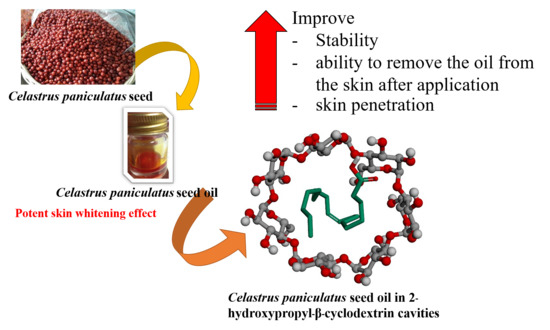
Skin Penetration and Stability Enhancement of Celastrus paniculatus Seed Oil by 2-Hydroxypropyl-β-Cyclodextrin Inclusion Complex for Cosmeceutical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the C. paniculatus Seed Oil
2.3. Determination of Oleic Acid Contents by HPLC
2.4. Encapsulation of C. paniculatus Seed Oil in HPβCD
2.5. Physicochemical Characteristics of Encapsulated HPβCD
2.5.1. Determination of Maximum Encapsulation
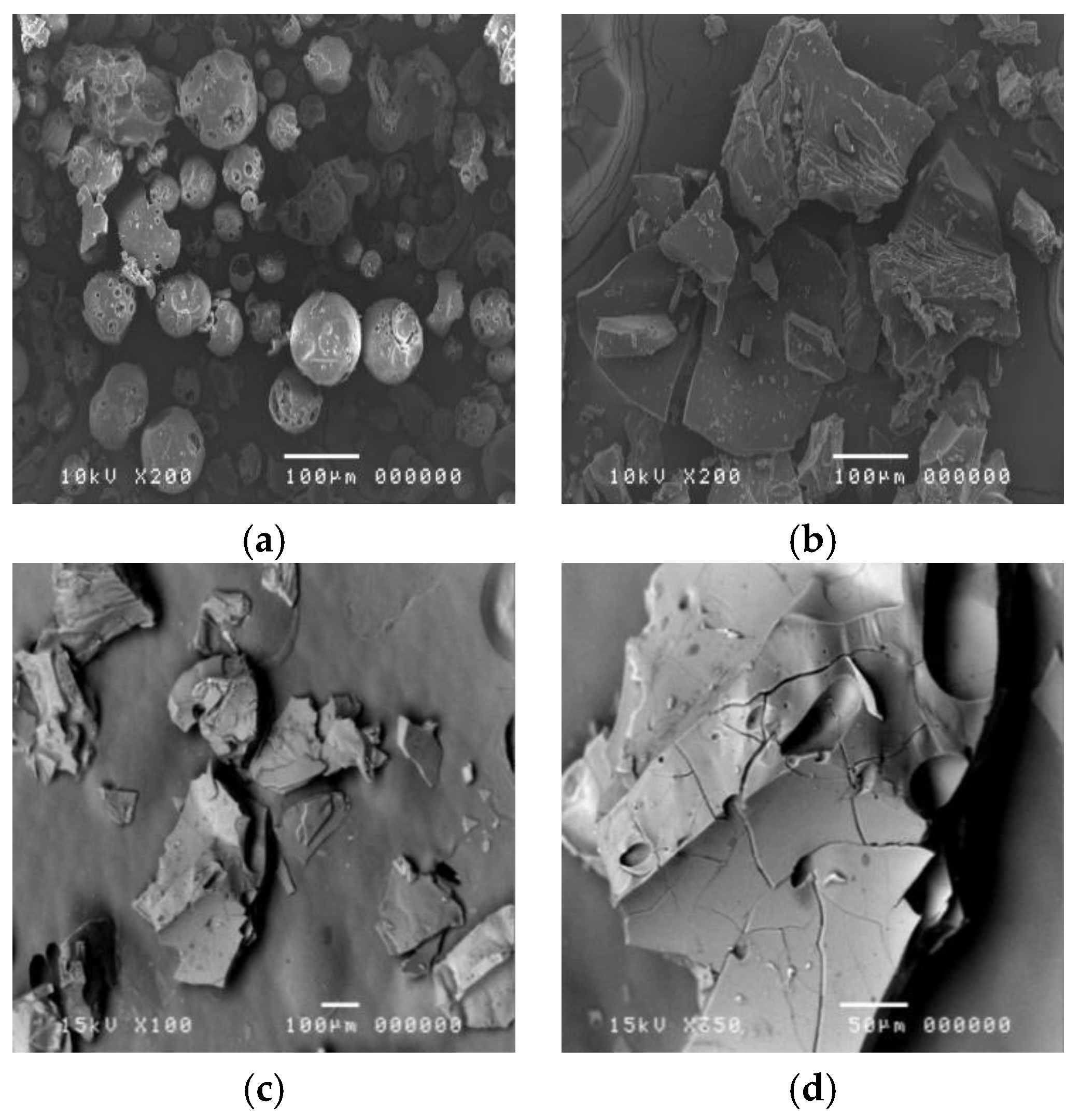
2.5.2. Morphology and Particle Size of Inclusion Complex
2.6. Biological Studies of the CPSO-HPβCD Inclusion Complex
2.7. Preparation of Formulations
2.8. Stability of CPSO and CPSO-HPβCD Inclusion Complex Formulations
2.9. In Vitro Skin Permeation Study
2.9.1. Skin Sample
2.9.2. CPSO Sample Preparation
2.9.3. Skin-Permeation Study
2.10. Statistical Analysis
3. Results and Discussion
3.1. Physical Characteristics of C. paniculatus Seed Oil (CPSO)
3.2. Physical Characteristics of the CPSO-HPβCD Inclusion Complex
3.3. Biological Activities of CPSO-HPβCD Inclusion Complex
3.4. Physical Characteristics of CPSO-HPβCD Inclusion-Complex Formulations
3.5. Chemical Characteristics of Oleic Acid in CPSO-HPβCD Inclusion Complex and CPSO-HPβCD Formulations
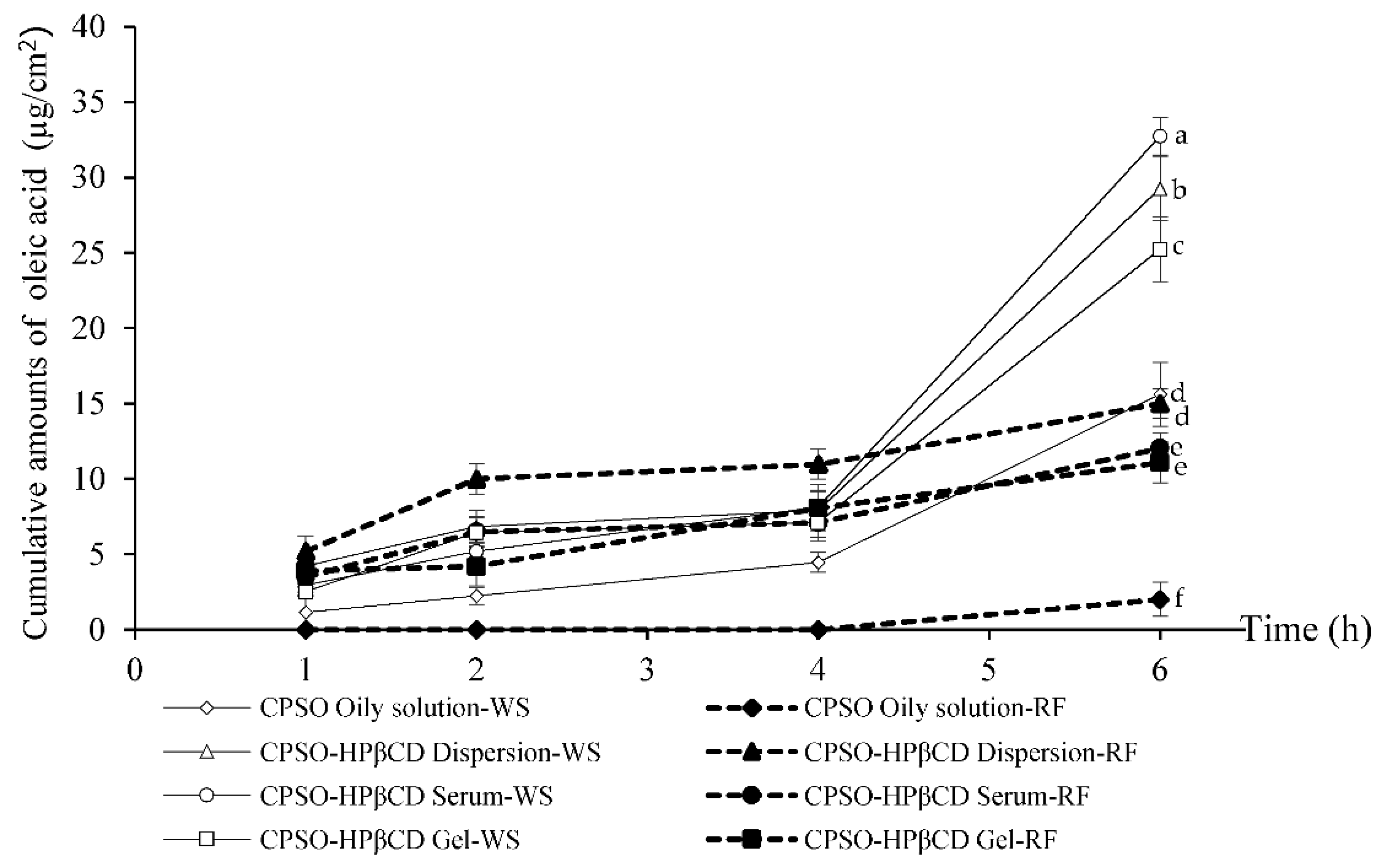
3.6. Skin Permeation of CPSO-HPβCD Inclusion-Complex Formulations
4. Conclusions
Author contributions
Funding
Acknowledgments
Conflicts of interest
References
- Chatterjee, A.; Prakashi, S.C. The Treatise on Indian Medicinal Plants; Niscair: New Delhi, India, 2003; Volume 3, p. 160. [Google Scholar]
- Singh, U.; Wadhawani, A.M.; Johri, B.M. Dictionary of Economical Plants of India; Indian council of Agricultural Research: New Delhi, India, 1996. [Google Scholar]
- Nadkarni, K.M.; Nadkarni, A.K.; Nadkarni, K.M. Indian Plants and Drugs; Popular Bombay Prakashan Pvt Ltd.: Mumbai, India, 1976; Volume 1, p. 296. [Google Scholar]
- Rana, V.S.; Das, M. Fatty acid and non-fatty acid components of the seed oil of Celastrus paniculatus willd. Int. J. Fruit Sci. 2017, 17, 407–414. [Google Scholar] [CrossRef]
