Synthesis, Structure, and Analgesic Properties of Halogen-Substituted 4-Hydroxy-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxanilides
Abstract
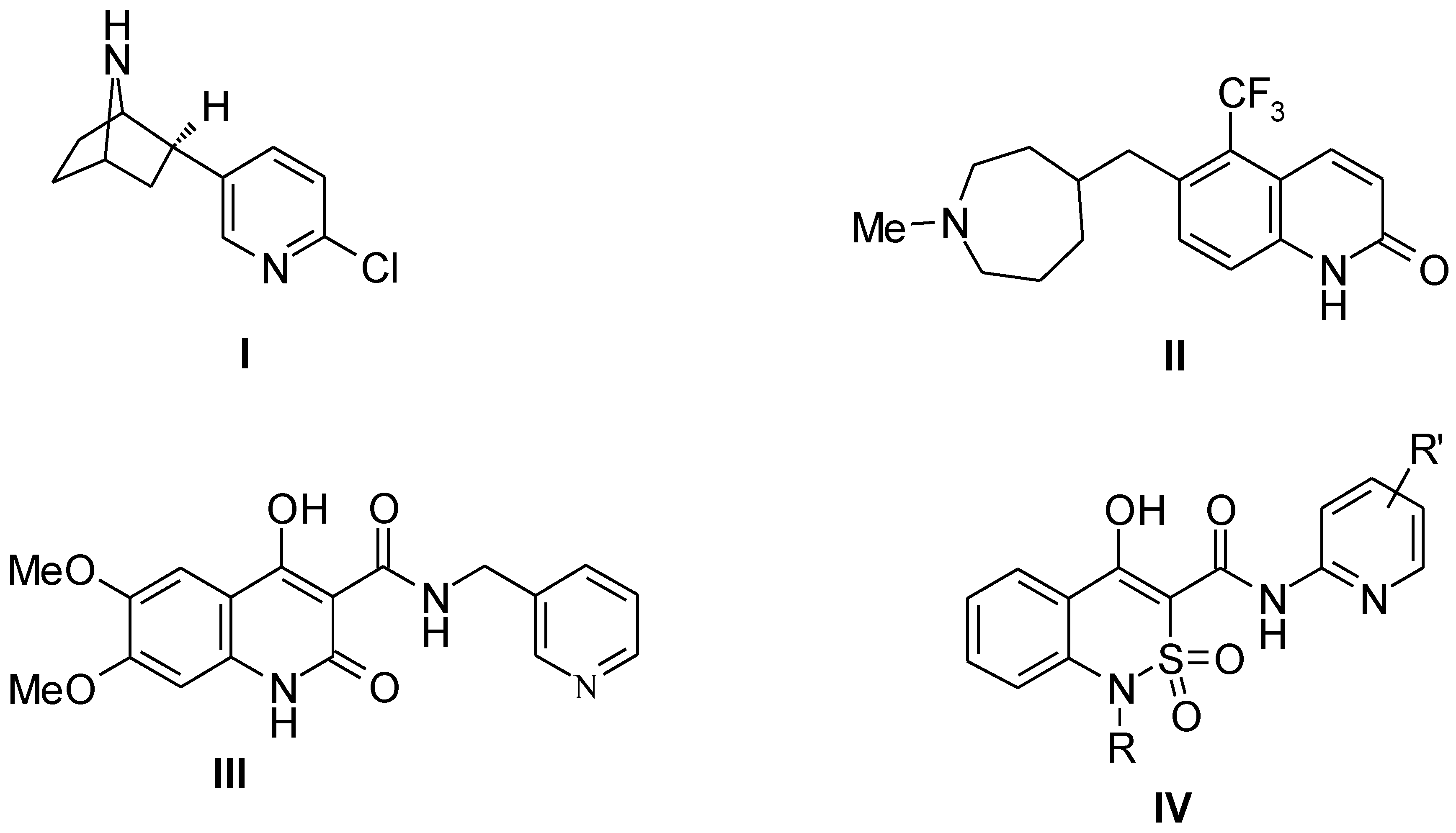
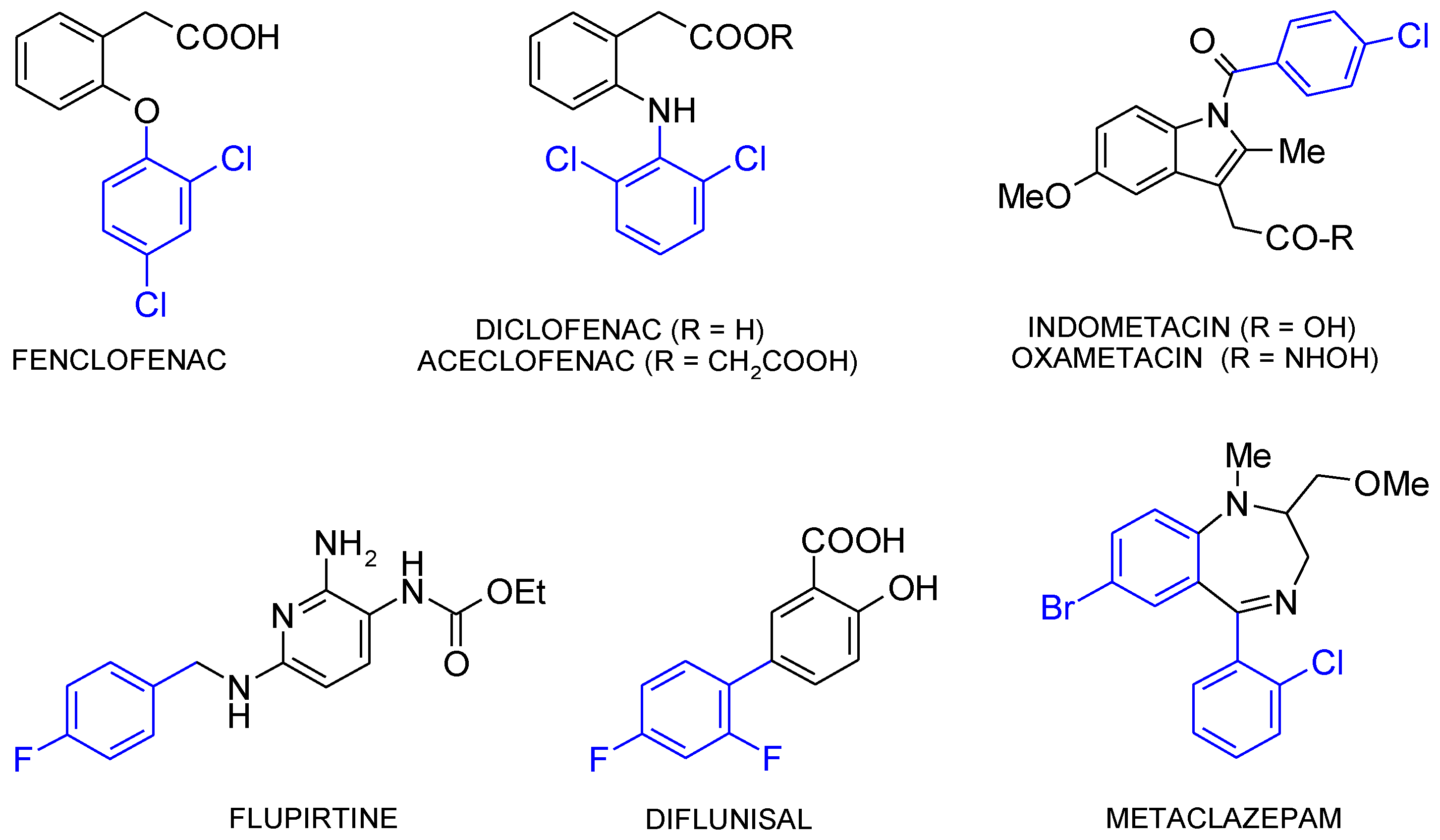
:1. Introduction
2. Materials and Methods
2.1. Chemistry
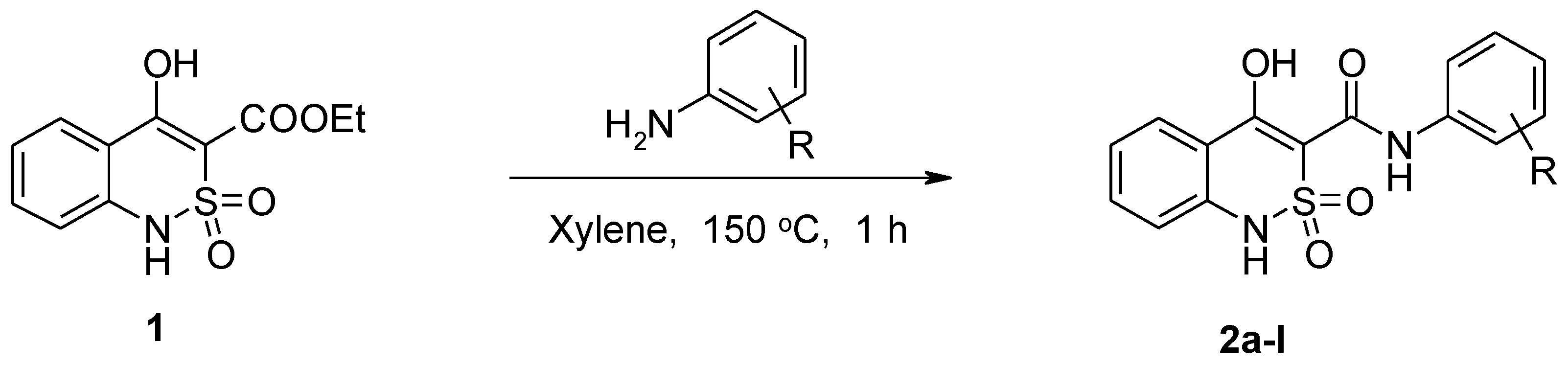
2.2. General Procedure for the Synthesis of N-aryl-4-hydroxy-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamides (2a–l)
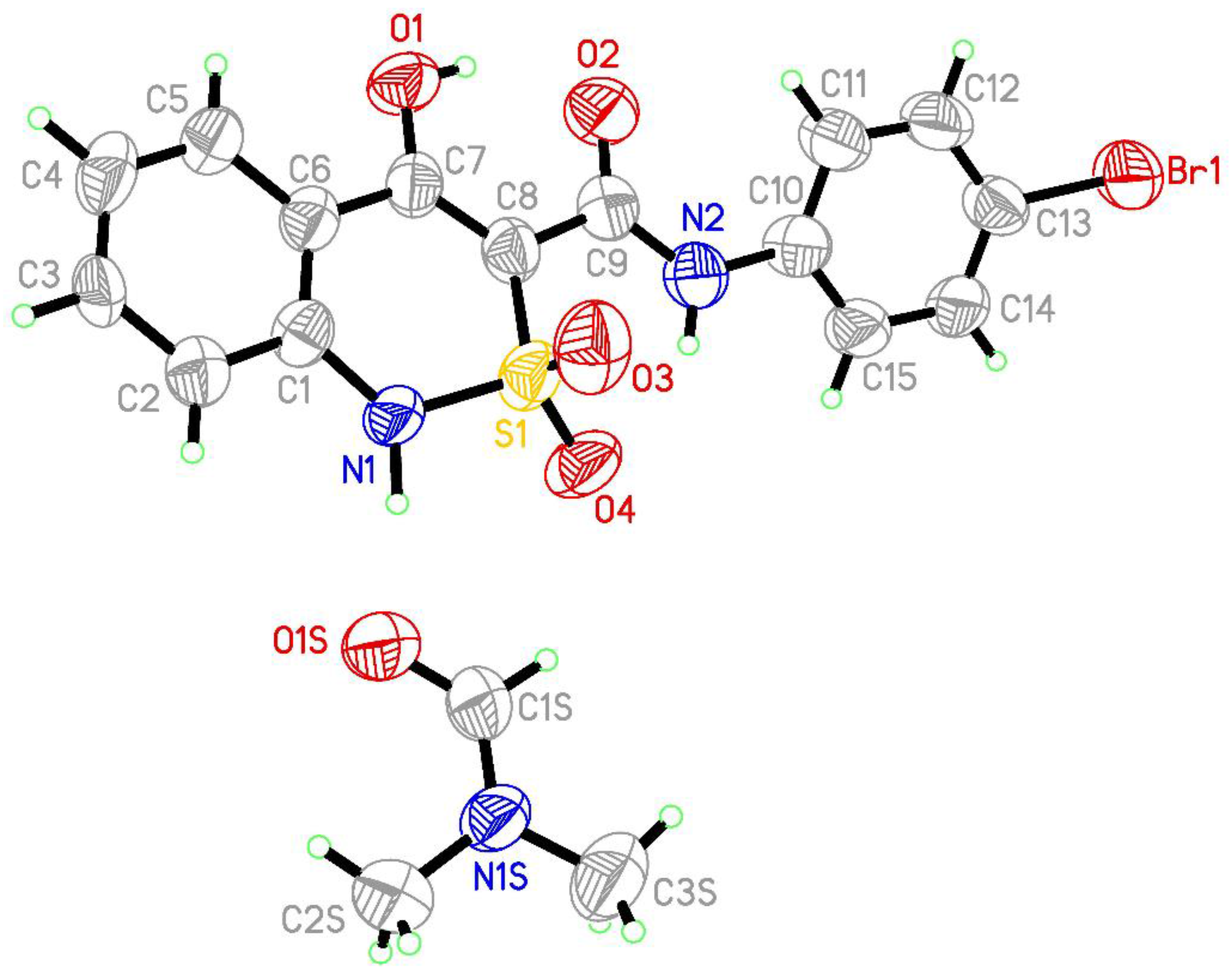
2.3. X-ray Structural Analysis
2.4. Pharmacology
3. Results and Discussion
3.1. Chemistry
3.2. Evaluation of the Analgesic Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bach, T.B.; Jensen, A.A.; Petersen, J.G.; Sørensen, T.E.; Della Volpe, S.; Liu, J.; Blaazer, A.R.; van Muijlwijk-Koezen, J.E.; Balle, T.; Frølund, B. Exploration of the molecular architecture of the orthosteric binding site in the α4β2nicotinicacetylcholinereceptor with analogs of 3-(dimethylamino)butyl dimethylcarbamate (DMABC) and 1-(pyridin-3-yl)-1,4-diazepane. Eur. J. Med. Chem. 2015, 102, 425–444. [Google Scholar] [CrossRef] [PubMed]
- Carroll, F.I.; Navarro, H.A.; Mascarella, S.W.; Castro, A.H.; Luetje, C.W.; Wageman, C.R.; Marks, M.J.; Jackson, A.; Damaj, M.I. In vitro and in vivo neuronalnicotinicreceptor properties of (+)- and (−)-pyrido[3,4]homotropane [(+)- and (−)-PHT]: (+)-PHT is a potent and selective full agonist at α6β2 containingneuronalnicotinic acetylcholine receptors. ACS Chem. Neurosci. 