Trimester-Specific Reference Intervals of Thyroid Function Testing in Pregnant Women from Basrah, Iraq Using Electrochemiluminescent Immunoassay
Abstract
:1. Introduction
Objective
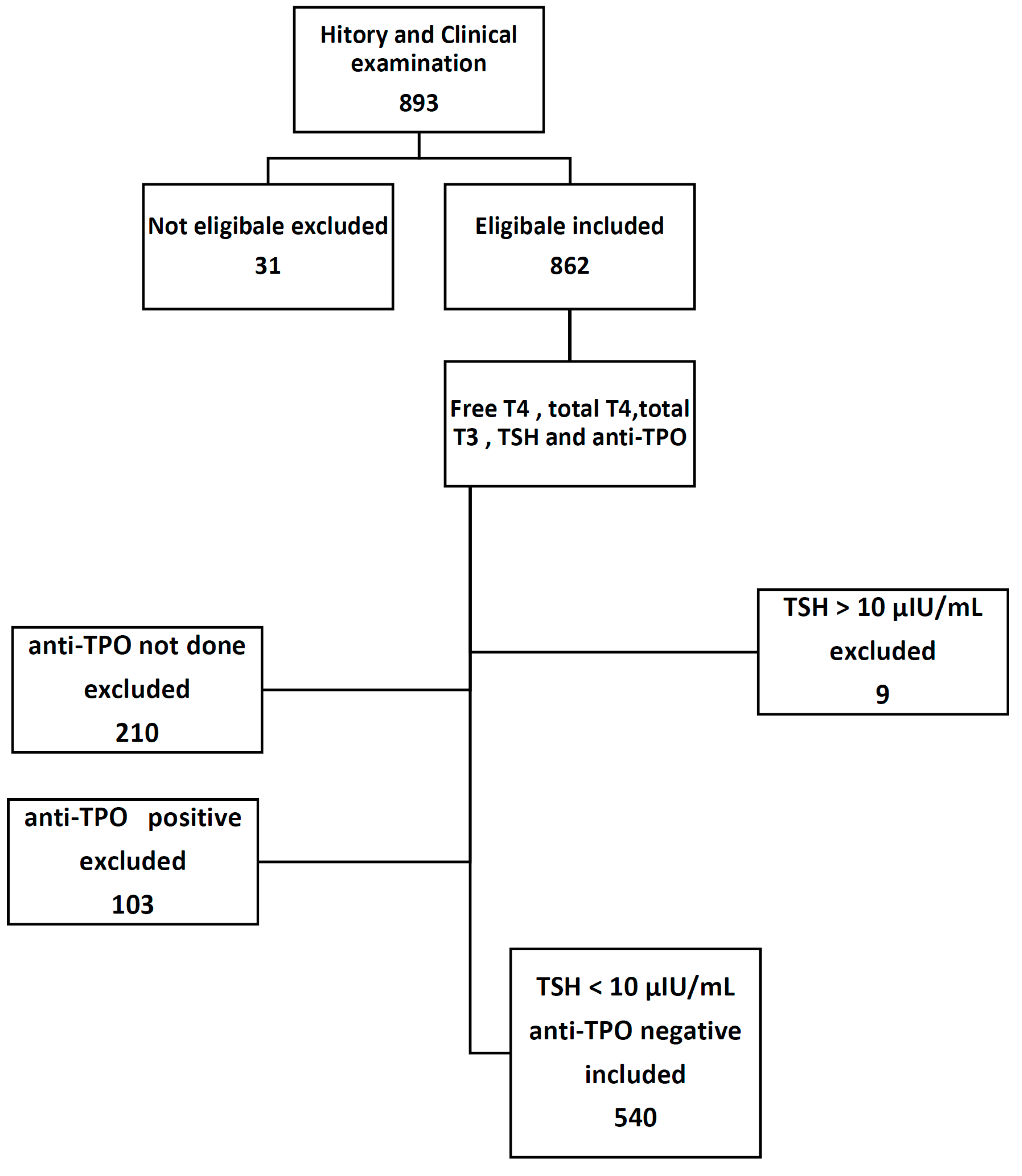
2. Material and Methods
2.1. Setting
2.2. Subjects
2.3. Exclusion Criteria
- History of hyperemesis gravidarum, thyroid illness or use of medication known to affect thyroid function like amiodarone, lithium, steroids, and non-steroidal anti-inflammatory drugs.
