Specialized Diagnostic Investigations to Assess Ocular Status in Hypertensive Diseases of Pregnancy
Abstract
:1. Introduction
2. Fluorescein Angiography and Indocyanine Green Angiography in Hypertensive Diseases of Pregnancy
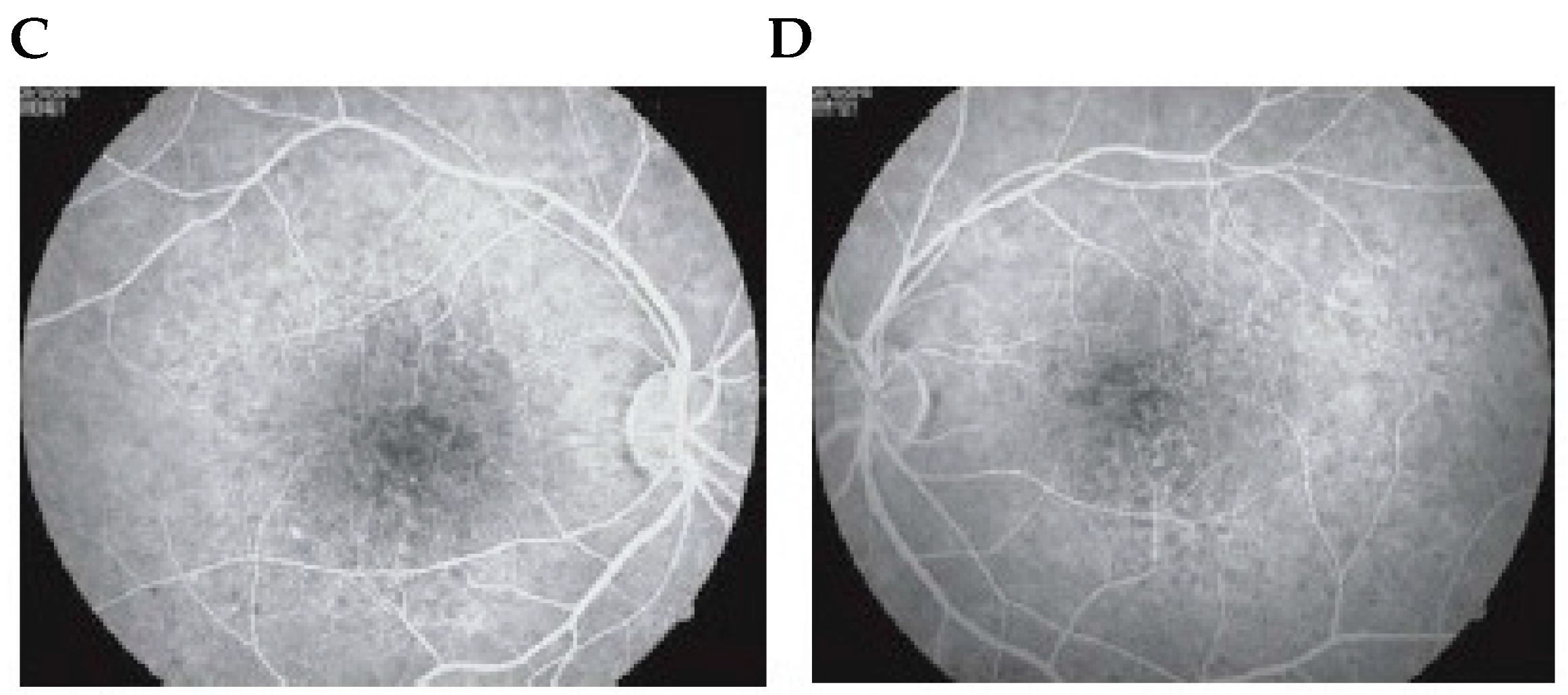
2.1. Fluorescein Angiography
2.2. Indocyanine Green Angiography
3. Ocular Fluorophotometric Findings in Toxemia of Pregnancy
4. Ophthalmodynamometry in Toxemia of Pregnancy
5. Imaging Techniques
5.1. Computed Axial Tomography Scan
5.2. Magnetic Resonance Imaging (MRI)
5.2.1. Imaging Sequences in MRI
5.2.2. Magnetic Resonance Spectroscopy
5.2.3. FMRI, PET, and SPECT
5.2.4. Angiography
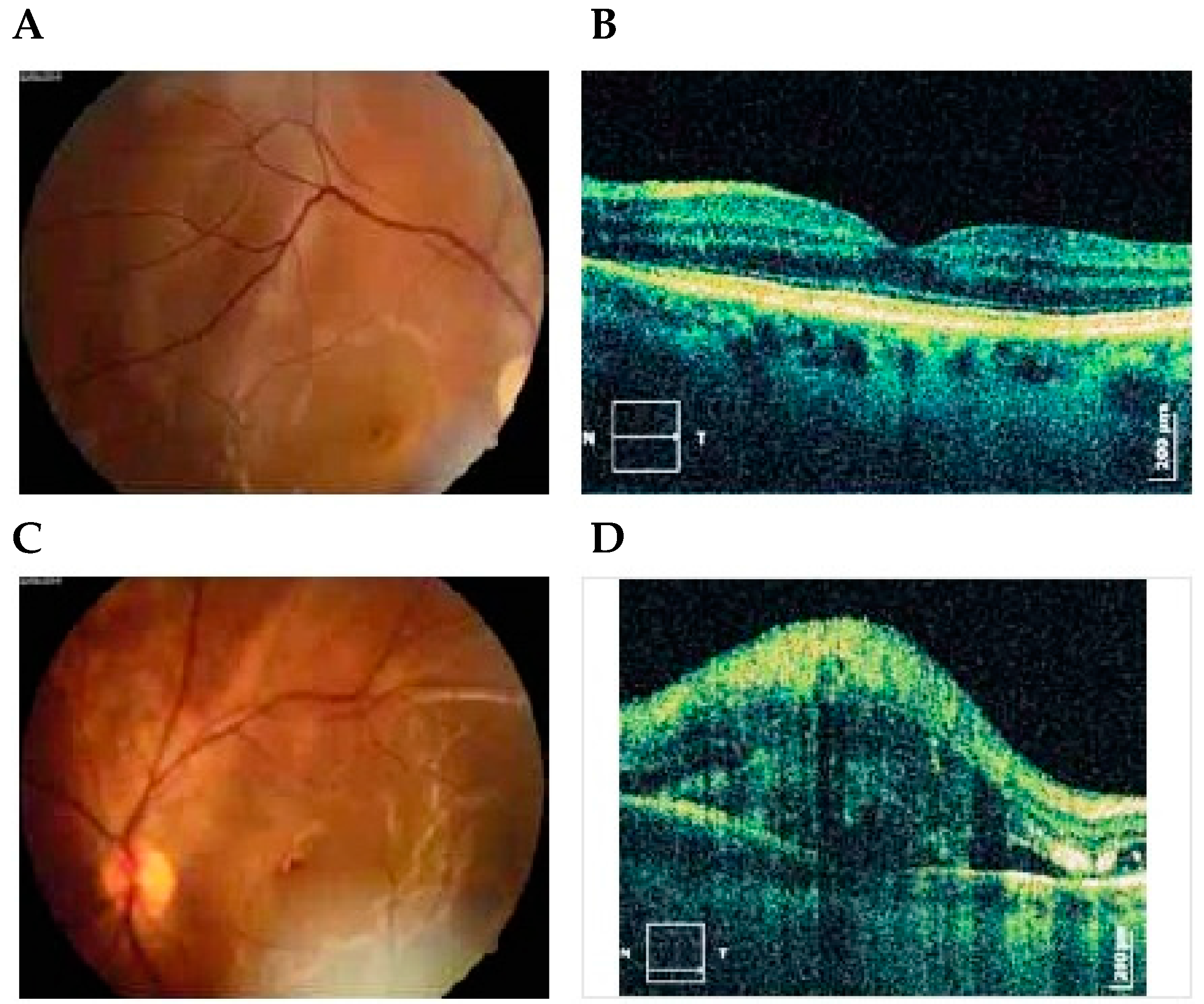
6. OCT
Multinodal Imaging
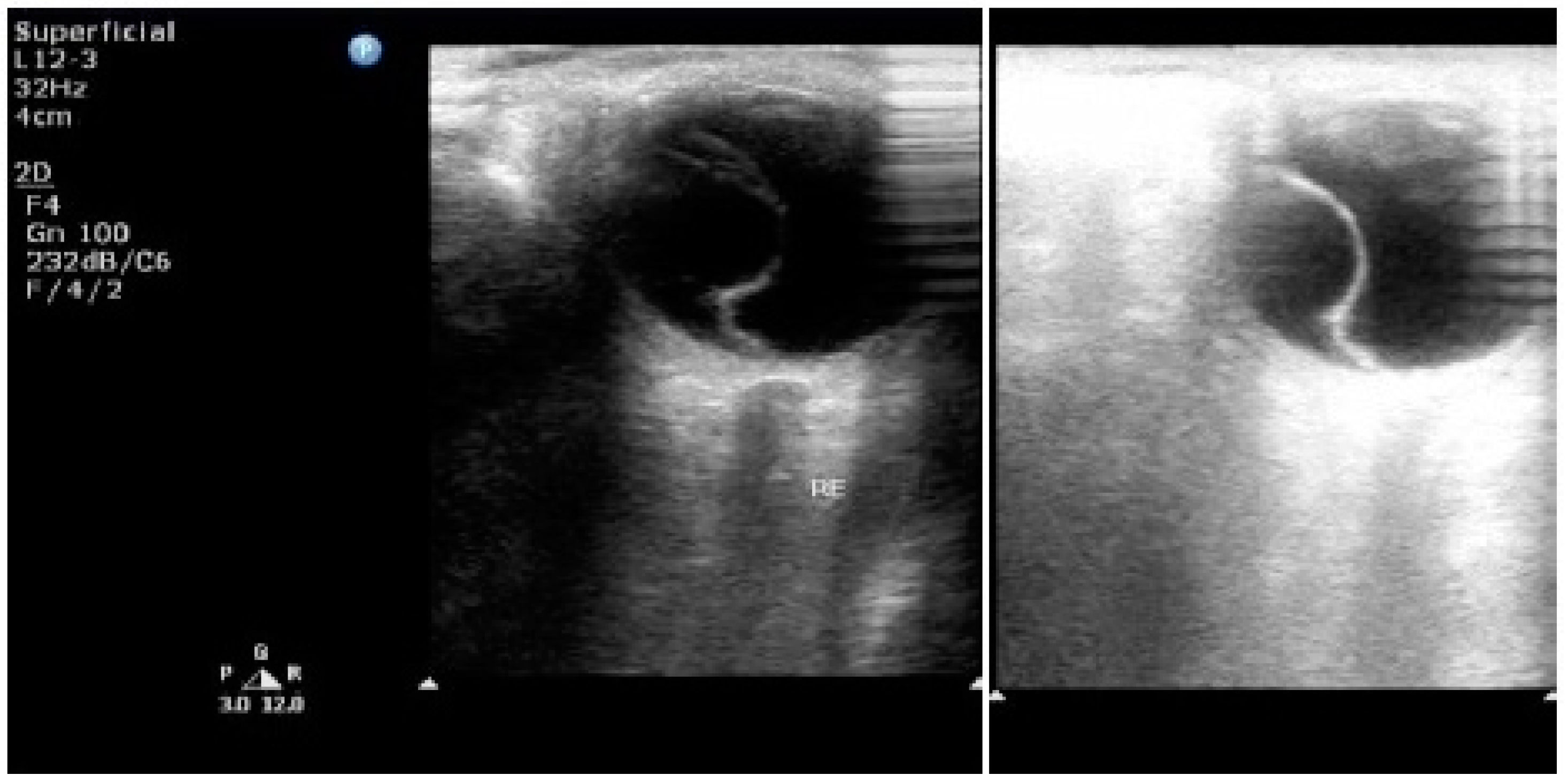
7. Ultrasonography
8. Doppler Velocimetry of the Ophthalmic Artery
9. Laboratory Investigations
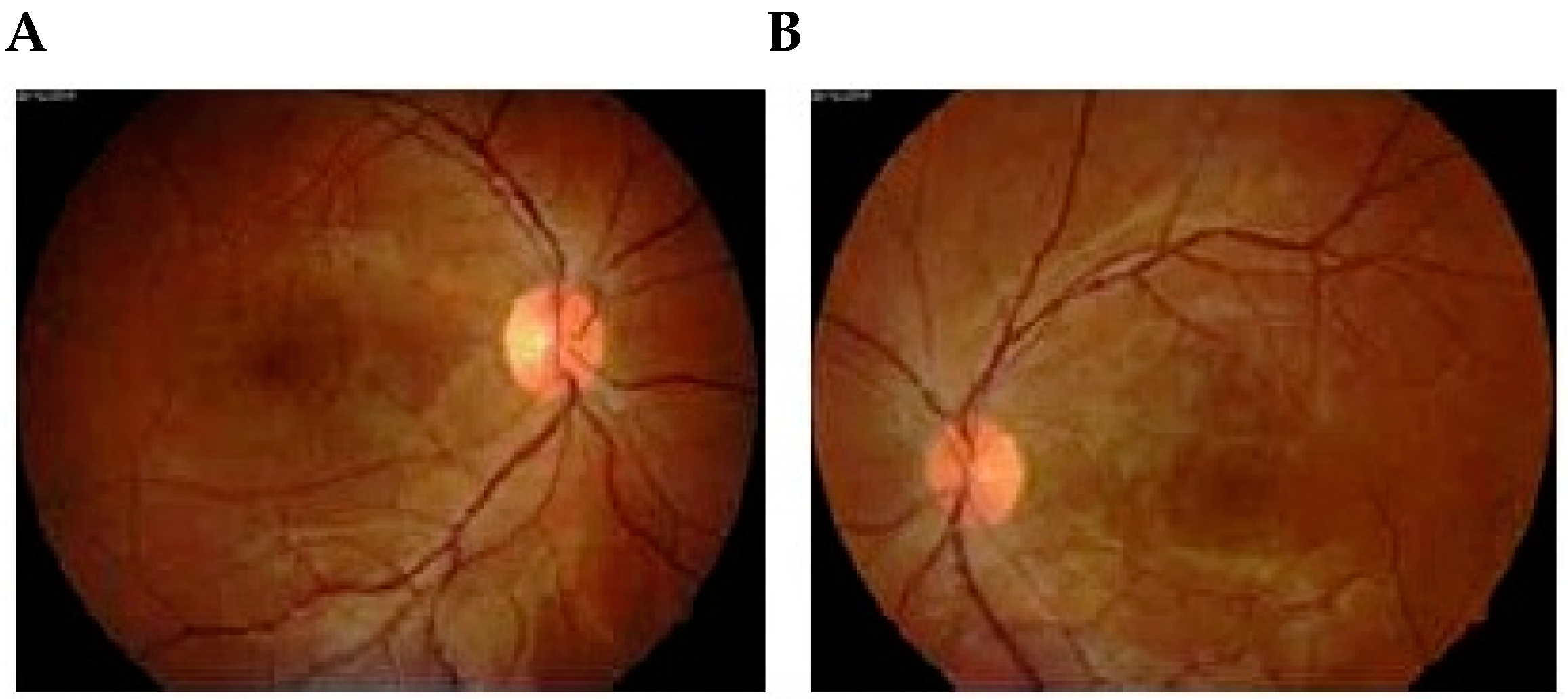
10. Ophthalmoscopy
11. Conclusions
Acknowledgments
Conflicts of Interest
References
- Davey, D.A.; MacGillivray, I. The classification and definition of the hypertensive disorders of pregnancy. Am. J. Obstet. Gynecol. 1988, 158, 892–898. [Google Scholar] [CrossRef]
- Duley, L. Maternal mortality associated with hypertensive disorders of pregnancy in Africa, Asia, Latin America and the Caribbean. Br. J. Obstet. Gynaecol. 1992, 99, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; Buddle, M.L. Hypertension in pregnancy: Maternal and fetal outcomes according to laboratory and clinical features. Med. J. Aust. 1996, 165, 360–365. [Google Scholar] [PubMed]
