Alginate-Based Composite Sponges as Gastroretentive Carriers for Curcumin-Loaded Self-Microemulsifying Drug Delivery Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Liquid Curcumin-Loaded Self-Microemulsifying Drug Delivery Systems
2.3. Preparation of Alginate-Based Sponges Loaded with Curcumin-Loaded Self-Microemulsifying Drug Delivery Systems
2.3.1. Alginate Sponges
2.3.2. Alginate-Colloidal Silicon Dioxide Sponges
2.3.3. Alginate-Based Composite Sponges
2.4. Investigation of Morphology
2.5. Weight Variation
2.6. Emulsion Droplet Size Analysis
2.7. Drug Content and Drug Entrapment Efficiency
2.8. Water Sorption Capacity of Alginate-Based Sponges
2.9. Buoyancy
2.10. In Vitro Drug Release from Alginate-Based Sponges
2.11. Stability Studies
3. Results and Discussion
3.1. Alginate-Based Sponges Containing Curcumin-Loaded Self-Microemulsifying Drug Delivery Systems
3.1.1. Alginate Sponges
3.1.2. Alginate-Colloidal Silicon Dioxide Sponges
3.1.3. Alginate-Based Composite Sponges
3.2. Morphology and Pore Size of Composite Sponges
3.3. Measurement of the Self-Microemulsifying Drug Delivery Systems Droplet Size in Composite Sponges
3.4. Sorption Behavior of Composite Sponges in Simulated Gastric Fluid
3.5. Buoyancy of Alginate-Based Sponges
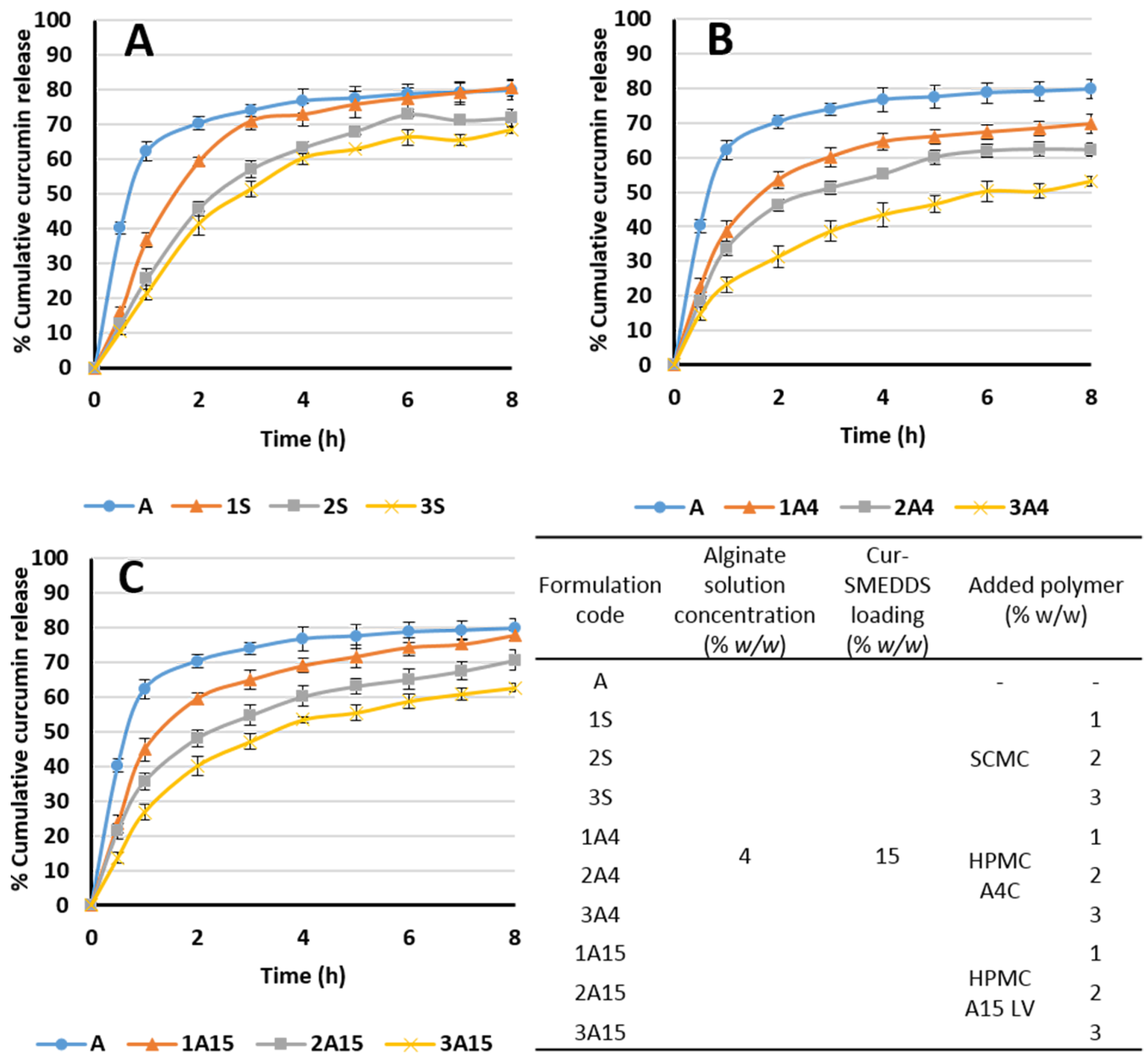
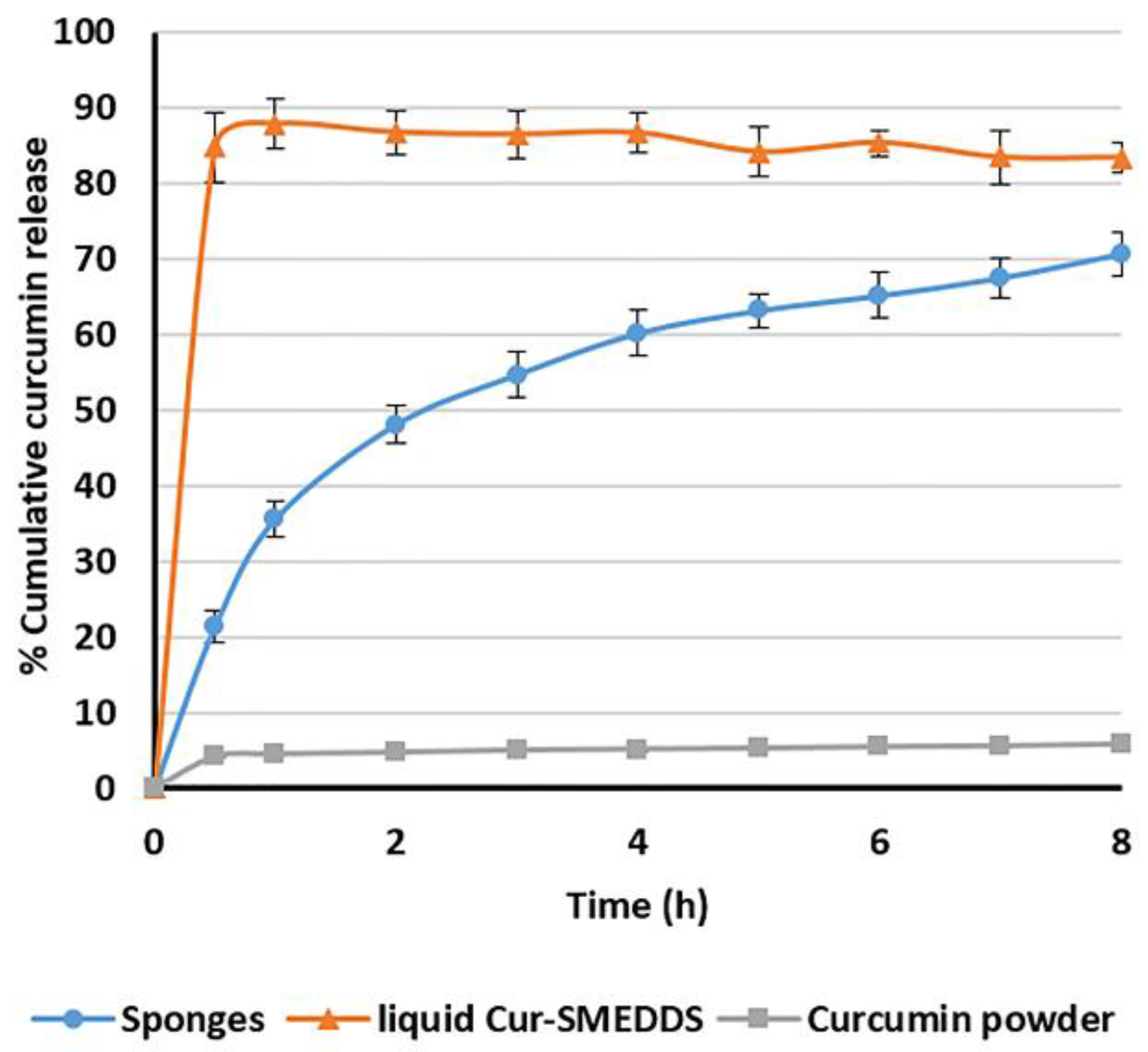
3.6. In Vitro Release of Curcumin from Alginate-Based Sponges
3.7. The Optimized Composite Sponges
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Somparn, P.; Phisalaphong, C.; Nakornchai, S.; Unchern, S.; Morales, N. Comparative antioxidant activities of curcumin and its demethoxy and hydrogenated derivatives. Biol. Pharm. Bull. 2007, 30, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Chainani-Wu, N. Safety and anti-inflammatory activity of curcumin: A component of tumeric (Curcuma longa). J. Altern. Complement. Med. 2003, 9, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jiang, Y.; Wang, Y.; Huang, M.; Ho, C.; Huang, Q. Enhancing anti-inflammation activity of curcumin through o/w nanoemulsions. Food Chem. 2008, 108, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Mun, S.H.; Joung, D.K.; Kim, Y.S.; Kang, O.H.; Kim, S.B.; Seo, Y.S.; Kim, Y.C.; Lee, D.S.; Shin, D.W.; Kweon, K.T.; et al. Synergistic antibacterial effect of curcumin against methicillin-resistant Staphylococcus aureus. Phytomedicine 2013, 20, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as “Curecumin”: From kitchen to clinic. Biochem. Pharmacol. 2008, 75, 787–809. [Google Scholar] [CrossRef] [PubMed]
