The In Vitro Anti-Proliferative Interaction of Flavonoid Quercetin and Toxic Metal Cadmium in the 1321N1 Human Astrocytoma Cell Line
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Stock Solutions and Concentrations Used for Treating the Cells
2.3. Treatments of the 1321N1 Cells with QE and Cd
3. Results
3.1. Evaluation of the Anti-Proliferative Effect of the Vehicle (0.5%) DMSO
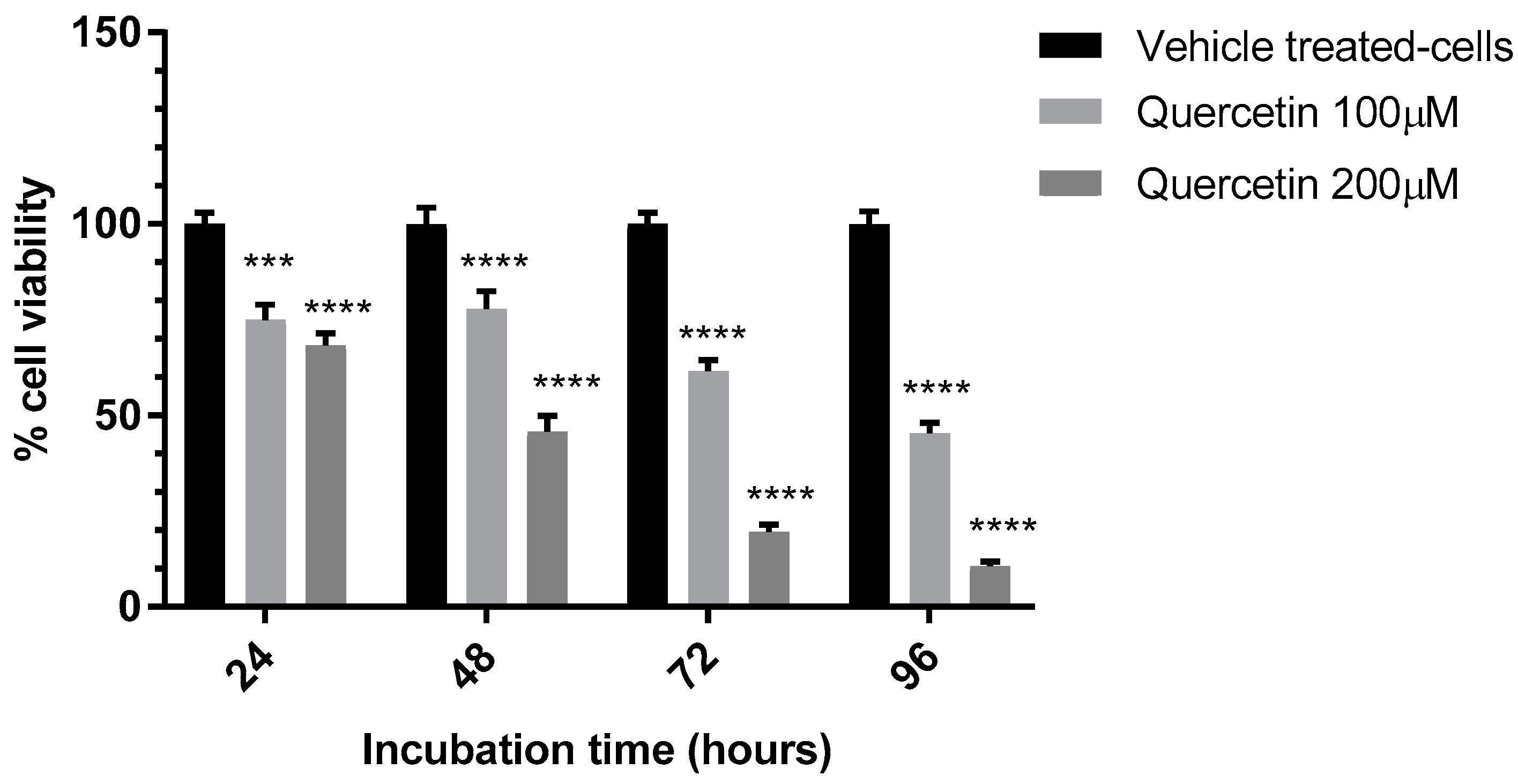
3.2. Evaluation of the Anti-Proliferative Effect of QE
3.3. Evaluation of the Anti-Proliferative Effect of Cd
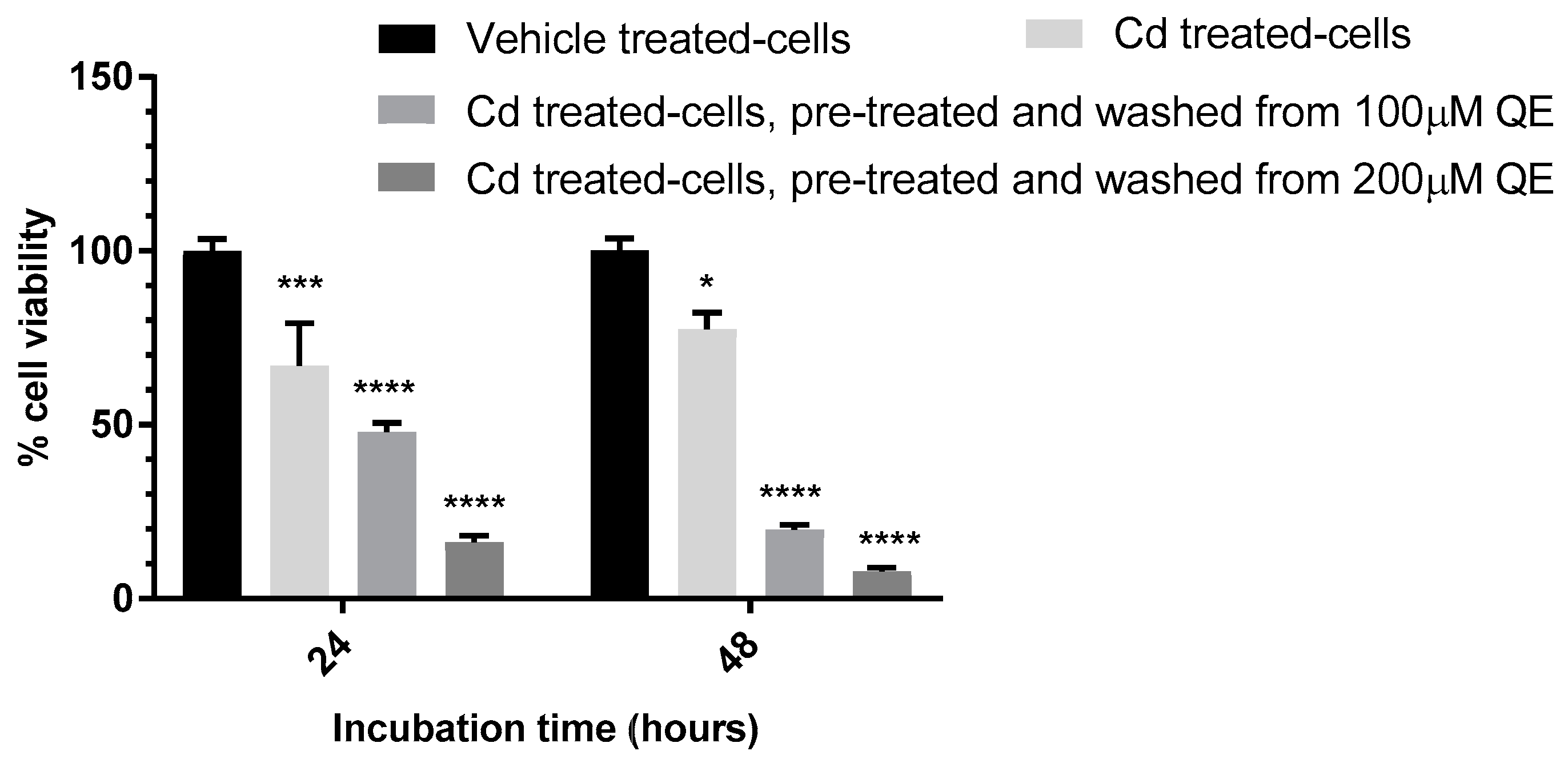
3.4. Evaluation of the Anti-Proliferative Effect of QE and Cd in Co-Treatment
4. Discussion
5. Conclusions
Author Contributions
Acknowledgment
Conflicts of Interest
References
- Arita, A.; Costa, M. Epigenetics in metal carcinogenesis: Nickel, arsenic, chromium and cadmium. Metallomics 2009, 1, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Jarup, L.; Akesson, A. Current status of cadmium as an environmental health problem. Toxicol. Appl. Pharmacol. 2009, 238, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.K.; Athersuch, T.J.; Thomas, L.D.; Teichert, F.; Perez-Trujillo, M.; Svendsen, C.; Spurgeon, D.J.; Singh, R.; Järup, L.; Bundy, J.G.; et al. Metabolic profiling detects early effects of environmental and lifestyle exposure to cadmium in a human population. BMC Med. 2012, 10, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huff, J.; Lunn, R.M.; Waalkes, M.P.; Tomatis, L.; Infante, P.F. Cadmium-induced cancers in animals and in humans. Int. J. Occup. Environ. Health 2007, 13, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qu, W.; Kadiiska, M.B. Role of oxidative stress in cadmium toxicity and carcinogenesis. Toxicol. Appl. Pharmacol. 