- Twin pregnancy.
- The family history of thyroid illness.
- The presence of more than mild goiter on clinical ground.
- Overt hypothyroidism or hyperthyroidism.
- Women with significant acute or chronic diseases were also excluded, leaving only healthy or apparently healthy pregnant women to participate in the study.
- TSH >10 (nine pregnant women excluded).
- Anti-TPO positive pregnant women.
2.4. Main Outcome Measure
2.5. Biochemical Tests
2.6. Research Instruments
Cobas e 411 Analyzer
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Strachan, M.W.J.; Newell-Price, J. Endocrine disease. In Davidson Principles and Practice of Medicine, 22nd ed.; Walker, B.R., Ed.; Churchill Livingstone: Edinburgh, UK, 2014; pp. 738–740. [Google Scholar]
- Rajput, R.; Goel, V.; Nanda, S.; Rajput, M.; Seth, S. Prevalence of thyroid dysfunction among women during the first trimester of pregnancy at a tertiary care hospital in Haryana. Indian J. Endocrinol. MeTable 2015, 19, 416–419. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.Z.; Haddow, J.E.; Faix, J.D.; Brown, R.S.; Hermos, R.J.; Pulkkinen, A.; Mitchell, M.L. Prevalence of thyroid deficiency in pregnant women. Clin. Endocrinol. 1991, 35, 41–46. [Google Scholar] [CrossRef]
- Lazzarus, J. Thyroid regulation and dysfunction in the pregnant patient. Available online: https://www.thyroidmanager.org (accessed on 8 October 2015).
- Jameson, J.L.; Weetman, A.P. Disorders of the thyroid gland. In Harrison Principles of Internal Medicine, 17th ed.; Fauci, A.S., Ed.; Mc Graw Hill: New York, NY, USA, 2008; pp. 2224–2229. [Google Scholar]
- Mannisto, T.; Vaarasmaki, M.; Pouta, A.; Hartikainen, A.L.; Ruokonen, A.; Surcel, H.M.; Bloigu, A.; Järvelin, M.R.; Suvanto, E. Thyroid dysfunction and autoantibodies during pregnancy as predictive factors of pregnancy complications and maternal morbidity in later life. J. Clin. Endocrinol. MeTable 2010, 95, 1084–1094. [Google Scholar] [CrossRef] [PubMed]
- Matalon, S.; Sheiner, E.; Levy, A.; Mazor, M.; Wiznitzer, A. Relationship of treated maternal hypothyroidism and perinatal outcome. J. Reprod. Med. 2006, 51, 59–63. [Google Scholar] [PubMed]
- Su, P.Y.; Huang, K.; Hao, J.H.; Xu, Y.Q.; Yan, S.Q.; Li, T.; Xu, Y.H.; Tao, F.B. Maternal thyroid function in the first twenty weeks of pregnancy and subsequent fetal and infant development: A prospective population-based cohort study in China. J. Clin. Endocrinol. MeTable 2011, 96, 3234–3241. [Google Scholar] [CrossRef] [PubMed]
- Nazarpour, S.; Ramezani, T.F.; Simbar, M.; Azizi, F. Thyroid dysfunction and pregnancy outcomes. Iran J. Reprod. Med. 2015, 13, 387–396. [Google Scholar] [PubMed]
- American Thyroid Association Taskforce on Thyroid Disease during Pregnancy and Postpartum; Stagnaro-Green, A.; Abalovich, M.; Alexander, E.; Azizi, F.; Mestman, J.; Negro, R.; Nixon, A.; Pearce, E.N.; Soldin, O.P.; et al. Guidelines of the American thyroid association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid 2011, 21, 1081–1125. [Google Scholar] [CrossRef] [PubMed]
- Garber, J.R.; Cobin, R.H.; Gharib, H.; Hennessey, J.V.; Klein, I.; Mechanick, J.I.; Pessah-Pollack, R.; Singer, P.A.; Woeber, K.A.; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults: Cosponsored by the American association of clinical endocrinologists and the American thyroid association. Endocr. Pract. 2012, 18, 988–1028. [Google Scholar] [CrossRef] [PubMed]
- Bahn Chair, R.S.; Burch, H.B.; Cooper, D.S.; Garber, J.R.; Greenlee, M.C.; Klein, I.; Laurberg, P.; McDougall, I.R.; Montori, V.M.; Rivkees, S.A.; et al. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocinologists. Thyroid 2011, 21, 593–646. [Google Scholar] [PubMed]
- Cleary-Goldman, J.; Malone, F.D.; Lambert-Messerlian, G.; Sullivan, L.; Canick, J.; Porter, T.F.; Luthy, D.; Gross, S.; Bianchi, D.W.; D’Alton, M.E. Maternal thyroid hypofunction and pregnancy outcome. Obstet. Gynecol. 2008, 112, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Glinoer, D.; Riahi, M.; Grün, J.P.; Kinthaert, J. Risk of subclinical hypothyroidism in pregnant women with asymptomatic autoimmune thyroid disorders. J. Clin. Endocrinol. MeTable 1994, 79, 197–204. [Google Scholar]
- De Groot, L.; Abalovich, M.; Alexander, E.K.; Amino, N.; Barbour, L.; Cobin, R.H.; Eastman, C.J.; Lazarus, J.H.; Luton, D.; Mandel, S.J.; et al. Management of thyroid dysfunction during pregnancy and postpartum: An endocrine society clinical practice guideline. J. Clin. Endocrinol. MeTable 2012, 97, 2543–2565. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, B.; Anthony, S.; Bilous, M.; Shields, B.; Drury, J.; Hutchison, S.; Bilous, R. Detection of thyroid dysfunction in early pregnancy: Universal screening or targeted high-risk case finding? J. Clin. Endocrinol. MeTable 2007, 92, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Thevarajah, M.; Chew, Y.Y.; Lim, S.C.; Sabir, N.; Sickan, J. Determination of trimester specific reference intervals for thyroid hormones during pregnancy in Malaysian women. Malays. J Pathol. 2009, 31, 23–37. [Google Scholar] [PubMed]
- Marwaha, R.K.; Chopra, S.; Gopalakrishnan, S.; Sharma, B.; Kanwar, R.S.; Sastry, A.; Singh, S. Establishment of reference range for thyroid hormones in normal pregnant Indian women. BJOG 2008, 115, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Maji, R.; Nath, S.; Lahiri, S.; Saha Das, M.; Bhattacharyya, A.R.; Das, H.N. Establishment of trimester-specific reference intervals of serum TSH & fT4 in a pregnant Indian population at North Kolkata. Indian J. Clin. Biochem. 2014, 29, 167–173. [Google Scholar] [PubMed]
- Mehran, L.; Amouzegar, A.; Delshad, H.; Askari, S.; Hedayati, M.; Amirshekari, G.; Azizi, F. Trimester-specific reference ranges for thyroid hormones in Iranian pregnant women. J. Thyroid Res. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Zarghami, N.; Rohbani Noubar, M.; Khosrowbeygi, A. Thyroid hormones status during pregnancy in normal Iranian women. Indian J. Clin. Biochem. 2005, 20, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Ekinci, E.I.; Lu, Z.X.; Sikaris, K.; Bittar, I.; Cheong, K.Y.; Lam, Q. Longitudinal assessment of thyroid function in pregnancy. Ann. Clin. Biochem. 2013, 50 Pt 6, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.W.; Chung, H.J.; Park, C.M.; Hur, M.; Yun, Y.M. Establishment of trimester specific reference intervals for thyroid hormones in Korean pregnant women. Ann. Lab. Med. 2015, 35, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Wang, Q.W.; Huang, R.P.; Cao, F.; Zhu, Z.Q.; Sun, D.C. Establishment of self-sequential longitudinal reference intervals of maternal thyroid function during pregnancy. Exp. Biol. Med. 2010, 235, 1212–1215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, W.; Chen, Q.B.; Liu, L.Y.; Zhang, W.; Liu, M.Y.; Wang, Y.T.; Li, W.Y.; Zeng, L.Z. Establishment of trimester-specific thyroid stimulating hormone and free thyroxine reference interval in pregnant Chinese women using the Beckman Coulter UniCel™ DxI 600. Clin. Chem. Lab. Med. 2015, 53, 1409–1414. [Google Scholar] [CrossRef] [PubMed]
- Bocos-Terraz, J.P.; Izquierdo-Alvarez, S.; Bancalero-Flores, J.L.; Alvarez-Lahuerta, R.; Aznar-Sauca, A.; Real-López, E.; Ibáñez-Marco, R.; Bocanegra-García, V.; Rivera-Sánchez, G. Thyroid hormones according to gestational age in pregnant Spanish women. BMC Res. Notes 2009, 26, 237. [Google Scholar] [CrossRef] [PubMed]
- Sriphrapradang, C.; Pavarangkoon, S.; Jongjaroenprasert, W.; Chailurkit, L.O.; Ongphiphadhanakul, B.; Aekplakorn, W. Reference ranges of serum TSH, FT4 and thyroid autoantibodies in the Thai population: The national health examination survey. Clin. Endocrinol. 2014, 80, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Karakosta, P.; Chatzi, L.; Bagkeris, E.; Daraki, V.; Alegakis, D.; Castanas, E.; Kogevinas, M.; Kampa, M. First- and second-trimester reference intervals for thyroid hormones during pregnancy in “Rhea” mother-child cohort, crete, Greece. J. Thyroid Res. 2011, 2011, 490783. [Google Scholar] [CrossRef] [PubMed]
- Medici, M.; Korevaar, T.; Visser, W.E.; Visser, T.J.; Peeters, R.P. Thyroid function in pregnancy: What is normal? Clin. Chem. 2015, 61, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Glinoer, D.; de Nayer, P.; Bourdoux, P.; Lemone, M.; Robyn, C.; van Steirteghem, A.; Kinthaert, J.; Lejeune, B. Regulation of maternal thyroid during pregnancy. J. Clin. Endocrinol. MeTable 1990, 71, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Soldin, O.P.; Soldin, D.; Sastoque, M. Gestation-specific thyroxine and thyroid stimulating hormone levels in the United States and worldwide. Ther. Drug Monit. 2007, 29, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Mehran, L.; Tohidi, M.; Sarvghadi, F.; Delshad, H.; Amouzegar, A.; Soldin, O.P.; Azizi, F. Management of thyroid peroxidase antibody euthyroid women in pregnancy: Comparison of the american thyroid association and the endocrine society guidelines. J. Thyroid Res. 2013, 2013, 542692. [Google Scholar] [CrossRef] [PubMed]
- Reference Intervals for Children and Adults Elecsys Thyroid Tests. Available online: https://www.google.iq/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&cad=rja&uact=8&ved=0CCEQFjAAahUKEwihquHK4e_HAhXnvHIKHdAlBro&url=http%3A%2F%2Frochediagnostics.cz%2FNews%2FDocuments%2FEnewsArchiv%2FRef_Intervals_Thyroid.pdf&usg=AFQjCNH_ui24MM0stwA69qfFREEDr-2NgQ&sig2=ufYbEr3w6Gjyr71ix5BSig&bvm=bv.102537793,d.bGQ (accessed on 11 January 2014).

| Age in Years | Number (%) |
|---|---|
| <20 | 76 (13.9) |
| 20–30 | 242 (45.2) |
| 30–40 | 181 (33.6) |
| >40 | 41 (7.3) |
| Total | 540 (100) |
| Trimester | TSH μIU/mL Mean ± SD | p Value | FreeT4 ng/dL Mean ± SD | p Value | TotalT4 μg/dL Mean ± SD | p Value | TotalT3 ng/mL Mean ± SD | p Value |
|---|---|---|---|---|---|---|---|---|
| First | 1.51 ± 1.16 | 0.011 | 1.15 ± 0.23 | <0.0001 | 11.07 ± 2.62 | <0.0001 | 1.62 ± 0.47 | <0.0001 |
| Second | 1.58 ± 0.94 | 0.97 ± 0.16 | 13.02 ± 2.59 | 1.99 ± 0.47 | ||||
| Third | 1.87 ± 1.11 | 0.90 ± 0.16 | 12.43 ± 3.0 | 1.99 ± 0.44 |
| Tests | Trimester | 5th Centile | 95th Centile |
|---|---|---|---|
| TSH μIU/mL | First trimester | 0.04 | 3.77 |
| Second trimester | 0.30 | 3.21 | |
| Third trimester | 0.60 | 4.50 | |
| FreeT4 ng/dL | First trimester | 0.80 | 1.53 |
| Second trimester | 0.70 | 1.20 | |
| Third trimester | 0.70 | 1.20 | |
| Total T4 μg/dL | First trimester | 7.31 | 15.0 |
| Second trimester | 8.92 | 17.38 | |
| Third trimester | 7.98 | 17.70 | |
| Total T3 ng/mL | First trimester | 0.90 | 2.51 |
| Second trimester | 1.30 | 2.87 | |
| Third trimester | 1.20 | 2.70 |
| Anti-TPO Level IU/mL | Number (%) |
|---|---|
| Missed values | 210 (24.6) |
| 0–34 | 540 (63.3) |
| >34 | 103 (12.1) |
| Total | 853 (100) |
| Country, Year | Thyroid Test | Sample Size | First Trimester | Second Trimester | Third Trimester | Methods/Instrument |
|---|---|---|---|---|---|---|
| Basrah, Iraq, 2015 5th–95th centile | TSH μIU/mL | 540 | 0.04–3.77 | 0.30–3.21 | 0.6–4.5 | ECL/cobas e411 analyzer |
| Mean ± SD | 1.51 ± 1.16 | 1.58 ± 0.94 | 1.87 ± 1.11 | |||
| 5th–95th centile | FreeT4 ng/dL | 0.8–1.53 | 0.70–1.20 | 0.70–1.20 | ||
| Mean ± SD | 1.15 ± 0.23 | 0.97 ± 0.16 | 0.90 ± 0.16 | |||
| 5th–95th centile | Total T4 μg/dL | 7.31–15.0 | 8.92–17.38 | 7.98–17.7 | ||
| Mean ± SD | 11.07 ± 2.62 | 13.02 ± 2.59 | 12.43 ± 3.0 | |||
| 5th–95th centile | Total T3 ng/mL | 0.90–2.51 | 1.30–2.87 | 1.20–2.70 | ||
| Mean ± SD | 1.62 ± 0.47 | 1.99 ± 0.47 | 1.99 ± 0.44 | |||
| Ref. Current study | ||||||
| Malaysia, 2009 Mean ± SD | TSH MIU/L | 626 | 1.04 ± 0.08 | 1.82 + 0.07 mIU/L | 1.92 + 0.06 | Abbott AxSYM immunoassay platform. |
| Mean ± SD | FreeT4 pmol/L | 13.86 ± 5.9 | 9.35 + 2.07 | 8.40 + 1.30 | ||
| Mean ± SD | Total T4 nmol/L | 143.56 ± 38.26 | 140.89 + 26.99 | 138.03 + 22.79 | ||
| Mean ± SD | Total T3 nmol/L | 1.18 ± 0.38 | 1.29 + 0.24 | 1.29 + 0.30 | ||
| Ref. [17] | ||||||
| New Delhi, India, 2008 5th–95th centile | TSH μIU/mL | 541 | 0.6–5 | 0.435–5.78 | 0.74–5.7 | ECL/Elecsys 1010 analyzer |
| 5th–95th centile | FreeT4 pmol/L | 12–19.45 | 9.48–19.58 | 11.3–17.71 | ||
| Ref. [18] | ||||||
| North Kolkata, West Bengal, India, 2014 Mean ± SD | TSH μIU/mL | * 402 | 0.25–3.35 | 0.78–4.96 | 0.9–4.6 | ELISA |
| Mean ± SD | FreeT4 ng/dL | 0.64–2.0 | 0.53–2.02 | 0.64–1.99 | ||
| Ref. [19] * | ||||||
| Tehran, Iran, 2013 5th–95th centile | TSH μIU/mL | *152 | 0.2–3.9 | 0.5–4.1 | 0.6–4.1 | Immunoenzymometric assay (IRMA) /Wizard, Wallac Oy, Turku, Finland). |
| 5th–95th centile | Total T4 (μg/dL) | 8.2–18.5 | 10.1–20.6 | 9.0–19.4 | ||
| 5th–95th centile | Total T3 (ng/dL) | 138–278 | 155–328 | 137–324 | ||
| Ref. [20] * | ||||||
| Tabriz, Iran, 2005 Mean + SD | TSH μIU/mL | 229 | 1.71 + 1.38 | 1.89 + 1.24 | 2.12 ± 0.77 | Radio immunoassay/Gammamatic II gammacounter (Contron, Switzerland). |
| Mean + SD | FreeT4 pmol/L | 14.90 ± 4.67 | 13.07 ± 3.06 | 6.91 + 3.20 | ||
| Mean + SD | Total T4 nmol/L | 87.98 + 40.87 | 94.30 ± 41.70 | 123.80 + 50.50 | ||
| Mean + SD | TT3 nmol/L | 2.54 + 1.41 | 3.15 + 1.76 | 2.90 ± 1.5 | ||
| Ref. [21] | ||||||
| Australia, 2013 5th–95th centile | TSH μIU/mL | 130 | 0.05–2.33 | 0.47–2.71 | 0.42–2.65 | Beckman Dxl 800 analysers |
| Mean + SD | FreeT4 pmol/L | 5.9–15.5 | 4.9–11.3 | 4.5–11.0 | ||
| Ref. [22] | ||||||
| Korea, 2012 Mean + SD | TSH μIU/mL | 531 | 0.01–4.10 | 0.01–4.26 | 0.15–4.57 | ECL/Elecsys thyroid tests, Roche Diagnostics |
| Mean + SD | FreeT4 ng/dL | 0.83–1.65 | 0.71–1.22 | 0.65–1.13 | ||
| Ref. [23] | ||||||
| Jiangsu, China, 2010 2.5th–95th centile | TSH μIU/mL | 301 | 0.02–3.65 | 0.36–3.46 | 0.44–5.04 | Electrochemistry immunoassay (ECL)/COBAS e601 |
| 2.5th–95th centile | FreeT4 pmol/L | 11.85–21.51 | 9.45–6.26 | 9.30–17.14 | ||
| Ref. [24] | ||||||
| Shanghai, China, 2013 2.5th–95th centile | TSH mIU/L | 2743 | 0.06–3.13 | 0.07–4.13 | 0.15–5.02 | Beckman Coulter UniCel™ DxI 600. |
| 2.5th–95th centile | FreeT4 pmol/L | 8.72–15.22 | 7.10–13.55 | 6.16–12.03 | ||
| Ref. [25] | ||||||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almomin, A.M.S.; Mansour, A.A.; Sharief, M. Trimester-Specific Reference Intervals of Thyroid Function Testing in Pregnant Women from Basrah, Iraq Using Electrochemiluminescent Immunoassay. Diseases 2016, 4, 20. https://doi.org/10.3390/diseases4020020
Almomin AMS, Mansour AA, Sharief M. Trimester-Specific Reference Intervals of Thyroid Function Testing in Pregnant Women from Basrah, Iraq Using Electrochemiluminescent Immunoassay. Diseases. 2016; 4(2):20. https://doi.org/10.3390/diseases4020020
Chicago/Turabian StyleAlmomin, Ammar Mohammed Saeed, Abbas Ali Mansour, and Maysoon Sharief. 2016. "Trimester-Specific Reference Intervals of Thyroid Function Testing in Pregnant Women from Basrah, Iraq Using Electrochemiluminescent Immunoassay" Diseases 4, no. 2: 20. https://doi.org/10.3390/diseases4020020