- Yoysungnoen, P.; Wirachwong, P.; Changtam, C.; Suksamrarn, A.; Patumraj, S. Anti-cancer and anti-angiogenic effects of curcumin and tetrahydrocurcumin on implanted hepatocellular carcinoma in nude mice. World J. Gastroenterol. 2008, 14, 2003–2009. [Google Scholar] [CrossRef] [PubMed]
- Kaminaga, Y.; Nagatsu, A.; Akiyama, T.; Sugimoto, N.; Yamazaki, T.; Maitani, T.; Mizukami, H. Production of unnatural glucosides of curcumin with drastically enhanced water solubility by cell suspension cultures of Catharanthus roseus. FEBS Lett. 2003, 555, 311–316. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.; Newman, R.; Aggarwal, B. Bioavailability of Curcumin: Problems and Promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Kakran, M.; Sahoo, N.G.; Tan, I.L.; Li, L. Preparation of nanoparticles of poorly water-soluble antioxidant curcumin by antisolvent precipitation methods. Nanopart Res. 2012, 14, 757. [Google Scholar] [CrossRef]
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Recent Developments in Delivery, Bioavailability, Absorption and Metabolism of Curcumin: The Golden Pigment from Golden Spice. Cancer Res. Treat. 2014, 46, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Setthacheewakul, S.; Mahattanadul, S.; Phadoongsombut, N.; Pichayakorn, W.; Wiwattanapatapee, R. Development and evaluation of self-microemulsifying liquid and pellet formulations of curcumin, and absorption studies in rats. Eur. J. Pharm. Biopharm. 2010, 76, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Sermkaew, N.; Wiwattanawongsa, K.; Ketjinda, W.; Wiwattanapatapee, R. Development, Characterization and Permeability Assessment Based on Caco-2 Monolayers of Self-Microemulsifying Floating Tablets of Tetrahydrocurcumin. AAPS PharmSciTech 2013, 14, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Sriraksa, S.; Sermkaew, N.; Setthacheewakul, S.; Wiwattanapatapee, R. Floating Alginate Beads as Carriers for Self-Emulsifying System Containing Tetrahydrocurcumin. Adv. Mater. Res. 2012, 506, 517–520. [Google Scholar] [CrossRef]