2009, 238, 209–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filipic, M. Mechanisms of cadmium induced genomic instability. Mutat. Res. 2012, 733, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.P.; Kumari, R.; Pevey, C.; Jackson, D.; DuMond, J.W. Long duration exposure to cadmium leads to increased cell survival, decreased DNA repair capacity, and genomic instability in mouse testicular Leydig cells. Cancer Lett. 2009, 279, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Venza, M.; Visalli, M.; Biondo, C.; Oteri, R.; Agliano, F.; Morabito, S.; Caruso, G.; Caffo, M.; Teti, D.; Venza, I. Epigenetic effects of cadmium in cancer: Focus on melanoma. Curr. Genom. 2014, 15, 420–435. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Ma, S.; Qi, Y.; Wei, X.; Cai, H.; Dong, L.; Lu, Y.; Zhang, Y.; Guo, Q. Quercetin inhibited cadmium-induced autophagy in the mouse kidney via inhibition of oxidative stress. J. Toxicol. Pathol. 2016, 29, 247–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harnly, J.M.; Doherty, R.F.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Bhagwat, S.; Gebhardt, S. Flavonoid content of U.S. fruits, vegetables, and nuts. J. Agric. Food Chem. 2006, 54, 9966–9977. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, G. Quercetin: A flavonol with multifaceted therapeutic applications? Fitoterapia 2015, 106, 256–271. [Google Scholar] [CrossRef] [PubMed]
- Chirumbolo, S. Quercetin in cancer prevention and therapy. Integr. Cancer Ther. 2013, 12, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Gorlach, S.; Fichna, J.; Lewandowska, U. Polyphenols as mitochondria-targeted anticancer drugs. Cancer Lett. 2015, 366, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Bruning, A. Inhibition of mTOR signalingby quercetin in cancer treatment and prevention. Anticancer Agents Med. Chem. 2013, 13, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Sak, K. Site-specific anticancer effects of dietary flavonoid quercetin. Nutr. Cancer 2014, 66, 177–193. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.L.; Russo, M.; Spagnuolo, C.; Tedesco, I.; Bilotto, S.; Iannitti, R.; Palumbo, R. Quercetin: A pleiotropic kinase inhibitor against cancer. Cancer Treat. Res. 2014, 159, 185–205. [Google Scholar] [CrossRef] [PubMed]