- Seidman, D.S.; Serr, D.M.; Ben-Rafael, Z. Renal and ocular manifestations of hypertensive diseases of pregnancy. Obstet. Gynecol. Surv. 1991, 46, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Conde-Agudelo, A.; Lede, R.; Belizán, J. Evaluation of methods used in the prediction of hypertensive disorders of pregnancy. Obstet. Gynecol. Surv. 1994, 49, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Schreyer, P.; Tzadok, J.; Sherman, D.J.; Herman, A.; Bar-Itzhak, R.; Caspi, E. Fluorescein angiography in hypertensive pregnancies. Int. J. Gynaecol. Obstet. 1991, 34, 127–132. [Google Scholar] [CrossRef]
- Sreckovic, S.B.; Janicijevic-Petrovic, M.A.; Stefanovic, I.B.; Petrovic, N.T.; Šarenac, T.S.; Paunovic, S.S. Bilateral retinal detachment in a case of preeclampsia. Bosn. J. Basic Med. Sci. 2011, 11, 129–131. [Google Scholar] [PubMed]
- Tayade, S.; Wattamwar, A. Bilateral retinal detachment in pregnancy complicated by preeclampsia, eclampsia and placental abruption. Int. J. Biomed. Res. 2014, 5, 780–782. [Google Scholar]
- Fastenbery, D.M.; Fetkenhour, C.L.; Choromokos, E.; Shoch, D.E. Choroidal vascular changes in toxaemia of pregnancy. Am. J. Ophthalmol. 1980, 89, 362–368. [Google Scholar] [CrossRef]
- Sood, P.; Saxena, N.; Talwar, D. OCT angiography: An upcoming tool for diagnosis and treatment of retinal vascular diseases. DJO 2015, 26, 125–130. [Google Scholar] [CrossRef]
- Valluri, S.; Adelberg, D.A.; Curtis, R.S.; Olk, R.J. Diagnostic indocyanine green angiography in preeclampsia. Am. J. Ophthalmol. 1996, 122, 672–677. [Google Scholar] [CrossRef]