- Petchsomrit, A.; Sermkaew, N.; Wiwattanapatapee, R. Hydroxypropylmethyl cellulose-based sponges loaded self-microemulsifying curcumin: Preparation, characterization, and in vivo oral absorption studies. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Nazzal, S.; Smalyukh, I.I.; Lavrentovich, O.D.; Khan, M.A. Preparation and in vitro characterization of a eutectic based semisolid self-nanoemulsified drug delivery system (SNEDDS) of ubiquinone: Mechanism and progress of emulsion formation. Int. J. Pharm. 2002, 235, 247–265. [Google Scholar] [CrossRef]
- Oh, D.H.; Kang, J.H.; Kim, D.W.; Lee, B.J.; Kim, J.O.; Yong, C.S.; Choi, H.G. Comparison of solid self-microemulsifying drug delivery system (solid SMEDDS) prepared with hydrophilic and hydrophobic solid carrier. Int. J. Pharm. 2011, 420, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Shao, A.; Chen, G.; Jiang, N.; Li, Y.; Zhang, X.; Wen, L.; Yang, F.; Wei, S. Development and evaluation of self-microemulsifying liquid and granule formulations of Brucea javanica oil. Arch. Pharm. Res. 2013, 36, 993–1003. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Kang, J.H.; Oh, D.H.; Yong, C.S.; Choi, H.G. Development of novel flurbiprofen-loaded solid self-microemulsifying drug delivery system using gelatin as solid carrier. J. Microencapsul. 2012, 29, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, S.A. Therapeutic potential of curcumin in gastrointestinal diseases. World J. Gastrointest. Pathophysiol. 2011, 2, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N.; Kim, K.H. Floating Drug Delivery System—An Approach To Oral Controlled Drug Delivery. J. Control Release 2000, 63, 235–259. [Google Scholar] [CrossRef]
- Arora, S.; Ali, J.; Ahuja, A.; Khar, R.K.; Baboota, S. Floating Drug Delivery Systems: A Review. AAPS PharmSciTech 2005, 6, E372–E390. [Google Scholar] [CrossRef] [PubMed]
- Shishu; Gupta, N.; Aggarwal, N. Bioavailability Enhancement and Targeting of Stomach Tumors Using Gastro-Retentive Floating Drug Delivery System of Curcumin—“A Technical Note”. AAPS PharmSciTech 2008, 9, 810–813. [Google Scholar] [CrossRef]