2015, 6, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Papke, R.L.; Bagdas, D.; Kulkarni, A.R.; Gould, T.; AlSharari, S.D.; Thakur, G.A.; Damaj, M.I. The analgesic-like properties of the alpha7nAChR silent agonist NS6740 is associated with non-conducting conformations of the receptor. Neuropharmacology 2015, 91, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Nencini, A.; Castaldo, C.; Comery, T.A.; Dunlop, J.; Genesio, E.; Ghiron, C.; Haydar, S.; Maccari, L.; Micco, I.; Turlizzi, E.; et al. Design and synthesis of a hybrid series of potent and selective agonists of α7 nicotinicacetylcholine receptor. Eur. J. Med. Chem. 2014, 78, 401–418. [Google Scholar] [CrossRef] [PubMed]
- Piao, M.H.; Liu, Y.; Wang, Y.S.; Qiu, J.P.; Feng, C.S. Volatile anesthetic isoflurane inhibits LTP induction of hippocampal CA1 neurons through α4β2nAChR subtype-mediated mechanisms. Ann. Fr. Anesth. Reanim. 2013, 32, e135–e141. [Google Scholar] [CrossRef] [PubMed]
- Loram, L.C.; Taylor, F.R.; Strand, K.A.; Maier, S.F.; Speake, J.D.; Jordan, K.G.; James, J.W.; Wene, S.P.; Pritchard, R.C.; Green, H.; et al. Systemic administration of an alpha-7 nicotinic acetylcholine agonist reverses neuropathic pain in male Sprague Dawley rats. J. Pain 2012, 13, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, S.; Maskos, U. Role of the nicotinic acetylcholinereceptor in Alzheimer’s disease pathology and treatment. Neuropharmacology 2015, 96, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Russo, P.; Del Bufalo, A.; Frustaci, A.; Fini, M.; Cesario, A. Beyond acetylcholinesterase inhibitors for treating Alzheimer’s disease: α7-nAChR agonistsin human clinical trials. Curr. Pharm. Des. 2014, 20, 6014–6021. [Google Scholar] [CrossRef] [PubMed]
- Quik, M.; Perez, X.A.; Bordia, T. Nicotine as a potential neuroprotective agent for Parkinson’s disease. Mov. Disord. 2012, 27, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Fehér, A.; Juhász, A.; Rimanóczy, A.; Csibri, E.; Kálmán, J.; Janka, Z. Association between a genetic variant of the alpha-7 nicotinic acetylcholinereceptor subunit and four types of dementia. Dement. Geriatr. Cogn. Disord. 2009, 28, 56–62. [Google Scholar] [PubMed]
- Spande, T.F.; Garraffo, H.M.; Edwards, M.W.; Yeh, H.J.C.; Pannell, L.; Daly, J.W. Epibatidine: A novel (chloropyridyl)azabicycloheptane with potent analgesic activity from an Ecuadoran poison frog. J. Am. Chem. Soc. 1992, 114, 3475–3478. [Google Scholar] [CrossRef]
- Tolstikov, G.A.; Dembitskii, V.M.; Tolstikova, T.G.; Shults, E.E. Epibatidine and the problem of opioid analgesics. In Selected Methods for Synthesis and Modification of Heterocycles; Kartsev, V.G., Ed.; IBS Press: Moscow, Russia, 2003; Volume 1, pp. 418–449. [Google Scholar]