- Tanojo, H.; Boelsma, E.; Junginger, H.E.; Ponec, M.; Bodde, H.E. In vivo human skin barrier modulation by topical application of fatty acids. Skin Pharmacol. Appl. Skin Physiol. 1998, 11, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Ziboh, V.A.; Miller, C.C.; Cho, Y. Metabolism of polyunsaturated fatty acids by skin epidermal enzymes: Generation of antiinflammatory and antiproliferative metabolites. Am. J. Clin. Nutr. 2000, 71 (Suppl. 1), 361S–366S. [Google Scholar] [CrossRef] [PubMed]
- Ruksiriwanich, W.; Sringarm, K.; Jantrawut, P. Stability enhancement of Celastrus paniculatus seed oil by loading in niosomes. Asian J. Pharm. Clin. Res. 2014, 7, 186–191. [Google Scholar]
- Choe, E.; Min, D.B. Mechanisms and factors for edible oil oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Duchêne, D.; Bochot, A.; Yu, S.C.; Pépin, C.; Seiller, M. Cyclodextrins and emulsions. Int. J. Pharm. 2003, 266, 85–90. [Google Scholar] [CrossRef]
- Schmid, G. Cyclodextrin glycosyltransferase production: Yield enhancement by overexpression of cloned genes. Trends Biotechnol. 1989, 7, 244–248. [Google Scholar] [CrossRef]
- Kurkov, S.V.; Loftsson, T. Cyclodextrins. Int. J. Pharm. 2013, 453, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Citernesi, U.; Sciacchitano, M. Cyclodextrins in functional dermocosmetics. Cosmet. Toilet. 1995, 110, 53–61. [Google Scholar]
- Sarveiya, V.; Templeton, J.F.; Benson, H.A.E. Inclusion complexation of the sunscreen 2-hydroxy-4-methoxy benzophenone (oxybenzone) with hydroxypropyl-β-cyclodextrin: Effect on membrane diffusion. J. Incl. Phenom. Macrocycl. Chem. 2004, 49, 275–281. [Google Scholar] [CrossRef]
- Radhika, P.; Nagabhushanam, M.V.; Ramana, M.V. Preparation and characterization of Lornoxicam solid systems using cyclodextrins for improved bioavailability. Bull. Pharm. Res. 2015, 5, 101–107. [Google Scholar]
- Wang, J.; Cao, Y.; Sun, B.; Wang, C. Physicochemical and release characterisation of garlic oil-β-cyclodextrin inclusion complexes. Food Chem. 2011, 127, 1680–1685. [Google Scholar] [CrossRef]
- Tachibana, Y.; Kikuzaki, H.; Hj-Lajis, N.; Nakatani, N. Antioxidant activity of carbazoles from Murraya koenigii leaves. J. Agric. Food Chem. 2001, 49, 5589–5594. [Google Scholar] [CrossRef] [PubMed]