- Torelli-Souza, R.; Cavalcante Bastos, L.; Nunes, H.; Camara, C.; Amorim, R. Sustained release of an antitumoral drug from alginate-chitosan hydrogel beads and its potential use as colonic drug delivery. J. Appl. Polym. Sci. 2012, 126, E409–E418. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, H.; Kawazoe, N.; Chen, G. Pore size effect of collagen scaffolds on cartilage regeneration. Acta Biomater. 2014, 10, 2005–2013. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Ikeda, K.; Yamamoto, K.; Ishizaki, H.; Yoshizaw, Y.; Yanagiguchi, K.; Yamada, S.; Hayashi, Y. Fabrication and Characteristics of Chitosan Sponge as a Tissue Engineering Scaffold. Biomed. Res. Int. 2014, 2014, 786892. [Google Scholar] [CrossRef] [PubMed]
- Patel, Y.L.; Sher, P.; Pawar, A.P. The Effect of Drug Concentration and Curing Time on Processing and Properties of Calcium Alginate Beads Containing Metronidazole by Response Surface Methodology. AAPS PharmSciTech 2006, 7, E24–E30. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Xu, L.; Wang, Q.; Zhang, X.; Zhang, W.; Li, Y.; Jin, L.; Li, S. Development and evaluation of new sustained-release floating microspheres. Int. J. Pharm. 2008, 358, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Remya, K.S.; Beena, P.; Bijesh, P.V.; Sheeba, A. Formulation Development, Evaluation and Comparative Study of Effects of Super Disintegrants in Cefixime Oral Disintegrating Tablets. J. Young Pharm. 2010, 2, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Singhal, P.; Tomar, A.; Goel, K.; Pandey, M.; Saraf, S.A. Preparation and evaluation of stomach-specific ion tropically emulsion gelled alginate beads of tinidazole. Der Pharm. Lett. 2010, 2, 272–282. [Google Scholar]
- Ratanajiajaroen, P.; Ohshima, M. Synthesis, release ability and bioactivity evaluation of chitin beads incorporated with curcumin for drug delivery applications. J. Microencapsul. 2012, 29, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Strübing, S.; Metz, H.; Mäder, K. Characterization of poly(vinyl acetate) based floating matrix tablets. J. Control Release 2008, 126, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Bhimavarapu, R.; Nissankararao, S.; Nagavani, S.; Ramadevi, S.; Durga, P.L. Design, development and in vitro evaluation of gastro retentive alginate floating beads for ranitidine hydrochloride. J. Chem. Pharm. Res. 2013, 5, 377–381. [Google Scholar]
- Huynh-Ba, K. (Ed.) Understanding ICH Guidelines Applicable to Stability Testing. In Handbook of Stability Testing in Pharmaceutical Development: Regulations, Methodologies, and Best Practices; Springer: New York, NY, USA, 2009; pp. 21–41.