- Wolf, S.; Wald, K.J.; Elsner, A.E.; Staurenghi, G. Indocyanine green choroidal video angiography: A comparison of imaging analysis with the scanning laser ophthalmoscope and the fundus camera. Retina 1993, 13, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Hope-Ross, M.; Yannuzzi, L.A.; Gragoudas, E.S.; Guyer, D.R.; Slakter, J.S.; Sorenson, J.A.; Krupsky, S.; Orlock, D.A.; Puliafito, C.A. Adverse reactions due to indocyanine green. Ophthalmology 1994, 101, 529–533. [Google Scholar] [CrossRef]
- Fineman, M.S.; Maguire, J.I.; Fineman, S.W.; Benson, W.E. Safety of indocyanine green angiography during pregnancy: A survey of the retina, macula, and vitreous societies. Arch. Ophthalmol. 2001, 119, 353–355. [Google Scholar] [CrossRef] [PubMed]
- Freeman, W.R. Simultaneous indocyanine green and fluorescein angiography using a confocal scanning laser ophthalmoscope. Arch. Ophthalmol. 1998, 116, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Kayazawa, F.; Miyake, K. Ocular fluorophotometry in patients with essential hypertension. Arch. Ophthalmol. 1984, 102, 1169–1170. [Google Scholar] [CrossRef] [PubMed]
- Chaine, G.; Attali, P.; Gaudric, A.; Colin, M.C.; Quentel, G.; Coscas, G. Ocular fluorophotometric and angiographic findings in toxemia of pregnancy. Arch. Ophthalmol. 1986, 104, 1632–1635. [Google Scholar] [CrossRef] [PubMed]
- Raines, M.F. Vitreous fluorophotometry. Semin. Ophthalmol. 1986, 1, 153–183. [Google Scholar] [CrossRef]
- Tyagi, P.; Kadam, R.S.; Kompella, U.B. Comparison of suprachoroidal drug delivery with subconjuctival and intravitreal routes using noninvasive fluorophotometry. PLoS ONE 2012, 7, e48188. [Google Scholar] [CrossRef] [PubMed]