- Osawa, T.; Namiki, M. A novel type of antioxidant isolated from leaf wax of eucalyptus leaves. Agric. Biol. Chem. 1981, 45, 735–739. [Google Scholar] [CrossRef]
- Decker, E.A.; Welch, B. Role of ferritin as a lipid oxidation catalyst in muscle food. J. Agric. Food Chem. 1990, 38, 674–677. [Google Scholar] [CrossRef]
- Shimizu, K.; Kondo, R.; Sakai, K.; Lee, S.; Sato, H. The inhibitory components from Artocarpus incisus on melanin biosynthesis. Planta Med. 1998, 64, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Plessis, J.; Egbaria, K.; Weiner, N. Influence of formulation factors on the deposition of liposomal components into the different strata of the skin. J. Soc. Cosmet. Chem. 1992, 43, 93–100. [Google Scholar]
- Kabnurkar, R.B. Phytopharmaceutical studies of the topical formulations of Celastrus paniculatus Willd (CELASTRACEAE). J. Pharm. Biomed. Sci. 2012, 17, 1–4. [Google Scholar]
- Bhanumathy, M.; Chandrasekar, S.B.; Chandur, U.; Somasundaram, T. Phytopharmacology of Celastrus paniculatus: An overview. Int. J. Pharm. Sci. Drug Res. 2010, 2, 176–181. [Google Scholar]
- Arora, N.; Pandey-Rai, S. Celastrus paniculatus, an endangered indian medicinal plant with miraculous cognitive and other therapeutic properties: An overview. Int. J. Pharma Bio Sci. 2012, 3, 290–303. [Google Scholar]
- Salústio, P.J.; Feio, G.; Figueirinhas, J.L.; Pinto, J.F.; Cabral Marques, H.M. The influence of the preparation methods on the inclusion of model drugs in a β-cyclodextrin cavity. Eur. J. Pharm. Biopharm. 2009, 71, 377–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mourtzinos, I.; Salta, F.; Yannakopoulou, K.; Chiou, A.; Karathanos, V.T. Encapsulation of olive leaf extract in β-cyclodextrin. J. Agric. Food Chem. 2007, 55, 8088–8094. [Google Scholar] [CrossRef] [PubMed]
- Del Toro-Sánchez, C.; Ayala-Zavala, J.; Machi, L.; Santacruz, H.; Villegas-Ochoa, M.; Alvarez-Parrilla, E.; González-Aguilar, G. Controlled release of antifungal volatiles of thyme essential oil from β-cyclodextrin capsules. J. Incl. Phenom. Macrocycl. Chem. 2010, 67, 431–441. [Google Scholar] [CrossRef]
- Del Valle, E.M.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Szejtli, J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef] [PubMed]