- Maurya, A.K.; Vinayak, M. Modulation of PKC signaling and induction of apoptosis through suppression of reactive oxygen species and tumor necrosis factor receptor 1 (TNFR1): Key role of quercetin in cancer prevention. Tumour Biol. 2015, 36, 8913–8924. [Google Scholar] [CrossRef] [PubMed]
- Zargar, S.; Siddiqi, N.J.; Al Daihan, S.K.; Wani, T.A. Protective effects of quercetin on cadmium fluoride induced oxidative stress at different intervals of time in mouse liver. Acta Biochim. Pol. 2015, 62, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Sanchez, C.; Egido, J.; Sanchez-Gonzalez, P.D.; Perez-Barriocanal, F.; Lopez-Novoa, J.M.; Morales, A.I. Effect of the flavonoid quercetin on cadmium-induced hepatotoxicity. Food Chem. Toxicol. 2008, 46, 2279–2287. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.I.; Vicente-Sanchez, C.; Jerkic, M.; Santiago, J.M.; Sanchez-Gonzalez, P.D.; Perez-Barriocanal, F.; López-Novoa, J.M. Effect of quercetin on metallothionein, nitric oxide synthases and cyclooxygenase-2 expression on experimental chronic cadmium nephrotoxicity in rats. Toxicol. Appl. Pharmacol. 2006, 210, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.I.; Vicente-Sanchez, C.; Sandoval, J.M.; Egido, J.; Mayoral, P.; Arevalo, M.A.; Fernández-Tagarro, M.; López-Novoa, J.M.; Pérez-Barriocanal, F. Protective effect of quercetin on experimental chronic cadmium nephrotoxicity in rats is based on its antioxidant properties. Food Chem. Toxicol. 2006, 44, 2092–2100. [Google Scholar] [CrossRef] [PubMed]
- Renugadevi, J.; Prabu, S.M. Quercetin protects against oxidative stress-related renal dysfunction by cadmium in rats. Exp. Toxicol. Pathol. 