- Wunsh, S.E. Ophthalmodynamometry. N. Eng. J. Med. 1969, 281, 446. [Google Scholar]
- Koch, F.L. Retina in systemic vascular hypertension: A clinical study of the calibre of the retinal arterioles and the retinal arterial diastolic blood pressure. Arch. Ophthalmol. 1941, 26, 565–584. [Google Scholar] [CrossRef]
- Van dar Worllff, T.J. The pressure measured in ophthalmodynamometry. Arch. Ophthalmol. 1972, 87, 290–292. [Google Scholar]
- Stodtmeister, R.; Oppitz, T.; Spoerl, E.; Haustein, M.; Boehm, A.G. Contact lens dynamometry: The influence of age. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6620–6624. [Google Scholar] [CrossRef] [PubMed]
- Legler, U.; Jonas, J.B. Assessment of the spontaneous pulsations of the central retinal vein in daily ophthalmic practice. Clin. Exp. Ophthalmol. 2007, 35, 870–871. [Google Scholar] [CrossRef] [PubMed]
- Firsching, R.; Müller, C.; Pauli, S.U.; Voellger, B.; Röhl, F.W.; Behrens-Baumann, W. Noninvasive assessment of intracranial pressure with venous ophthalmodynamometry: Clinical article. J. Neurosurg. 2011, 115, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.G.; Johnson, M.C.; Policeni, B.A.; Smoker, W.R. Imaging for neuro-ophthalmic and orbital disease—A review. Clin. Exp. Ophthalmol. 2009, 37, 30–53. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.O.; Hadley, D.; Bone, I.; Symonds, E.M.; Worthington, B.S.; Rubin, P.C. Blindness in eclampsia: CT and MR imaging. J. Neurol. Neurosurg. Psychiatry 1989, 52, 899–902. [Google Scholar] [CrossRef] [PubMed]
- Vellody, D.; Waldron, R.L.; Abbott, D.C. Computed tomography in preeclampsia-eclampsia syndrome. AJNR Am. J. Neuroradiol. 1985, 6, 442–443. [Google Scholar]
- Lee, A.G.; Hayman, L.A.; Ross, A.W. Neuroimaging contrast agents in ophthalmology. Surv. Ophthalmol. 2000, 45, 237–253. [Google Scholar] [CrossRef]
- Lan, S.P.C.; Chan, F.L.; Yu, Y.L.; Woo, E.; Huang, C.Y. Cortical blindness in toxaemia of pregnancy: Findings on computed tomography. Br. J. Radiol. 1987, 60, 347–349. [Google Scholar]
- Moodley, J.; Bobat, S.M.; Hoffman, M.; Bill, P.L.A. Electroencephalogram and computerized cerebral tomography findings in eclampsia. Br. J. Obstet. Gynaecol. 1993, 100, 984–988. [Google Scholar] [CrossRef] [PubMed]
- Drayer, B.P.; Rosenbaum, A.E. Brain edema defined by cranial computed tomography. J. Comput. Assist. Tomogr. 1979, 3, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Zunker, P.; Ley-Pozo, J.; Louwen, F.; Schuierer, G.; Holzgreve, W.; Ringelstein, E.B. Cerebral hemodynamics in preeclampsia/eclampsia syndrome. Ultrasound Obstet. Gynecol. 1995, 6, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, R.B.; Jones, K.M.; Kalina, P.; Bajakian, R.L.; Mantello, M.T.; Garada, B.; Holman, B.L. Hypertensive encephalopathy: Findings on CT, MR imaging, and SPECT imaging in 14 cases. AJR Am. J. Roentgenol. 