- Bilia, A.R.; Guccione, C.; Isacchi, B.; Righeschi, C.; Firenzuoli, F.; Bergonzi, M.C. Essential oils loaded in nanosystems: A developing strategy for a successful therapeutic approach. Evid. Based Complement. Alternat. Med. 2014, 2014, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Arora, N.; Pandey-Rai, S. GC–MS analysis of the essential oil of Celastrus paniculatus Willd seeds and antioxidant, anti-inflammatory study of its various solvent extracts. Ind. Crops Prod. 2014, 61, 345–351. [Google Scholar] [CrossRef]
- Khatib, S.; Nerya, O.; Musa, R.; Shmuel, M.; Tamir, S.; Vaya, J. Chalcones as potent tyrosinase inhibitors: The importance of a 2, 4-substituted resorcinol moiety. Bioorg. Med. Chem. 2005, 13, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Kouba, M.; Mourot, J. A review of nutritional effects on fat composition of animal products with special emphasis on n-3 polyunsaturated fatty acids. Biochimie 2011, 93, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Szente, L.; Szejtli, J.; Szemán, J.; Kató, L. Fatty acid-cyclodextrin complexes: Properties and applications. J. Incl. Phenom. Macrocycl. Chem. 1993, 16, 339–354. [Google Scholar] [CrossRef]
- Godin, B.; Touitou, E. Transdermal skin delivery: Predictions for humans from in vivo, ex vivo and animal models. Adv. Drug Deliv. Rev. 2007, 59, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.E.; Mueller, K.R. Comparisons of in vitro nitroglycerin (TNG) flux across Yucatan pig, hairless mouse, and human skins. Pharm. Res. 1990, 7, 673–676. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Sugibayashi, K.; Morimoto, Y. Species differences in percutaneous absorption of nicorandil. J. Pharm. Sci. 1991, 80, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Imai, T.; Ariyoshi, S.; Ohura, K.; Sawada, T.; Nakada, Y. Expression of carboxylesterase isozymes and their role in the behavior of a fexofenadine prodrug in rat skin. J. Pharm. Sci. 2016, 105, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Krishnaiah, Y.S.R.; Satyanarayana, V.; Karthikeyan, R.S. Penetration enhancing effect of menthol on the percutaneous flux of nicardipine hydrochloride through excised rat epidermis from hydroxypropyl cellulose gels. Pharm. Dev. Technol. 