2010, 62, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lin, S.Q.; He, Y.L.; Liu, G.; Wang, Z.Y. Protective effects of quercetin on cadmium-induced cytotoxicity in primary cultures of rat proximal tubular cells. Biomed. Environ. Sci. 2013, 26, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Bu, T.; Mi, Y.; Zeng, W.; Zhang, C. Protective effect of quercetin on cadmium-induced oxidative toxicity on germ cells in male mice. Anat. Rec. 2011, 294, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Lin, J.; Mi, Y.; Zhang, C. Quercetin attenuates cadmium-induced oxidative damage and apoptosis in granulosa cells from chicken ovarian follicles. Reprod. Toxicol. 2011, 31, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Kanter, M.; Aktoz, T.; Aktas, C.; Ozen, F.; Yarali, O.; Kanter, B. Role of quercetin in cadmium-induced oxidative stress, testicular damage, and apoptosis in rats. Anal. Quant. Cytopathol. Histpathol. 2016, 38, 45–51. [Google Scholar] [PubMed]
- Nna, V.U.; Usman, U.Z.; Ofutet, E.O.; Owu, D.U. Quercetin exerts preventive, ameliorative and prophylactic effects on cadmium chloride—Induced oxidative stress in the uterus and ovaries of female Wistar rats. Food Chem. Toxicol. 2017, 102, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Shukla, L.P.; Chandravanshi, L.P.; Srivastava, P.; Dhuriya, Y.K.; Shanker, J.; Singh, M.P.; Pant, A.B.; Khanna, V.K. Protective role of quercetin in cadmium-induced cholinergic dysfunctions in rat brain by modulating mitochondrial integrity and MAP kinase signaling. Mol. Neurobiol. 2017, 54, 4560–4583. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.; Kar, R.; Galav, V.; Mehta, A.K.; Bhattacharya, S.K.; Mediratta, P.K.; Banerjee, B.D. Cadmium exposure during lactation causes learning and memory-impairment in F1 generation mice: Amelioration by quercetin. Drug Chem. Toxicol. 2016, 39, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, F.H.; Cardoso, A.M.; Pereira, L.B.; Schmatz, R.; Goncalves, J.F.; Stefanello, N.; Fiorenza, A.M.; Gutierres, J.M.; Serres, J.D.; Zanini, D.; et al. Neuroprotective effect of quercetin in ectoenzymes and acetylcholinesterase activities in cerebral cortex synaptosomes of cadmium-exposed rats. Mol. Cell. Biochem. 2013, 381, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, F.H.; Schmatz, R.; Cardoso, A.M.; Carvalho, F.B.; Baldissarelli, J.; de Oliveira, J.S.; Rosa, M.M.; Goncalves Nunes, M.A.; Rubin, M.A.; da Cruz, I.B.; et al. Quercetin protects the impairment of memory and anxiogenic-like behavior in rats exposed to cadmium: Possible involvement of the acetylcholinesterase and Na+,K+-ATPase activities. Physiol. Behav. 2014, 135, 152–167. [Google Scholar] [CrossRef] [PubMed]
- Kanter, M.; Unsal, C.; Aktas, C.; Erboga, M. Neuroprotective effect of quercetin against oxidative damage and neuronal apoptosis caused by cadmium in hippocampus. Toxicol. Ind. Health 2016, 32, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Unsal, C.; Kanter, M.; Aktas, C.; Erboga, M. Role of quercetin in cadmium-induced oxidative stress, neuronal damage, and apoptosis in rats. Toxicol. Ind. Health 2015, 31, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Milton Prabu, S.; Muthumani, M.; Shagirtha, K. Quercetin potentially attenuates cadmium induced oxidative stress mediated cardiotoxicity and dyslipidemia in rats. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 582–595. [Google Scholar] [PubMed]
- Prabu, S.M.; Shagirtha, K.; Renugadevi, J. Amelioration of cadmium-induced oxidative stress, impairment in lipids and plasma lipoproteins by the combined treatment with quercetin and alpha-tocopherol in rats. J. Food Sci. 2010, 75, T132–T140. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, S.; Halagowder, D.; Sivasithambaram, N.D. Quercetin suppresses Twist to induce apoptosis in MCF-7 breast cancer cells. PLoS ONE 2015, 10, e0141370. [Google Scholar] [CrossRef] [PubMed]
- Okoko, T.; Ere, D. Some bioactive potentials of two biflavanols isolated from Garcinia kola on cadmium-induced alterations of raw U937 cells and U937-derived macrophages. Asian Pac. J. Trop. Med. 2013, 6, 43–48. [Google Scholar] [CrossRef]
- Carmichael, J.; DeGraff, W.G.; Gazdar, A.F.; Minna, J.D.; Mitchell, J.B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: Assessment of chemosensitivity testing. Cancer Res. 1987, 47, 936–942. [Google Scholar] [PubMed]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Arsenic, metals, fibres, and dusts. In IARC Monographs Evaluating Carcinogenic Risks to Humans; IARC: Lyon, France, 2012; Volume 100, pp. 11–465. [Google Scholar]
- Dajas, F. Life or death: Neuroprotective and anticancer effects of quercetin. J. Ethnopharmacol. 2012, 143, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Santel, T.; Pflug, G.; Hemdan, N.Y.; Schafer, A.; Hollenbach, M.; Buchold, M.; Hintersdorf, A.; Lindner, I.; Otto, A.; Bigl, M.; et al. Curcumin inhibits glyoxalase 1: A possible link to its anti-inflammatory and anti-tumor activity. PLoS ONE 2008, 3, e3508. [Google Scholar] [CrossRef] [PubMed]
- Lawal, A.O.; Ellis, E. Differential sensitivity and responsiveness of three human cell lines HepG2, 1321N1 and HEK 293 to cadmium. J. Toxicol. Sci. 2010, 35, 465–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawal, A.O.; Ellis, E.M. Nrf2-mediated adaptive response to cadmium-induced toxicity involves protein kinase C delta in human 1321N1 astrocytoma cells. Environ. Toxicol. Pharmacol. 2011, 32, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Chopikashvili, L.V.; Bobyleva, L.A.; Shapiro, A.N. The genetic action of ammonium molybdate and cadmium chloride and iodide. Tsitol. Genet. 1991, 25, 63–67. (In Russian) [Google Scholar] [PubMed]
- Rabkova, L.M. On the use of cadmium iodide and semicarbazide Hc1 in the treatment of bladder cancers. Vop. Onkol. 1963, 9, 125–126. (In Russian) [Google Scholar]
- Vilner, L.S. Data on the results of treatment, at the united hospital of the Moscow district of Leningrad, of cancer patients with cadmium iodide and semicarbazide Hc1. Vop. Onkol. 1963, 9, 120–125. (In Russian) [Google Scholar]
- Kruglova, G.V. Results of the clinical trial of treating cancer patients with cadmium iodide and semicarbazide Hc1. Vop. Onkol. 1963, 9, 104–113. (In Russian) [Google Scholar]
- Chou, T.C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Ellis, M.E.; Oduse, K.A. The roles of phytochemicals in red wine as a protective agent against alcohol damage. Int. Food Res. J. 2013, 20, 1191–1197. [Google Scholar]


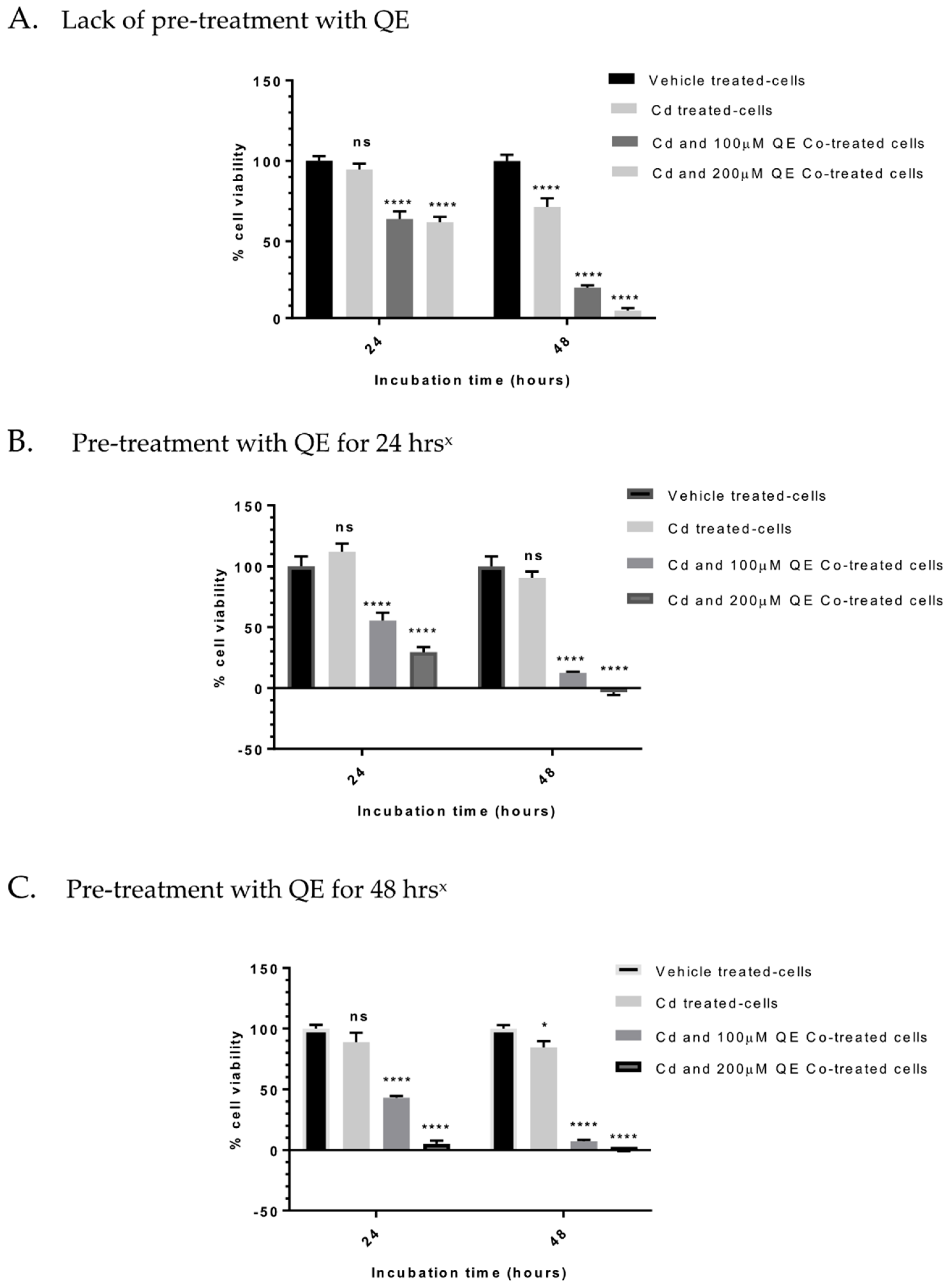
| QE Pre-Incubation | Pre-Incubation with QE | Cd Incubations for 24 and 48 h | QE and Cd Incubations for 24 and 48 h | Remarks |
|---|---|---|---|---|
| - | - | - | √ | - |
| √ | 24 h | - | √ | - |
| √ | 48 h | - | √ | - |
| √ | 48 h | √ | - | QE washed off with HBSS * or medium |
| Concentration of Quercetin (QE) and/or Cadmium (Cd) | Viability | Combination Index (CI) *** | ||
|---|---|---|---|---|
| 24 h | 48 h | 24 h | 48 h | |
| QE 100 μM | 74.9 ± 4.0 | 77.9 ± 4.6 | ||
| QE 200 μM | 68.3 ± 3.2 | 45.8± 4.2 | ||
| Cd 16 μM | 94.7 ± 3.7 | 71.4 ± 5.4 | ||
| QE 100 μM + Cd 16 μM | 64.0 ± 4.8 * | 21.4 ± 1.3 * | 0.91 | 0.50 |
| QE 200 μM + Cd 16 μM | 62.1 ± 3.1 ** | 6.9 ± 1.6 * | 1.01 | 0.41 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Hasawi, N.A.; Amine, S.A.; Novotny, L. The In Vitro Anti-Proliferative Interaction of Flavonoid Quercetin and Toxic Metal Cadmium in the 1321N1 Human Astrocytoma Cell Line. Sci. Pharm. 2018, 86, 36. https://doi.org/10.3390/scipharm86030036
Al-Hasawi NA, Amine SA, Novotny L. The In Vitro Anti-Proliferative Interaction of Flavonoid Quercetin and Toxic Metal Cadmium in the 1321N1 Human Astrocytoma Cell Line. Scientia Pharmaceutica. 2018; 86(3):36. https://doi.org/10.3390/scipharm86030036
Chicago/Turabian StyleAl-Hasawi, Nada A., Sanaa A. Amine, and Ladislav Novotny. 2018. "The In Vitro Anti-Proliferative Interaction of Flavonoid Quercetin and Toxic Metal Cadmium in the 1321N1 Human Astrocytoma Cell Line" Scientia Pharmaceutica 86, no. 3: 36. https://doi.org/10.3390/scipharm86030036