- Young, T.G.; Broad, L.M.; Zwart, R.; Astles, P.C.; Bodkin, M.; Sher, E.; Millar, N.S. Species selectivity of a nicotinic acetylcholine receptor agonist is conferred by two adjacent extracellular β4 amino acids that are implicated in the coupling of binding to channel gating. Mol. Pharmacol. 2007, 71, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Ukrainets, I.V.; Gorokhova, O.V.; Jaradat, N.A.; Petrushova, L.A.; Mospanova, E.V.; Savchenkova, L.V.; Kuz’min, V.E.; Lyahovsky, A.V. 4-Hydroxyquinolin-2-ones and their close structural analogues as a new source of highly effective pain-killers. In Pain and Treatment; Racz, G.B., Noe, C.E., Eds.; InTech: Rijeka, Croatia, 2014; pp. 21–73. [Google Scholar]
- Ukrainets, I.V.; Petrushova, L.A.; Dzyubenko, S.P.; Liu, Y. 2,1-Benzothiazine 2,2-dioxides. 4. Synthesis, structure, and analgesic properties of 4-hydroxy-1-methyl-2,2-dioxo-N-(pyridin-2-yl)-1H-2λ6,1-benzothiazine-3-carboxamides. Chem. Heterocycl. Compd. 2014, 50, 564–572. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Petrushova, L.A.; Gorokhova, O.V.; Davidenko, A.A. The study of structure—Analgesic activity relationships in a series of 4-hydroxy-N-(pyridin-2-yl)-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamides. Zh. Org. Farm. Khim. (J. Org. Pharm. Chem.) 2015, 13, 39–44. [Google Scholar]