- Bertram, U.; Bodmeier, R. In situ gelling, bioadhesive nasal inserts for extended drug delivery: In vitro characterization of a new nasal dosage form. Eur. J. Pharm. Sci. 2006, 27, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Liew, C.V.; Chan, L.W.; Ching, A.L.; Heng, P.W. Evaluation of sodium alginate as drug release modifier in matrix tablets. Int. J. Pharm. 2006, 309, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Trinkle, D.; Derbin, G.; Martin, K.; Desai, D.; Sharif, S.; Timmins, P. Moisture adsorption and desorption properties of colloidal silicon dioxide and its impact on layer adhesion of a bilayer tablet formulation. J. Excip. Food Chem. 2014, 5, 21–31. [Google Scholar]
- Sadeghi, F.; Mosaffa, F.; Garekani, H.A. Effect of Particle Size, Compaction Force and Presence of Aerosil 200 on the Properties of Matrices Prepared from Physical Mixture of Propranolol Hydrochloride and Eudragit RS or RL. Iran. J. Basic Med. Sci. 2007, 10, 197–205. [Google Scholar]
- Mahattanadul, S.; Reanmongkol, W.; Yano, S.; Panichayupakaranant, P.; Phdoongsombut, N. Preventive and curative effects of curcumin on the development of gastric inflammatory diseases in rats. J. Nat. Med. 2006, 60, 191–197. [Google Scholar] [CrossRef]
- Mujtaba, A.; Kohli, K. In vitro/in vivo evaluation of HPMC/alginate based extended-release matrix tablets of cefpodoxime proxetil. Int. J. Biol. Macromolec. 2016, 89, 434–441. [Google Scholar] [CrossRef] [PubMed]

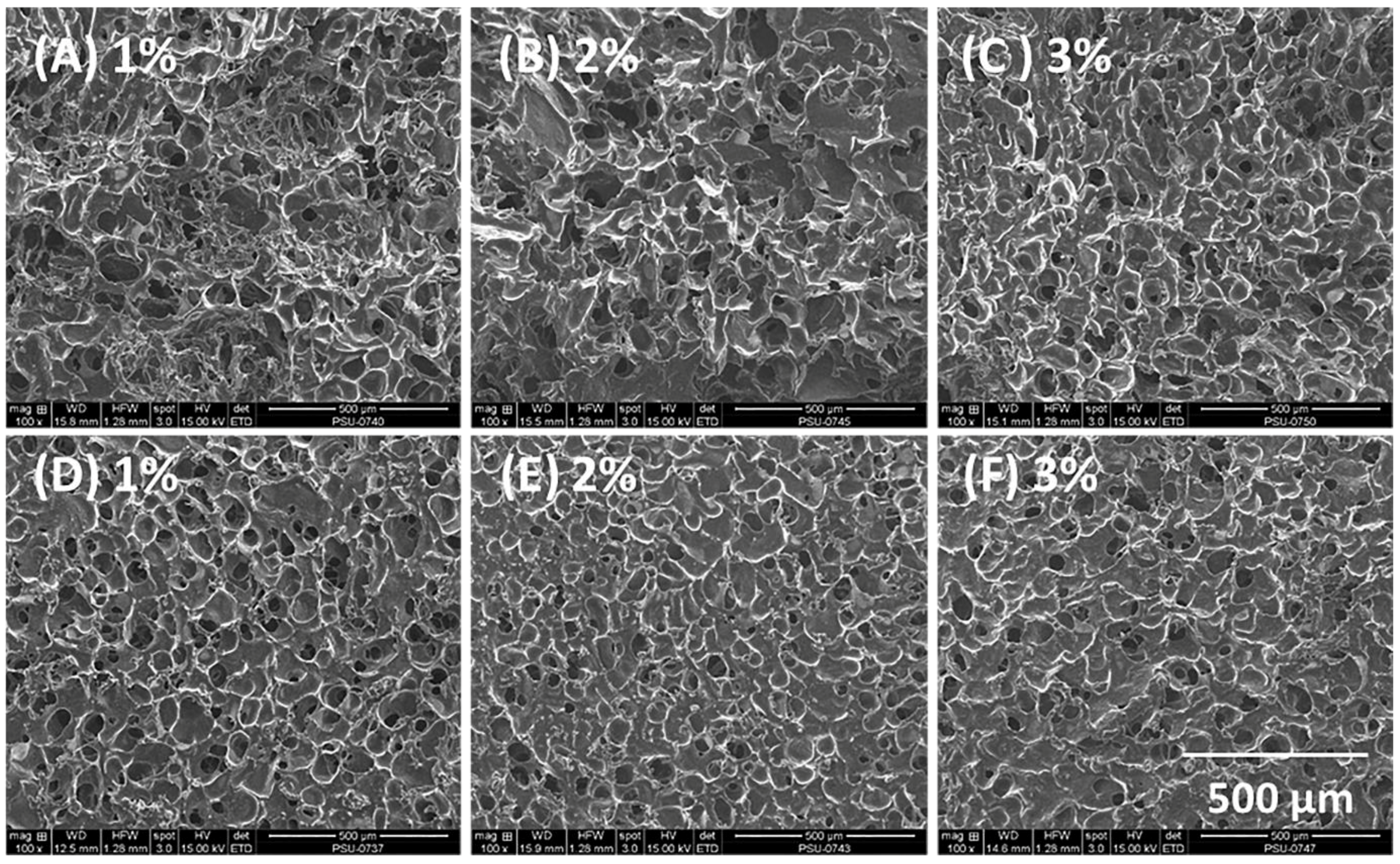
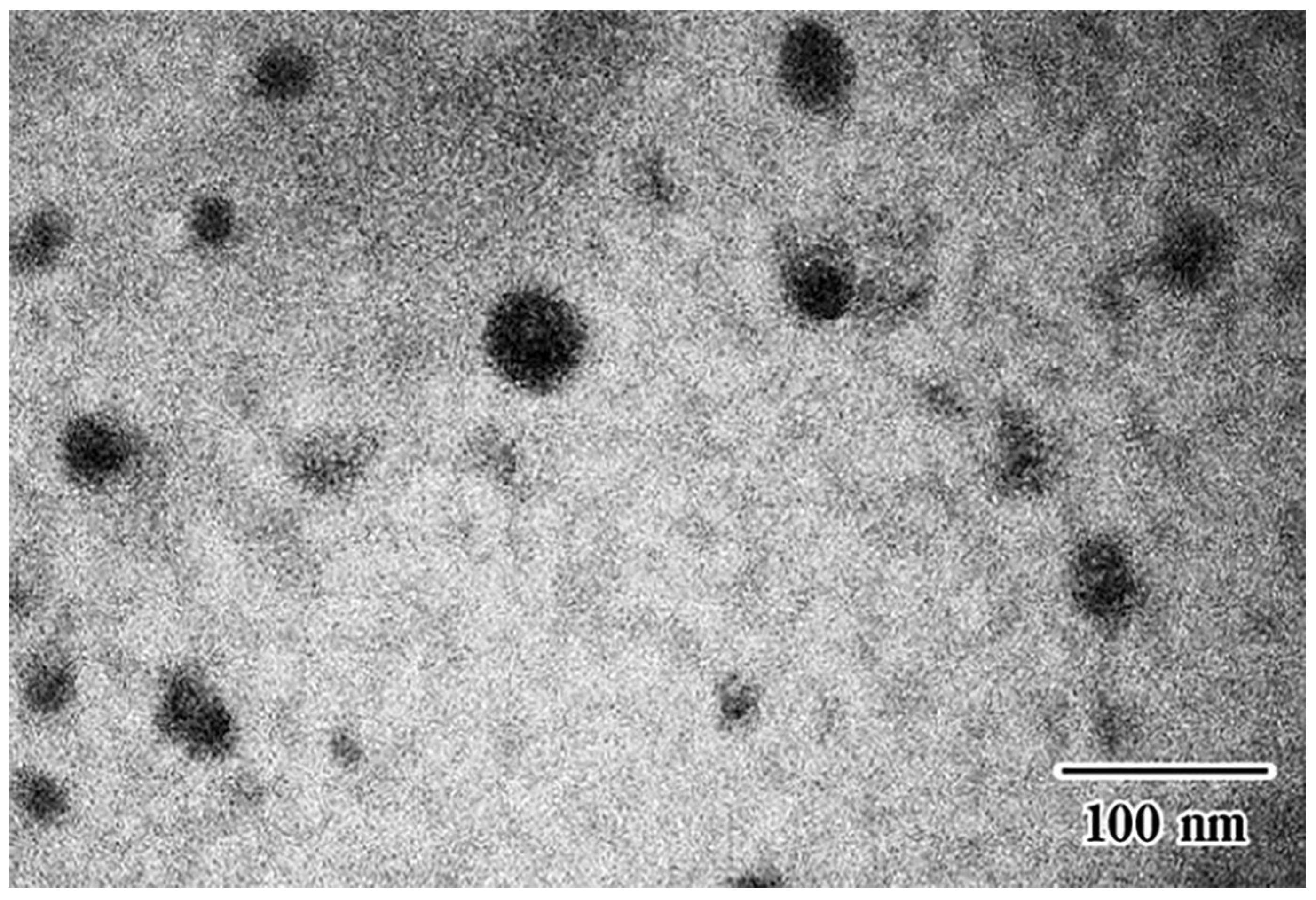


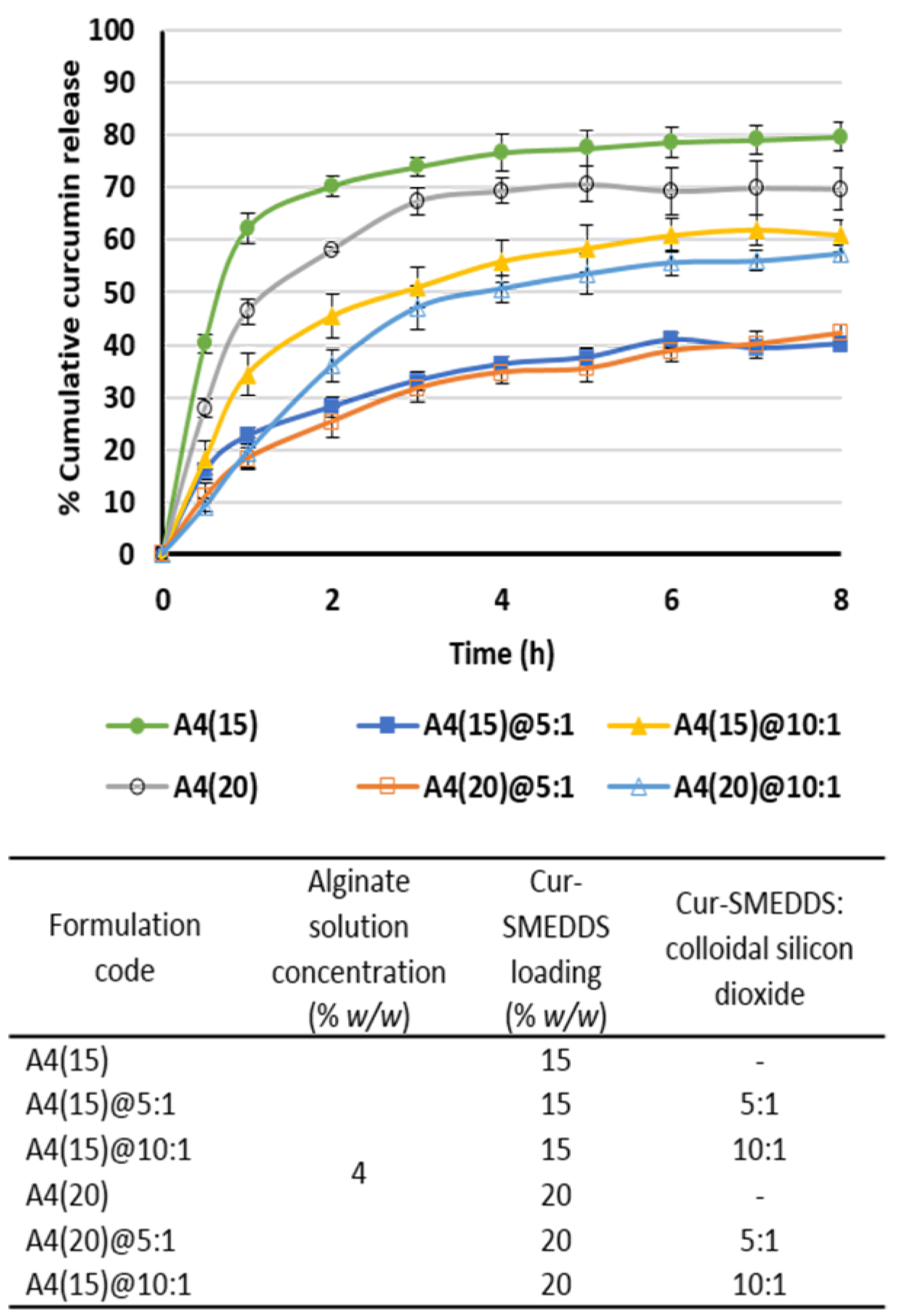
| Alginate Solution Concentration (% w/w) | Cur-SMEDDS Loading (% w/w) | Oil Leakage | Weight (mg ± SD) | Curcumin Content (% w/w) | Encapsulation Efficiency (% ± SD) |
|---|---|---|---|---|---|
| 3 | 5 | no | 19.1 ± 0.5 | 2.20 ± 0.02 | 83 ± 0.9 |
| 10 | no | 30.6 ± 0.7 | 2.77 ± 0.03 | 85 ± 0.9 | |
| 15 | yes | 44.4 ± 1.1 | 3.21 ± 0.01 | 90 ± 0.3 | |
| 20 | yes | 56.1 ± 1.0 | 3.37 ± 0.01 | 91 ± 0.4 | |
| 4 | 5 | no | 21.0 ± 0.3 | 1.88 ± 0.01 | 80 ± 0.5 |
| 10 | no | 32.9 ± 0.3 | 2.66 ± 0.03 | 88 ± 1.0 | |
| 15 | no | 45.2 ± 0.8 | 3.09 ± 0.04 | 92 ± 1.2 | |
| 20 | yes | 58.5 ± 1.9 | 3.24 ± 0.03 | 91 ± 1.0 | |
| 5 | 5 | no | 23.7 ± 0.6 | 1.68 ± 0.04 | 79 ± 1.8 |
| 10 | no | 35.9 ± 0.6 | 2.46 ± 0.05 | 87 ± 1.9 | |
| 15 | no | 46.3 ± 3.5 | 2.85 ± 0.14 | 89 ± 4.4 | |
| 20 | no | 57.6 ± 5.5 | 3.07 ± 0.07 | 90 ± 1.9 |
| Alginate Solution Concentration (% w/w) | Cur-SMEDDS Loading (% w/w) | Cur-SMEDDS:Colloidal Silicon Dioxide | Oil Leakage | Weight (mg ± SD) | Curcumin Content (% w/w) | Encapsulation Efficiency (% ± SD) |
|---|---|---|---|---|---|---|
| 4 | 15 | 5:1 | no | 54.6 ± 0.9 | 2.49 ± 0.01 | 86 ± 0.3 |
| 20 | no | 69.4 ± 1.0 | 2.66 ± 0.01 | 87 ± 0.3 | ||
| 4 | 15 | 10:1 | no | 50.4 ± 0.8 | 2.68 ± 0.02 | 86 ± 0.8 |
| 20 | no | 63.5 ± 0.7 | 2.90 ± 0.03 | 88 ± 0.8 | ||
| 25 | no | 79.6 ± 0.7 | 2.96 ± 0.02 | 89 ± 0.6 |
| Alginate Solution Concentration (% w/w) | Cur-SMEDDS Loading (% w/w) | Added Polymer (% w/w) | Sponge Weight (mg ± SD) | Curcumin Content (% w/w) | Encapsulation Efficiency (% ± SD) | |
|---|---|---|---|---|---|---|
| 4 | 15 | SCMC | 1 | 48.4 ± 1.5 | 2.92 ± 0.03 | 88 ± 1.1 |
| 2 | 51.3 ± 1.5 | 2.88 ± 0.02 | 89 ± 0.6 | |||
| 3 | 53.3 ± 1.9 | 2.82 ± 0.03 | 97 ± 1.1 | |||
| HPMC A4C | 1 | 47.1 ± 0.9 | 2.95 ± 0.05 | 85 ± 1.5 | ||
| 2 | 51.7 ± 2.5 | 2.71 ± 0.02 | 87 ± 0.7 | |||
| 3 | 54.0 ± 1.0 | 2.56 ± 0.01 | 88 ± 0.3 | |||
| HPMC A15 LV | 1 | 47.6 ± 2.7 | 2.89 ± 0.05 | 91 ± 1.6 | ||
| 2 | 49.5 ± 2.7 | 2.83 ± 0.04 | 93 ± 1.2 | |||
| 3 | 52.5 ± 2.9 | 2.78 ± 0.02 | 96 ± 0.8 | |||
| Kinetic Model | Zero-order | First-order | Higuchi |
| Qt = K0t | logQt = logQ0 − K1t/2.303 | Q = KH t1/2 | |
| R2 | 0.7865 | 0.9009 | 0.9546 |
| Time (Months) | Oil Leakage | Emulsion Droplet Size (nm) | PDI | Curcumin Content (% w/w) |