2002, 7, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Kunta, J.R.; Goskonda, V.R.; Brotherton, H.O.; Khan, M.A.; Reddy, I.K. Effect of menthol and related terpenes on the percutaneous absorption of propranolol across excised hairless mouse skin. J. Pharm. Sci. 1997, 86, 1369–1373. [Google Scholar] [PubMed]
- Herman, A.; Herman, A.P. Essential oils and their constituents as skin penetration enhancer for transdermal drug delivery: A review. J. Pharm. Pharmacol. 2015, 67, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Suetake, T.; Sasai, S.; Zhen, Y.X.; Tagami, H. Effects of silicone gel sheet on the stratum corneum hydration. Br. J. Plast. Surg. 2000, 53, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Arellano, A.; Santoyo, S.; Martın, C.; Ygartua, P. Influence of propylene glycol and isopropyl myristate on the in vitro percutaneous penetration of diclofenac sodium from carbopol gels. Eur. J. Pharm. Sci. 1999, 7, 129–135. [Google Scholar] [CrossRef]
- Goodman, M.; Barry, B.W. Action of penetration enhancers on human skin as assessed by the permeation of model drugs 5-fluorouracil and estradiol I Infinite dose technique. J. Investig. Dermatol. 1988, 91, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Sarpotdar, P.P.; Zatz, J.L. Evaluation of penetration enhancement of lidocaine by nonionic surfactants through hairless mouse skin in vitro. J. Pharm. Sci. 1986, 75, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Uekama, K.; Hirayama, F.; Irie, T. Cyclodextrin drug carrier systems. Chem. Rev. 1998, 98, 2045–2076. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Brewster, M.E. Cyclodextrin-based pharmaceutics: Past, present and future. Nat. Rev. Drug Discov. 2004, 3, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Buschmann, H.J.; Schollmeyer, E. Application of cyclodextrins in cosmetic products: A review. J. Cosmet. Sci. 2002, 53, 185–191. [Google Scholar] [PubMed]
- Veiga, M.D.; Díaz, P.J.; Ahsan, F. Interactions of griseofulvin with cyclodextrins in solid binary systems. J. Pharm. Sci. 1998, 87, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Lutka, A. Effect of cyclodextrin complexation on aqueous solubility and photostability of phenothiazine. Die Pharm. 2000, 55, 120–123. [Google Scholar]