1992, 159, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.F.; Pioccini, P. Applications of positron emission tomography (PET) in neurology. J. Neurol. Neurosurg. Psychiatry 2004, 75, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Mayer, T.E.; Hamann, G.F.; Baranczyk, J.; Rosengarten, B.; Klotz, E.; Wiesmann, M.; Missler, U.; Schulte-Altedorneburg, G.; Brieckmann, H.J. Dynamic CT perfusion imaging of acute stroke. AJNR Am. J. Neuroradiol. 2000, 21, 1441–1449. [Google Scholar] [PubMed]
- Dahmus, M.A.; Barton, J.R.; Sibai, B.M. Cerebral imaging in eclampsia: Magnetic resonance imaging versus computed tomography. Am. J. Obstet. Gynecol. 1992, 167, 935–941. [Google Scholar] [CrossRef]
- Digre, K.B.; Varner, M.W.; Osborn, A.G.; Crawford, S. Cranial magnetic resonance imaging in severe preeclampsia vs. eclampsia. Arch. Neurol. 1993, 50, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Delfaut, E.M.; Beltran, J.; Johnson, G.; Rousseau, J.; Marchandise, X.; Cotten, A. Fat suppression in MR imaging: Techniques and pitfalls. Radiographics 1999, 19, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Arakia, Y.; Ashikaga, R.; Fujii, K.; Nishimura, Y.; Ueda, J.; Fujita, N. MR fluid-attenuated inversion recovery imaging as routine brain T2-weighted imaging. Eur. J. Radiol. 1999, 32, 136–143. [Google Scholar] [CrossRef]
- Wycliffe, N.D.; Choe, J.; Holshouser, B.; Oyoyo, U.E.; Haacke, E.M.; Kido, D.K. Reliability in detection of hemorrhage in acute stroke by a new 3-D gradient recalled echo susceptibility-weighted imaging technique compared to computed tomography: A retrospective study. J. Magn. Reson. Imaging 2004, 20, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Valentini, V.; Gaudino, S.; Spagnolo, P. Diffusion and perfusion MR imaging. Rays 2003, 28, 29–43. [Google Scholar] [PubMed]
