1H NMR-Based Metabolomic Analysis of Sub-Lethal Perfluorooctane Sulfonate Exposure to the Earthworm, Eisenia fetida, in Soil
Abstract
:1. Introduction
2. Results and Discussion
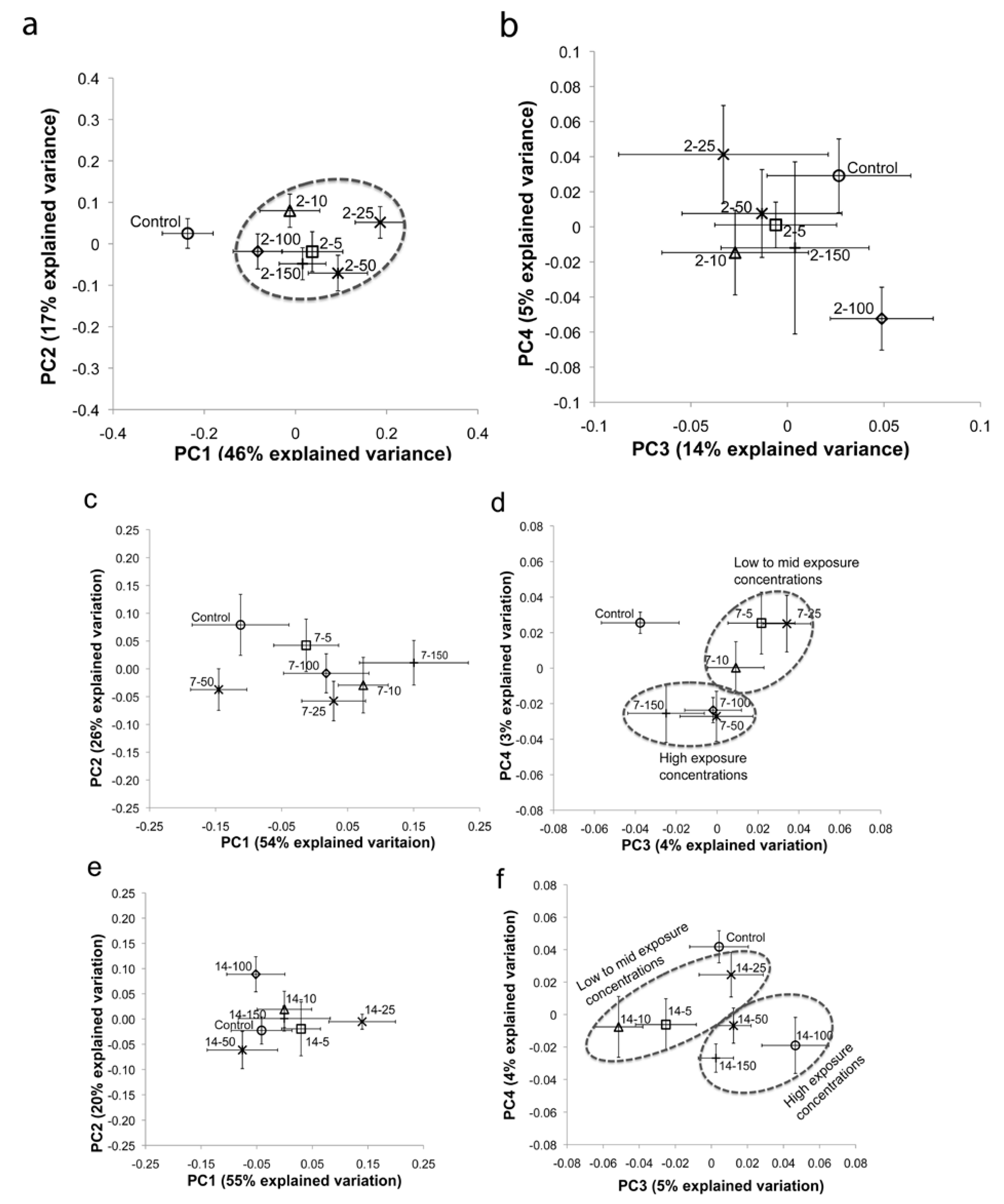
2.1. Multivariate Statistical Analysis
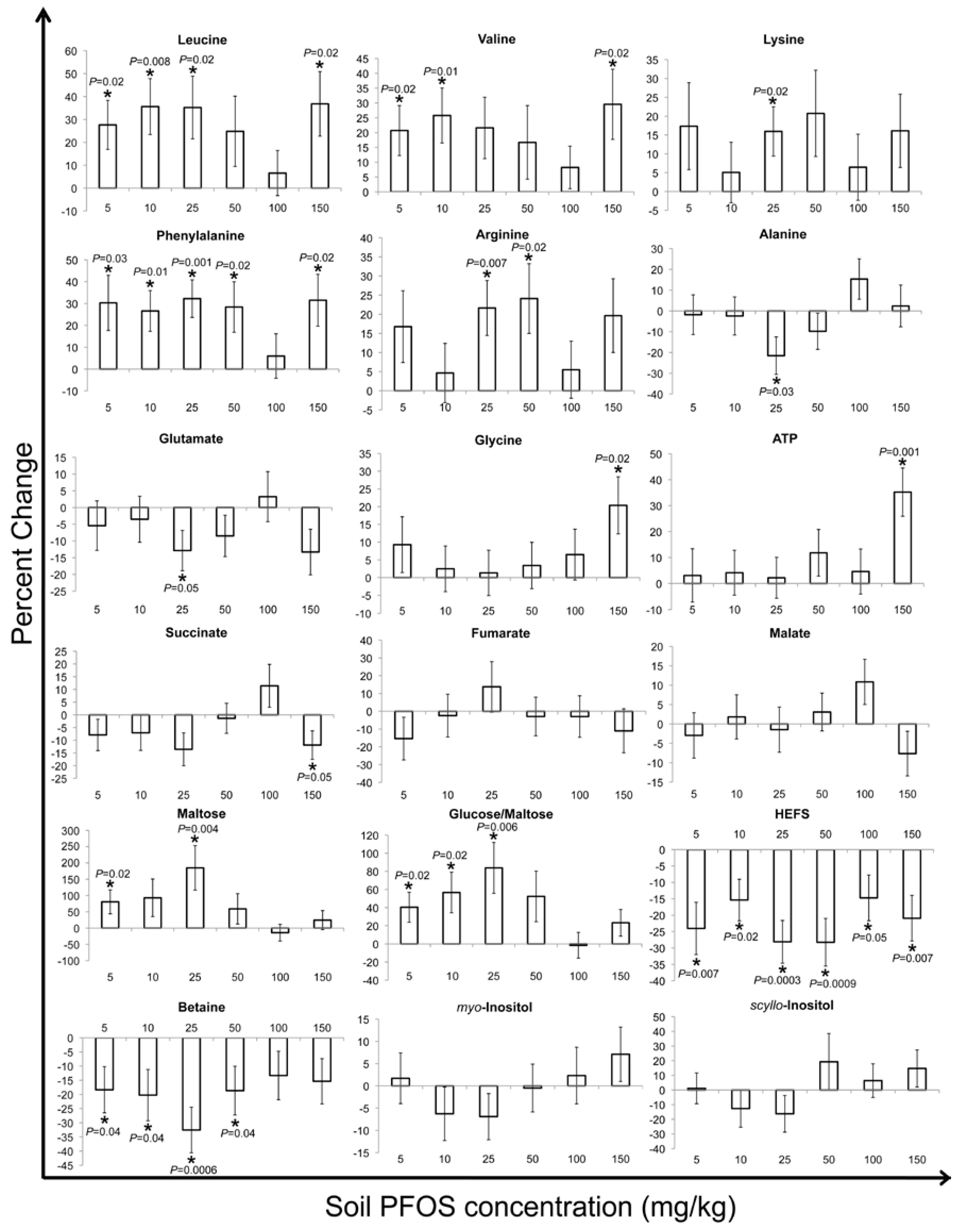
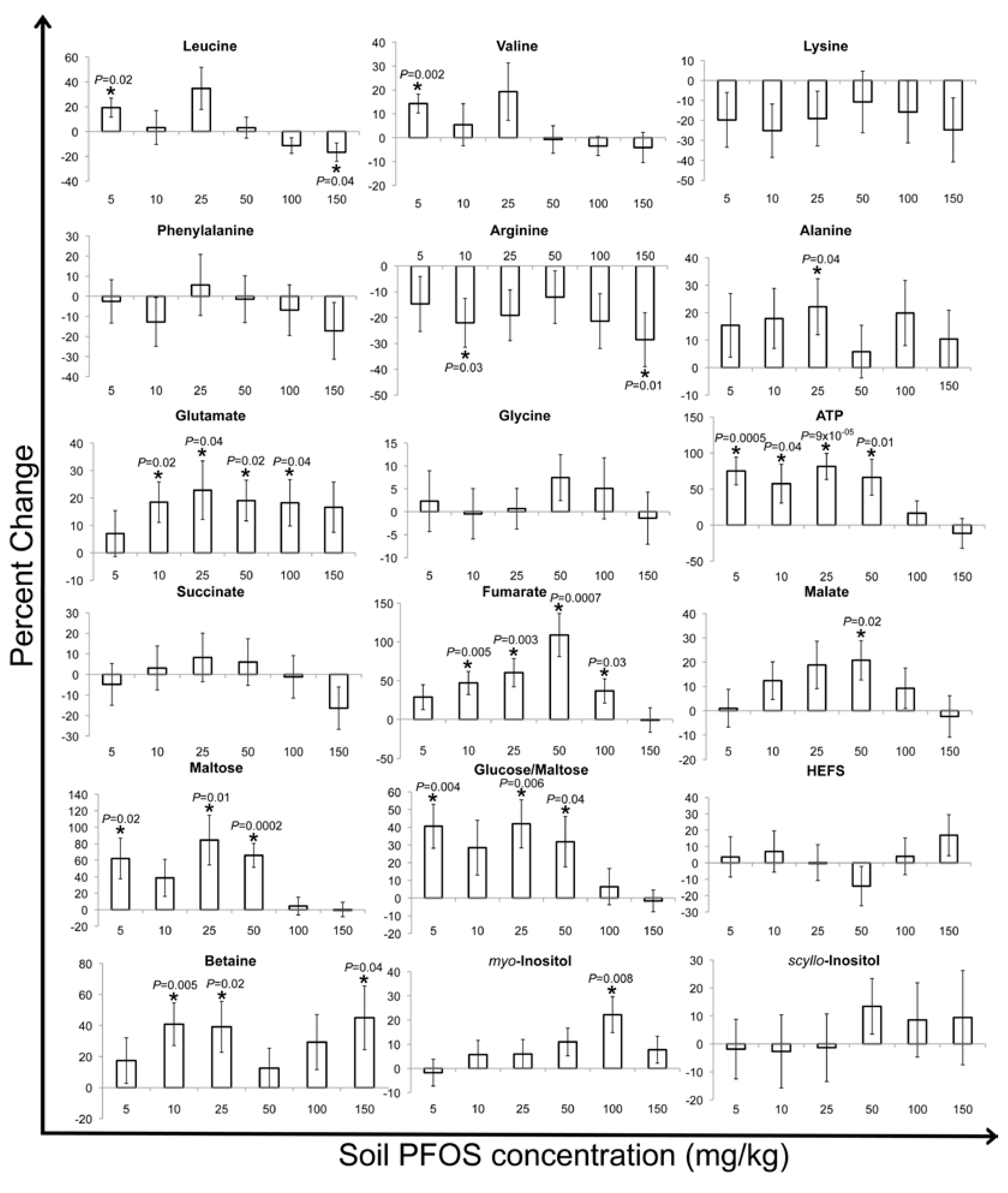
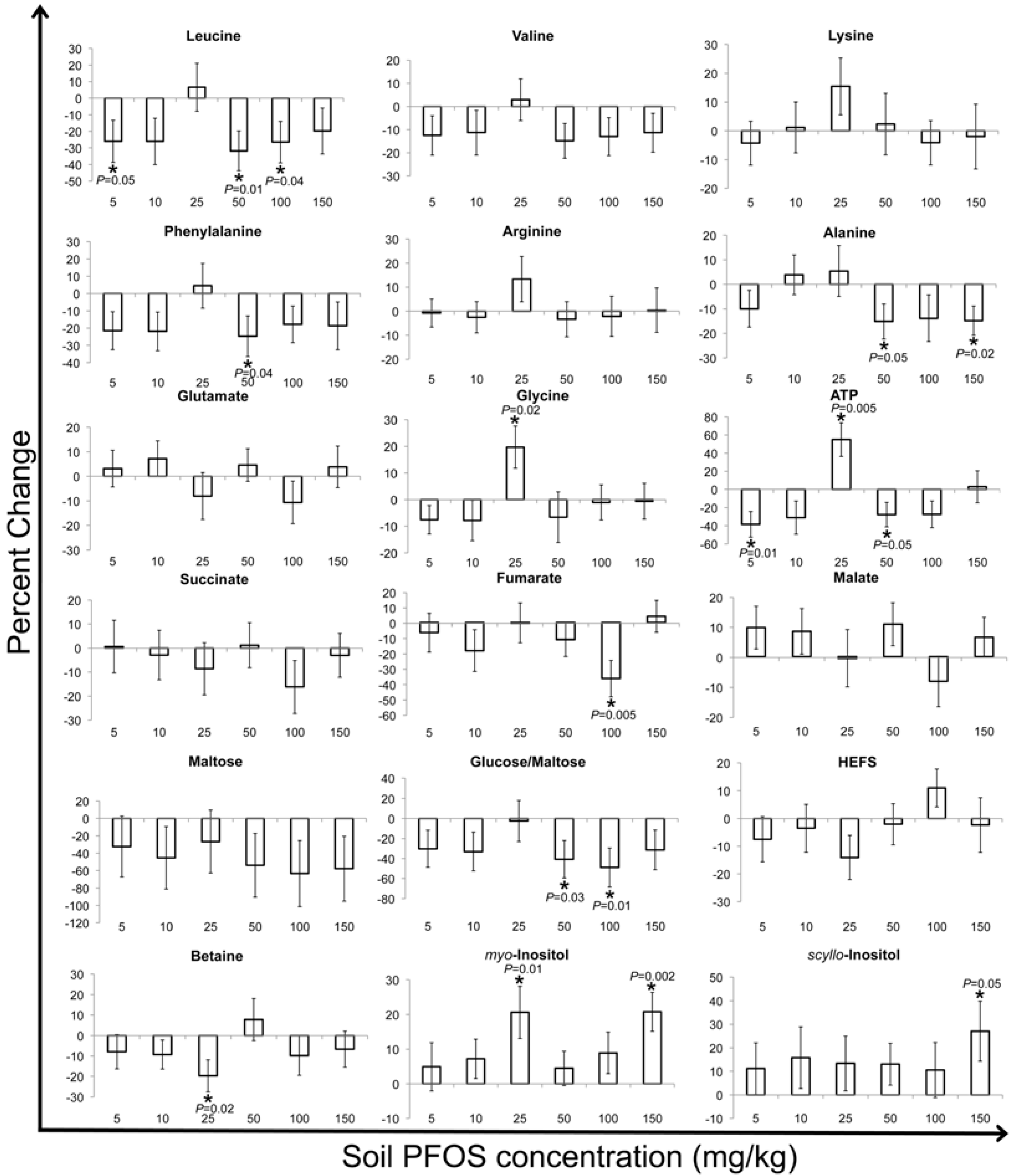
2.2. Metabolic Changes in Response to PFOS Exposure


3. Experimental Section
3.1. Soil Spiking and Total Soil PFOS Concentrations
3.2. Earthworm Exposure and Tissue Extraction
3.3. 1H-NMR Spectroscopy
3.4. Data and Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Kissa, E. Fluorinated Surfactants and Repellents, 2nd ed.; Marcel Dekker: New York, NY, USA, 2001; pp. 349–352. [Google Scholar]
- Lau, C.; Anitole, K.; Hodes, C.; Lai, D.; Pfahles-Hutchens, A.; Seed, J. Perfluoroalkyl acids: A review of monitoring and toxicological findings. Toxicol. Sci. 2007, 99, 366–394. [Google Scholar] [CrossRef]
- Lindstrom, A.B.; Strynar, M.J.; Libelo, E.L. Polyfluorinated compounds: Past, present, and future. Environ. Sci. Technol. 2011, 45, 7954–7961. [Google Scholar] [CrossRef]
- Renner, R. Growing concern over perfluorinated chemicals. Environ. Sci. Technol. 2001, 35, 154A–160A. [Google Scholar] [CrossRef]
- Zhao, Y.G.; Wong, C.K.; Wong, M.H. Environmental contamination, human exposure and body loadings of perfluorooctane sulfonate (PFOS), focusing on Asian countrie. Chemosphere 2012, 89, 355–368. [Google Scholar] [CrossRef]
- Guruge, K.S.; Taniyasu, S.; Yamashita, N.; Wijeratna, S.; Mohotti, K.M.; Seneviratne, H.R.; Kannan, K.; Yamanaka, N.; Miyazaki, S. Perfluorinated organic compounds in human blood serum and seminal plasma: A study of urban and rural tea worker populations in Sri Lanka. J. Environ. Monit. 2005, 7, 371–377. [Google Scholar] [CrossRef]
- Karrman, A.; Ericson, I.; van Bavel, B.; Darnerud, P.O.; Aune, M.; Glynn, A.; Lignell, S.; Lindstrom, G. Exposure of perfluorinated chemicals through lactation: Levels of matched human milk and serum and a temporal trend, 1996–2004, in Sweden. Environ. Health Persp. 2007, 115, 226–230. [Google Scholar]
- Sohlenius, A.K.; Lundgren, B.; Depierre, J.W. Perfluorooctanoic acid has persistent effects on peroxisome proliferation and related parameters in mouse-liver. J. Biochem. Toxicol. 1992, 7, 205–212. [Google Scholar] [CrossRef]
- Seacat, A.M.; Thomford, P.J.; Hansen, K.J.; Clemen, L.A.; Eldridge, S.R.; Elcombe, C.R.; Butenhoff, J.L. Sub-chronic dietary toxicity of potassium perfluorooctanesulfonate in rats. Toxicology 2003, 183, 117–131. [Google Scholar] [CrossRef]
- Seacat, A.M.; Thomford, P.J.; Hansen, K.J.; Olsen, G.W.; Case, M.T.; Butenhoff, J.L. Subchronic toxicity studies on perfluorooctanesulfonate potassium salt in cynomolgus monkeys. Toxicol. Sci. 2002, 68, 249–264. [Google Scholar] [CrossRef]
- Governments Unite to Step-Up Reduction on Global DDT Reliance and Add Nine New Chemicals under inTernational Treaty; Stockholm Convention Secretariat: Geneva, Switzerland, 2009.
- Joung, K.E.; Jo, E.H.; Kim, H.M.; Choi, K.; Yoon, J. Toxicological effects of PFOS and PFOA on earthwrom, Eisenia fetida. Environ. Health Toxicol. 2010, 25, 181. [Google Scholar]
- Renner, R. EPA finds record PFOS, PFOA levels in Alabama grazing fields. Environ. Sci. Technol. 2009, 43, 1245–1246. [Google Scholar] [CrossRef]
- Zareitalabad, P.; Siemens, J.; Hamer, M.; Amelung, W. Perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) in surface waters, sediments, soils and wastewater - A review on concentrations and distribution coefficients. Chemosphere 2013, 91, 725–732. [Google Scholar] [CrossRef]
- Cho, C.R.; Eom, I.; Kim, E.J.; Kim, S.; Choi, K.; Cho, H.; Yoon, J. Evaluation of the level of PFOS and PFOA in environmental media from industrial area and major river basin. J. Korea Soc. Environ. Anal. 2009, 12, 296–306. [Google Scholar]
- Li, F.; Zhang, C.J.; Qu, Y.; Chen, J.; Chen, L.; Liu, Y.; Zhou, Q. Quantitative characterization of short- and long-chain perfluorinated acids in solid matrices in Shanghai, China. Sci. Total Environ. 2010, 408, 617–623. [Google Scholar] [CrossRef]
- Sepulvado, J.G.; Blaine, A.C.; Hundal, L.S.; Higgins, C.P. Occurrence and fate of perfluorochemicals in soil following the land application of municipal biosolids. Environ. Sci. Technol. 2011, 45, 8106–8112. [Google Scholar] [CrossRef]
- Das, P.; Megharaj, M.; Naidu, R. Perfluorooctane sulfonate release pattern from soils of fire training areas in Australia and its bioaccumulation potential in the earthworm Eisenia fetida. Environ. Sci. Pollut. Res. 2013. [Google Scholar] [CrossRef]
- Edwards, C.A.; Bohlen, P.J. The effects of toxic-chemicals on earthworms. Rev. Environ. Contam. Toxicol. 1992, 125, 23–99. [Google Scholar] [CrossRef]
- Svendsen, C.; Spurgeon, D.J.; Hankard, P.K.; Weeks, J.M. A review of lysosomal membrane stability measured by neutral red retention: is it a workable earthworm biomarker? Ecotox. Environ. Saf. 2004, 57, 20–29. [Google Scholar] [CrossRef]
- Sindermann, A.B.; Porch, J.R.; Krueger, H.O.; van Hoven, R.L. PFOS: An acute toxicity study with the earthworm in an artificial soil substrate. In Project No. 454–111. EPA Docket AR226-1106; Wildlife International Ltd.: Easton, MD, USA, 2002. [Google Scholar]
- Mosleh, Y.Y.; Paris-Palacios, S.; Couderchet, M.; Vernet, G. Acute and sublethal effects of two insecticides on earthworms (Lumbricus terrestris L.) under laboratory conditions. Environ. Toxicol. 2003, 18, 1–8. [Google Scholar] [CrossRef]
- Neuhauser, E.F.; Callahan, C.A. Growth and reproduction of the earthworm Eisenia-Fetida exposed to sublethal concentrations of organic-chemicals. Soil Biol. Biochem. 1990, 22, 175–179. [Google Scholar] [CrossRef]
- Stubberud, H. Økotoksikologiske effecter av PFOS, PFOA og 6:2 FTS på meitemark (Eisenia fetida); (in Norwegian, English summary); Norwegian State Pollution Control Authority: Oslo, Norway, 2006. [Google Scholar]
- Lankadurai, B.P.; Wolfe, D.M.; Simpson, A.J.; Simpson, M.J. 1H NMR-based metabolomic analysis of the time-dependent response of Eisenia fetida after sub-lethal phenanthrene exposure. Environ. Pollut. 2011, 159, 2845–2851. [Google Scholar] [CrossRef]
- Beckwith-Hall, B.M.; Nicholson, J.K.; Nicholls, A.W.; Foxall, P.J.D.; Lindon, J.C.; Connor, S.C.; Abdi, M.; Connelly, J.; Holmes, E. Nuclear magnetic resonance spectroscopic and principal components analysis investigations into biochemical effects of three model hepatotoxins. Chem. Res. Toxicol. 1998, 11, 260–272. [Google Scholar] [CrossRef]
- Viant, M.R. Recent developments in environmental metabolomics. Mol. Biosyst. 2008, 4, 980–986. [Google Scholar] [CrossRef]
- Simpson, M.J.; McKelvie, J.R. Environmental metabolomics: new insights into earthworm ecotoxicity and contaminant bioavailability in soil. Anal. Bioanal. Chem. 2009, 394, 137–149. [Google Scholar] [CrossRef]
- Viant, M.R.; Bundy, J.G.; Pincetich, C.A.; de Ropp, J.S.; Tjeerdema, R.S. NMR-derived developmental metabolic trajectories: an approach for visualizing the toxic actions of trichloroethylene during embryogenesis. Metabolomics 2005, 1, 149–158. [Google Scholar] [CrossRef]
- Bundy, J.G.; Sidhu, J.K.; Rana, F.; Spurgeon, D.J.; Svendsen, C.; Wren, J.F.; Sturzenbaum, S.R.; Morgan, A.J.; Kille, P. “Systems toxicology” approach identifies coordinated metabolic responses to copper in a terrestrial non-model invertebrate, the earthworm Lumbricus rubellus. BMC Biol. 2008, 6, 25–46. [Google Scholar] [CrossRef]
- Ekman, D.R.; Teng, Q.N.; Villeneuve, D.L.; Kahl, M.D.; Jensen, K.M.; Durhan, E.J.; Ankley, G.T.; Collette, T.W. Profiling lipid metabolites yields unique information on sex- and time-dependent responses of fathead minnows (Pimephales promelas) exposed to 17 alpha-ethynylestradiol. Metabolomics 2009, 5, 22–32. [Google Scholar] [CrossRef]
- Lankadurai, B.P.; Simpson, A.J.; Simpson, M.J. H-1 NMR metabolomics of Eisenia fetida responses after sub-lethal exposure to perfluorooctanoic acid and perfluorooctane sulfonate. Environ. Chem. 2012, 9, 502–511. [Google Scholar] [CrossRef]
- Brown, S.A.E.; McKelvie, J.R.; Simpson, A.J.; Simpson, M.J. H-1 NMR metabolomics of earthworm exposure to sub-lethal concentrations of phenanthrene in soil. Environ. Pollut. 2010, 158, 2117–2123. [Google Scholar] [CrossRef]
- Whitfield Åslund, M.; Simpson, A.J.; Simpson, M.J. 1H NMR metabolomics of earthworm responses to polychlorinated biphenyl (PCB) exposure in soil. Ecotoxicology 2011, 20, 836–846. [Google Scholar] [CrossRef]
- Test No. 207: Earthworm, Acute Toxicity Tests. In OECD Guidelines for the Testing of Chemicals, Section 2: Effects on Biotic Systems; OECD Publishing: Paris, France, 1984.
- Soil Screening Levels for Perfluorooctanoic Acid (PFOA) and Perfluorooctyl Sulfonate (PFOS). In EPA Region 4; USEPA: USA, 2009.
- Brown, S.A.E.; Simpson, A.J.; Simpson, M.J. 1H NMR metabolomics of earthworm responses to sub-lethal PAH exposure. Environ. Chem. 2009, 6, 432–440. [Google Scholar] [CrossRef]
- Kaplan, F.; Kopka, J.; Haskell, D.W.; Zhao, W.; Schiller, K.C.; Gatzke, N.; Sung, D.Y.; Guy, C.L. Exploring the temperature-stress metabolome of Arabidopsis. Plant Physiol. 2004, 136, 4159–4168. [Google Scholar] [CrossRef]
- Bundy, J.G.; Lenz, E.M.; Bailey, N.J.; Gavaghan, C.L.; Svendsen, C.; Spurgeon, D.; Hankard, P.K.; Osborn, D.; Weeks, J.A.; Trauger, S.A. Metabonomic assessment of toxicity of 4-fluoroaniline, 3,5-difluoroaniline and 2-fluoro-4-methylaniline to the earthworm Eisenia veneta (Rosa): Identification of new endogenous biomarkers. Environ. Toxicol. Chem. 2002, 21, 1966–1972. [Google Scholar]
- Eriksson, L.; Johansson, E.; Kettaneh-Wold, N.; Trygg, J.; Wikstrom, C.; Wold, S. Multi- and Megavariate Data Analysis Part 1: Basic Principles and Applications, 2nd ed.; Umetrics AB: Umea, Sweden, 2006; pp. 63–98. [Google Scholar]
- Lankadurai, B.P.; Wolfe, D.M.; Whitfield Åslund, M.; Simpson, A.J.; Simpson, M.J. 1H NMR-based metabolomic analysis of polar and non-polar earthworm metabolites after sub-lethal exposure to phenanthrene. Metabolomics 2013, 9, 44–56. [Google Scholar]
- Sverdrup, L.E.; Nielsen, T.; Krogh, P.H. Soil ecotoxicity of polycyclic aromatic hydrocarbons in relation to soil sorption, lipophilicity, and water solubility. Environ. Sci. Technol. 2002, 36, 2429–2435. [Google Scholar] [CrossRef]
- Ekman, D.R.; Teng, Q.; Villeneuve, D.L.; Kahl, M.D.; Jensen, K.M.; Durhan, E.J.; Ankley, G.T.; Collette, T.W. Investigating compensation and recovery of fathead minnow (Pimephales promelas) exposed to 17 alpha-ethynylestradiol with metabolite profiling. Environ. Sci. Technol. 2008, 42, 4188–4194. [Google Scholar] [CrossRef]
- Yuk, J.; Simpson, A.J.; Simpson, M.J. 1D and 2D NMR metabolomics of earthworm responses to sub-lethal trifluralin and endosulfan exposure. Environ. Chem. 2011, 8, 281–294. [Google Scholar] [CrossRef]
- Lankadurai, B.P.; Wolfe, D.M.; Simpson, A.J.; Simpson, M.J. 1H NMR-based metabolomic observation of a two-phased toxic mode of action in Eisenia fetida after sub-lethal phenanthrene exposure. Environ. Chem. 2011, 8, 105–114. [Google Scholar] [CrossRef]
- Vanden Heuvel, J.P.; Thompson, J.T.; Frame, S.R.; Gillies, P.J. Differential activation of nuclear receptors by perfluorinated fatty acid analogs and natural fatty acids: A comparison of human, mouse, and rat peroxisome proliferator-activated receptor-alpha, -beta, and -gamma, liver X receptor-beta, and retinoid X receptor-alpha. Toxicol Sci. 2006, 92, 476–489. [Google Scholar] [CrossRef]
- Haughom, B.; Spydevold, O. The mechanism underlying the hypolipemic effect of perfluorooctanoic acid (PFOA), perfluorooctane sulfonic-acid (Pfosa) and clofibric acid. Biochim. Biophys. Acta 1992, 1128, 65–72. [Google Scholar] [CrossRef]
- Van Gilst, M.R.; Hadjivassiliou, H.; Jolly, A.; Yamamoto, K.R. Nuclear hormone receptor NHR-49 controls fat consumption and fatty acid composition in C. elegans. PLoS Biol. 2005, 3, e53. [Google Scholar] [CrossRef] [Green Version]
- Atherton, H.J.; Jones, O.A.H.; Malik, S.; Miska, E.A.; Griffin, J.L. A comparative metabolomic study of NHR-49 in Caenorhabditis elegans and PPAR-alpha in the mouse. FEBS Lett. 2008, 582, 1661–1666. [Google Scholar] [CrossRef]
- McKelvie, J.R.; Wolfe, D.M.; Celejewski, M.; Simpson, A.J.; Simpson, M.J. Correlations of Eisenia fetida metabolic responses to extractable phenanthrene concentrations through time. Environ. Pollut. 2010, 158, 2150–2157. [Google Scholar] [CrossRef]
- Vanwezel, A.P.; Opperhuizen, A. Narcosis due to environmental-pollutants in aquatic organisms—Residue-based toxicity, mechanisms, and membrane burdens. Crit. Rev. Toxicol. 1995, 25, 255–279. [Google Scholar]
- Jones, O.A.H.; Spurgeon, D.J.; Svendsen, C.; Griffin, J.L. A metabolomics based approach to assessing the toxicity of the polyaromatic hydrocarbon pyrene to the earthworm Lumbricus rubellus. Chemosphere 2008, 71, 601–609. [Google Scholar] [CrossRef]
- Keller, B.J.; Marsman, D.S.; Popp, J.A.; Thurman, R.G. Several Nongenotoxic Carcinogens Uncouple Mitochondrial Oxidative-Phosphorylation. Biochim. Biophys. Acta 1992, 1102, 237–244. [Google Scholar]
- O’Brien, T.M.; Wallace, K.B. Mitochondrial permeability transition as the critical target of N-acetyl perfluorooctane sulfonamide toxicity in vitro. Toxicol. Sci. 2004, 82, 333–340. [Google Scholar] [CrossRef]
- Starkov, A.A.; Wallace, K.B. Structural determinants of fluorochemical-induced mitochondrial dysfunction. Toxicol. Sci. 2002, 66, 244–252. [Google Scholar] [CrossRef]
- Cheng, W.; Yu, Z.; Feng, L.; Wang, Y. Perfluorooctane sulfonate (PFOS) induced embryotoxicity and disruption of cardiogenesis. Toxicol. in Vitro 2013, 27, 1503–1512. [Google Scholar] [CrossRef]
- Panaretakis, T.; Shabalina, I.G.; Grander, D.; Shoshan, M.C.; DePierre, J.W. Reactive oxygen species and mitochondria mediate the induction of apoptosis in human hepatoma HepG2 cells by the rodent peroxisome proliferator and hepatocarcinogen, perfluorooctanoic acid. Toxicol. Appl. Pharm. 2001, 173, 56–64. [Google Scholar] [CrossRef]
- Horton, H.R.; Moran, L.A.; Scrimgeour, K.G.; Perry, M.D.; Rawn, J.D. Principles of Biochemistry, 4th ed.; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2006. [Google Scholar]
- Rylander, C.; Dumeaux, V.; Olsen, K.S.; Waaseth, M.; Sandanger, T.M.; Lund, E. Usingdy blood gene signatures for assessing effects of exposure to perfluoroalkyl acids (PFAAs) in humans: The NOWAC postgenome study. Int. J. Mol. Epidemiol. Genet. 2011, 2, 207–216. [Google Scholar]
- Guruge, K.S.; Yeung, L.W.Y.; Yamanaka, N.; Miyazaki, S.; Lam, P.K.S.; Giesy, J.P.; Jones, P.D.; Yamashita, N. Gene expression profiles in rat liver treated with perfluorooctanoic acid (PFOA). Toxicol. Sci. 2006, 89, 93–107. [Google Scholar]
- Zhang, H.X.; Ding, L.N.; Fang, X.M.; Shi, Z.M.; Zhang, Y.T.; Chen, H.B.; Yan, X.Z.; Dai, J.Y. Biological responses to perfluorododecanoic acid exposure in rat kidneys as determined by integrated proteomic and metabonomic studies. PLoS One 2011, 6, e20862. [Google Scholar]
- Strange, K.; Morrison, R.; Heilig, C.W.; Dipietro, S.; Gullans, S.R. Up-regulation of inositol transport mediates inositol accumulation in hyperosmolar brain-cells. Am. J. Physiol. 1991, 260, C784–C790. [Google Scholar]
- Michell, R.H. Inositol derivatives: Evolution and functions. Nat. Rev. Mol. Cell Bio. 2008, 9, 151–161. [Google Scholar] [CrossRef]
- Santoro, M.M.; Liu, Y.; Khan, S.M.; Hou, L.X.; Bolen, D.W. Increased thermal stability of proteins in the presence of naturally occurring osmolytes. Biochemistry 1992, 31, 5278–5283. [Google Scholar] [CrossRef]
- Brinch, U.C.; Ekelund, F.; Jacobsen, C.S. Method for spiking soil samples with organic compounds. Appl. Environ. Microb. 2002, 68, 1808–1816. [Google Scholar] [CrossRef]
- McKelvie, J.R.; Yuk, J.; Xu, Y.P.; Simpson, A.J.; Simpson, M.J. H-1 NMR and GC/MS metabolomics of earthworm responses to sub-lethal DDT and endosulfan exposure. Metabolomics 2009, 5, 84–94. [Google Scholar] [CrossRef]
- Wu, H.F.; Southam, A.D.; Hines, A.; Viant, M.R. High-throughput tissue extraction protocol for NMR and MS-based metabolomics. Anal. Biochem. 2008, 372, 204–212. [Google Scholar] [CrossRef]
- Brown, S.A.E.; Simpson, A.J.; Simpson, M.J. Evaluation of sample preparation methods for nuclear magnetic resonance metabolic profiling studies with Eisenia fetida. Environ. Toxicol. Chem. 2008, 27, 828–836. [Google Scholar] [CrossRef]
- Simpson, A.J.; Brown, S.A. Purge NMR: Effective and easy solvent suppression. J. Magn. Reson. 2005, 175, 340–346. [Google Scholar] [CrossRef]
- Bundy, J.G.; Spurgeon, D.J.; Svendsen, C.; Hankard, P.K.; Weeks, J.M.; Osborn, D.; Lindon, J.C.; Nicholson, J.K. Environmental metabonomics: Applying combination biomarker analysis in earthworms at a metal contaminated site. Ecotoxicology 2004, 13, 797–806. [Google Scholar] [CrossRef]
- Boroujerdi, A.F.B.; Vizcaino, M.I.; Meyers, A.; Pollock, E.C.; Huynh, S.L.; Schock, T.B.; Morris, P.J.; Bearden, D.W. NMR-based microbial metabolomics and the temperature-dependent coral pathogen Vibrio coralliilyticus. Environ. Sci. Technol. 2009, 43, 7658–7664. [Google Scholar] [CrossRef]
- R Development Core Team, R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing: Vienna, Austria, 2009.
- Filzmoser, P.; Varmuza, K. Chemometrics: Multivariate Statistical Analysis in Chemometrics, R package Version 0:8, 2010.
- Varmuza, K.; Filzmoser, P. Introduction to Multivariate Statistical Analysis in Chemometrics; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Westerhuis, J.A.; Hoefsloot, H.C.J.; Smit, S.; Vis, D.J.; Smilde, A.K.; van Velzen, E.J.J.; van Duijnhoven, J.P.M.; van Dorsten, F.A. Assessment of PLSDA cross validation. Metabolomics 2008, 4, 81–89. [Google Scholar] [CrossRef]
- Alam, T.M.; Alam, N.K.; Neerathilingam, M.; Volk, D.E.; Sarkar, S.; Shakeel Ansari, G.A.; Luxon, B.A. 1H NMR metabonomic study of rat response to tri-phenyl phosphate and tri-butyl phosphate exposure. Metabolomics 2010, 6, 386–394. [Google Scholar] [CrossRef]
- Eriksson, L.; Jaworska, J.; Worth, A.P.; Cronin, M.T.; McDowell, R.M.; Gramatica, P. Methods for reliability and uncertainty assessment and for applicability evaluations of classification- and regression-based QSARs. Environ. Health Persp. 2003, 111, 1361–1375. [Google Scholar] [CrossRef]
- Rochfort, S.J.; Ezernieks, V.; Yen, A.L. NMR-based metabolomics using earthworms as potential indicators for soil health. Metabolomics 2009, 5, 95–107. [Google Scholar] [CrossRef]
- Higgins, C.P.; Luthy, R.G. Sorption of perfluorinated surfactants on sediments. Environ. Sci. Technol. 2006, 40, 7251–7256. [Google Scholar] [CrossRef]
- Sohlenius, A.K.; Eriksson, A.M.; Hogstrom, C.; Kimland, M.; Depierre, J.W. Perfluorooctane sulfonic-acid Is a potent inducer of peroxisomal fatty-acid beta-oxidation and other activities known to be affected by peroxisome proliferators in mouse-liver. Pharmacol. Toxicol. 1993, 72, 90–93. [Google Scholar] [CrossRef]
Supplementary Files
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lankadurai, B.P.; Furdui, V.I.; Reiner, E.J.; Simpson, A.J.; Simpson, M.J. 1H NMR-Based Metabolomic Analysis of Sub-Lethal Perfluorooctane Sulfonate Exposure to the Earthworm, Eisenia fetida, in Soil. Metabolites 2013, 3, 718-740. https://doi.org/10.3390/metabo3030718
Lankadurai BP, Furdui VI, Reiner EJ, Simpson AJ, Simpson MJ. 1H NMR-Based Metabolomic Analysis of Sub-Lethal Perfluorooctane Sulfonate Exposure to the Earthworm, Eisenia fetida, in Soil. Metabolites. 2013; 3(3):718-740. https://doi.org/10.3390/metabo3030718
Chicago/Turabian StyleLankadurai, Brian P., Vasile I. Furdui, Eric J. Reiner, André J. Simpson, and Myrna J. Simpson. 2013. "1H NMR-Based Metabolomic Analysis of Sub-Lethal Perfluorooctane Sulfonate Exposure to the Earthworm, Eisenia fetida, in Soil" Metabolites 3, no. 3: 718-740. https://doi.org/10.3390/metabo3030718