| Weight Ratio (CPSO:HPβCD) | Concentration of CPSO in the Inclusion Complex (% w/w) | Inclusion Ratio (% of Initial) | Total Recovery (% of Initial) |
|---|---|---|---|
| 1:1 | 50.0 | NA | NA |
| 1:2 | 33.3 | NA | NA |
| 1:3 | 25.0 | 92.4 ± 0.7 a | 87.5 ± 3.4 a |
| 1:4 | 20.0 | 95.4 ± 2.2 a | 90.3 ± 2.8 a |
| 1:5 | 16.7 | 94.2 ± 1.3 a | 89.6 ± 1.9 a |
| Sample | Antioxidative Activity (mg/mL) | Tyrosinase Inhibition Activity | ||
|---|---|---|---|---|
| DPPH Scavenging Activity | Metal Ion Chelating Activity | Inhibition of Lipid Peroxidation Activity | ||
| CPSO-HPβCD | 564.12 ± 17.14 a | 3587.21 ± 25.23 a | 464.87 ± 11.45 a | 0.19 ± 0.12 a |
| CPSO | 28.11 ± 1.97 b | 314.61 ± 12.45 a | 56.24 ± 8.85 b | 0.04 ± 0.03 b |
| Oleic acid | 70.45 ± 15.14 c | 26.28 ± 5.45 b | 11.25 ± 1.13 c | 9.14 ± 1.14 c |
| Ascorbic acid | 0.11 ± 0.02 d | NA | 0.83 ± 0.02 d | 0.09 ± 0.01 d |
| EDTA | NA | 0.48 ± 0.07 c | NA | NA |
| Kojic acid | NA | NA | NA | 0.07 ± 0.01 d |
| Arbutin | NA | NA | NA | 0.06 ± 0.01 d |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruksiriwanich, W.; Sirithunyalug, J.; Khantham, C.; Leksomboon, K.; Jantrawut, P. Skin Penetration and Stability Enhancement of Celastrus paniculatus Seed Oil by 2-Hydroxypropyl-β-Cyclodextrin Inclusion Complex for Cosmeceutical Applications. Sci. Pharm. 2018, 86, 33. https://doi.org/10.3390/scipharm86030033
Ruksiriwanich W, Sirithunyalug J, Khantham C, Leksomboon K, Jantrawut P. Skin Penetration and Stability Enhancement of Celastrus paniculatus Seed Oil by 2-Hydroxypropyl-β-Cyclodextrin Inclusion Complex for Cosmeceutical Applications. Scientia Pharmaceutica. 2018; 86(3):33. https://doi.org/10.3390/scipharm86030033
Chicago/Turabian StyleRuksiriwanich, Warintorn, Jakkapan Sirithunyalug, Chiranan Khantham, Krot Leksomboon, and Pensak Jantrawut. 2018. "Skin Penetration and Stability Enhancement of Celastrus paniculatus Seed Oil by 2-Hydroxypropyl-β-Cyclodextrin Inclusion Complex for Cosmeceutical Applications" Scientia Pharmaceutica 86, no. 3: 33. https://doi.org/10.3390/scipharm86030033