- Gregory, D.G.; Pelak, V.S.; Bennett, J.L. Diffusion-weighted magnetic resonance imaging and the evaluation of cortical blindness in preeclampsia. Surv. Ophthalmol. 2003, 48, 647–650. [Google Scholar] [CrossRef] [PubMed]
- Lamy, C.; Oppenheim, C.; Meder, J.F.; Mas, J.L. Neuroimaging in posterior reversible encephalopathy syndrome. J. Neuroimaging 2004, 14, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Castillo, M.; Kwock, L.; Mukherji, S.K. Clinical applications of proton MR spectroscopy. AJNR Am. J. Neuroradiol. 1996, 17, 1–15. [Google Scholar] [PubMed]
- Gore, J.C. Principles and practice of functional MRI of the human brain. J. Clin. Investig. 2003, 112, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Sakai, T.; Inagawa, S.; Utsu, M.; Bun, T. MR angiography of cerebral vasospasm in preeclampsia. AJNR Am. J. Neuroradiol. 1995, 16, 1344–1346. [Google Scholar] [PubMed]
- Goldman, J.P. New techniques and applications for magnetic resonance angiography. Mt. Sinai J. Med. N. Y. 2003, 70, 375–385. [Google Scholar]
- Kulkami, M. Constructive interference in steady-state/FIESTA-C clinical applications in neuroimaging. J. Med. Imaging Radiat. Oncol. 2011, 55, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Marsh, M.S.; Smith, S. The visual evoked potential in the assessment of central nervous system effects of preeclampsia: A pilot study. Br. J. Obstet. Gynaecol. 1994, 101, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.V.; Somanathan, N.; Radhakumari, R. Interictal EEG changes in eclampsia. Electroencephalogr. