. Two small (p)ppGpp synthases in Staphylococcus aureus mediate tolerance against cell envelope stress conditions. J. Bacteriol. 2014, 196, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.; Palmic, N.; Sanquer, S.; Lenoir, C.; Hauck, F.; Mongellaz, C.; Fabrega, S.; Nitschke, P.; Esposti, M.D.; Schwartzentruber, J.; et al. CTP synthase 1 deficiency in humans reveals its central role in lymphocyte proliferation. Nature 2014, 510, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Du, X.L.; Edelstein, D.; Rossetti, L.; Fantus, I.G.; Goldberg, H.; Ziyadeh, F.; Wu, J.; Brownlee, M. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc. Natl. Acad. Sci. USA 2000, 97, 12222–12226. [Google Scholar] [CrossRef] [PubMed]
- Marshall, S.; Bacote, V.; Traxinger, R.R. Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system. Role of hexosamine biosynthesis in the induction of insulin resistance. J. Biol. Chem. 1991, 266, 4706–4712. [Google Scholar] [PubMed]
- Olivier-Van Stichelen, S.; Guinez, C.; Mir, A.M.; Perez-Cervera, Y.; Liu, C.; Michalski, J.C.; Lefebvre, T. The hexosamine biosynthetic pathway and O-GlcNAcylation drive the expression of beta-catenin and cell proliferation. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E417–E424. [Google Scholar] [CrossRef] [PubMed]
- Hart, G.W.; Housley, M.P.; Slawson, C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature 2007, 446, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Butkinaree, C.; Park, K.; Hart, G.W. O-linked beta-N-acetylglucosamine (O-GlcNAc): Extensive crosstalk with phosphorylation to regulate signaling and transcription in response to nutrients and stress. Biochim. Biophys. Acta 2010, 1800, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Hanover, J.A.; Krause, M.W.; Love, D.C. The hexosamine signaling pathway: O-GlcNAc cycling in feast or famine. Biochim. Biophys. Acta 2010, 1800, 80–95. [Google Scholar] [CrossRef] [PubMed]
- Dörries, K.; Lalk, M. Metabolic Footprint Analysis Uncovers Strain Specific Overflow Metabolism and d-Isoleucine Production of Staphylococcus Aureus COL and HG001. PLoS ONE 2013, 8, e81500. [Google Scholar] [CrossRef] [PubMed]
- Liebeke, M.; Dörries, K.; Zühlke, D.; Bernhardt, J.; Fuchs, S.; Pane-Farre, J.; Engelmann, S.; Volker, U.; Bode, R.; Dandekar, T.; et al. A metabolomics and proteomics study of the adaptation of Staphylococcus aureus to glucose starvation. Mol. Biosyst. 2011, 7, 1241–1253. [Google Scholar] [CrossRef] [PubMed]
- Dörries, K.; Schlueter, R.; Lalk, M. Impact of antibiotics with various target sites on the metabolome of Staphylococcus aureus. Antimicrob. Agents Chemother. 2014, 58, 7151–7163. [Google Scholar] [CrossRef] [PubMed]
- Strelkov, S.; von Elstermann, M.; Schomburg, D. Comprehensive analysis of metabolites in Corynebacterium glutamicum by gas chromatography/mass spectrometry. Biol. Chem. 2004, 385, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.I.; Sharov, V.; White, J.; Li, J.; Liang, W.; Bhagabati, N.; Braisted, J.; Klapa, M.; Currier, T.; Thiagarajan, M.; et al. TM4: A free, open-source system for microarray data management and analysis. Biotechniques 2003, 34, 374–378. [Google Scholar] [PubMed]
- Wordle. Available online: http://www.wordle.net/ (accessed on 3 November 2016).




© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gierok, P.; Harms, M.; Methling, K.; Hochgräfe, F.; Lalk, M. Staphylococcus aureus Infection Reduces Nutrition Uptake and Nucleotide Biosynthesis in a Human Airway Epithelial Cell Line. Metabolites 2016, 6, 41. https://doi.org/10.3390/metabo6040041
Gierok P, Harms M, Methling K, Hochgräfe F, Lalk M. Staphylococcus aureus Infection Reduces Nutrition Uptake and Nucleotide Biosynthesis in a Human Airway Epithelial Cell Line. Metabolites. 2016; 6(4):41. https://doi.org/10.3390/metabo6040041
Chicago/Turabian StyleGierok, Philipp, Manuela Harms, Karen Methling, Falko Hochgräfe, and Michael Lalk. 2016. "Staphylococcus aureus Infection Reduces Nutrition Uptake and Nucleotide Biosynthesis in a Human Airway Epithelial Cell Line" Metabolites 6, no. 4: 41. https://doi.org/10.3390/metabo6040041