- Zefirova, O.N.; Zefirov, N.S. On the history emergence and development of the concept bioisosterism. Reports of Moscow State University.Ser. 2.Chem. 2002, 43, 251–256. [Google Scholar]
- Kleemann, A.; Engel, J.; Kutscher, B.; Reichert, D. Pharmaceutical Substances: Syntheses, Patents, Applications of the Most Relevant APIs, 5th ed.; Thieme: Stuttgart, Germany, 2008; p. 1800. [Google Scholar]
- Ukrainets, I.V.; Petrushova, L.A.; Dzyubenko, S.P.; Sim, G. 2,1-Benzothiazine 2,2-dioxides. 3. 4-Hydroxy-1-methyl-2,2-dioxo-N-(1,3-thiazol-2-yl)-1Н-2λ6,1-benzothiazine-3-carboxamides—A new group of potential analgetics. Chem. Heterocycl. Compd. 2014, 50, 103–110. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Petrushova, L.A.; Dzyubenko, S.P.; Grinevich, L.A. Synthesis and analgesic activity of N-(benzothiazol-2-yl)-4-hydroxy-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamides. Zh. Org. Farm. Khim. (J. Org. Pharm. Chem.) 2014, 12, 38–43. [Google Scholar]
- Ukrainets, I.V.; Petrushova, L.A.; Dzyubenko, S.P.; Sim, G.; Grinevich, L.A. The effective synthesis of N-(arylalkyl)-1-R-4-hydroxy-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamides as promising analgesics of a new chemical class. Sci.Pharm. 2015, 83, 549–566. [Google Scholar] [CrossRef] [PubMed]