|---|---|---|---|---|
| (A) 30 °C/65% RH | ||||
| 0 | no | 29.9 ± 0.2 | 0.089 ± 0.010 | 100.5 ± 1.4 |
| 3 | no | 31.5 ± 2.0 | 0.125 ± 0.035 | 99.7 ± 2.3 |
| 6 | no | 31.2 ± 1.4 | 0.118 ± 0.050 | 97.4 ± 2.5 |
| (B) 45 °C/75% RH | ||||
| 0 | no | 29.92 ± 0.24 | 0.089 ± 0.010 | 100.5 ± 1.4 |
| 3 | no | 31.39 ± 1.44 | 0.139 ± 0.045 | 99.7 ± 2.0 |
| 6 | no | 32.60 ± 0.77 | 0.136 ± 0.070 | 98.1 ± 1.8 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petchsomrit, A.; Sermkaew, N.; Wiwattanapatapee, R. Alginate-Based Composite Sponges as Gastroretentive Carriers for Curcumin-Loaded Self-Microemulsifying Drug Delivery Systems. Sci. Pharm. 2017, 85, 11. https://doi.org/10.3390/scipharm85010011
Petchsomrit A, Sermkaew N, Wiwattanapatapee R. Alginate-Based Composite Sponges as Gastroretentive Carriers for Curcumin-Loaded Self-Microemulsifying Drug Delivery Systems. Scientia Pharmaceutica. 2017; 85(1):11. https://doi.org/10.3390/scipharm85010011
Chicago/Turabian StylePetchsomrit, Arpa, Namfa Sermkaew, and Ruedeekorn Wiwattanapatapee. 2017. "Alginate-Based Composite Sponges as Gastroretentive Carriers for Curcumin-Loaded Self-Microemulsifying Drug Delivery Systems" Scientia Pharmaceutica 85, no. 1: 11. https://doi.org/10.3390/scipharm85010011