- Ukrainets, I.V.; Petrushova, L.A.; Dzyubenko, S.P. Methyl-substituted anilides of 4-hydroxy-1-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylic acid. Synthesis, spectral characteristics and biological properties. Zh. Org. Farm. Khim. (J. Org. Pharm. Chem.) 2014, 12, 53–58. [Google Scholar]
- Petrushova, L.A.; Ukrainets, I.V.; Dzyubenko, S.P.; Grinevich, L.A. Synthesis and the biological activity of 4-hydroxy-2,2-dioxo-1H-2λ6,1-benzothiazin-3-carboxylic acids trifluoromethyl-substituted anilides. Zh. Org. Farm. Khim. (J. Org. Pharm. Chem.) 2015, 13, 44–48. [Google Scholar]
- Ukrainets, I.V.; Petrushova, L.A.; Gorokhova, O.V.; Sidorenko, L.V. The synthesis and biological properties of hydroxy-(alkoxy) substituted anilides of 4-hydroxy-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylicacids. Zh. Org. Farm. Khim. (J. Org. Pharm. Chem.) 2016, 14, 67–73. [Google Scholar]
- Ukrainets, I.V.; Petrushova, L.A.; Sidorenko, L.V.; Davidenko, A.A.; Duchenko, M.A. The study of structure—Analgesic activity relationships in a series of 4-hydroxy-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylic acid toluidides and xylidides. Sci.Pharm. 2016, 84. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Cambridge Crystallographic Data Center. Available online: www.ccdc.ac.uk/data_request/cif CCDC 1474415 (accessed on 15 April 2016).
- Vogel, H.G. (Ed.) Drug Discovery and Evaluation: Pharmacological Assays, 2nd ed.; Springer: Berlin, Germany, 2008; pp. 1014–1016.
- Ukrainets, I.V.; Petrushova, L.A.; Dzyubenko, S.P. 2,1-Benzothiazine 2,2-dioxides. 1. Synthesis, structure, and analgesic activity of 1-R-4-hydroxy-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylic acid esters. Chem. Heterocycl. Compd. 2013, 49, 1378–1383. [Google Scholar] [CrossRef]
- Zefirov, N.S.; Palyulin, V.A.; Dashevskaya, E.E. Stereochemical studies. XXXIV. Quantitative description of ring puckering via torsional angles. The case of six-membered rings. J. Phys. Org. Chem. 1990, 3, 147–158. [Google Scholar] [CrossRef]
- Orpen, A.G.; Brammer, L.; Allen, F.H.; Kennard, O.; Watson, D.G.; Taylor, R. Typical interatomic distances in organic compounds and organometallic compounds and coordination complexes of the d- and f-block metals. In Structure Correlation; Burgi, H.-B., Dunitz, J.D., Eds.; Wiley-VCH: Weinheim, Germany, 1994; Volume 2, pp. 741–926. [Google Scholar]
- Terent’ev, P.B.; Stankiavichyus, A.P. Mass Spectrometry Bioactive Nitrogenous Bases; Mokslas: Vilnius, Lithuania, 1987; pp. 239–255. [Google Scholar]

| Entry | Product | R | Latent Period in 1 h after Introduction of the Compounds, s a | Change of the Latent Period, Compared to Control, % |
|---|---|---|---|---|
| 1 | 2a | H | 4.80 ± 0.11 | +24.8 |
| 2 | 2b | 2-F | 3.89 ± 0.10 | +1.1 |
| 3 | 2c | 3-F | 5.35 ± 0.13 | +39.0 |
| 4 | 2d | 4-F | 5.77 ± 0.14 | +49.9 |
| 5 | 2e | 3,4-(F)2 | 5.66 ± 0.12 | +47.1 |
| 6 | 2f | 2-Cl | 4.13 ± 0.13 | +7.4 |
| 7 | 2g | 3-Cl | 5.02 ± 0.11 | +30.3 |
| 8 | 2h | 4-Cl | 5.73 ± 0.14 | +48.9 |
| 9 | 2i | 2,5-(Cl)2 | 6.55 ± 0.16 | +70.1 |
| 10 | 2j | 2-Br | 4.34 ± 0.10 | +12.7 |
| 11 | 2k | 3-Br | 5.46 ± 0.14 | +41.8 |
| 12 | 2l | 4-Br | 4.17 ± 0.11 | +8.3 |
| 13 | Meloxicam | – | 5.71 ± 0.15 | +48.2 |
| 14 | Piroxicam | – | 4.77 ± 0.13 | +24.1 |
| 15 | Control | – | 3.85 ± 0.12 | – |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ukrainets, I.V.; Petrushova, L.A.; Shishkina, S.V.; Sidorenko, L.V.; Sim, G.; Kryvanych, O.V. Synthesis, Structure, and Analgesic Properties of Halogen-Substituted 4-Hydroxy-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxanilides. Sci. Pharm. 2016, 84, 523-535. https://doi.org/10.3390/scipharm84030523
Ukrainets IV, Petrushova LA, Shishkina SV, Sidorenko LV, Sim G, Kryvanych OV. Synthesis, Structure, and Analgesic Properties of Halogen-Substituted 4-Hydroxy-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxanilides. Scientia Pharmaceutica. 2016; 84(3):523-535. https://doi.org/10.3390/scipharm84030523
Chicago/Turabian StyleUkrainets, Igor V., Lidiya A. Petrushova, Svitlana V. Shishkina, Lyudmila V. Sidorenko, Galina Sim, and Olga V. Kryvanych. 2016. "Synthesis, Structure, and Analgesic Properties of Halogen-Substituted 4-Hydroxy-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxanilides" Scientia Pharmaceutica 84, no. 3: 523-535. https://doi.org/10.3390/scipharm84030523







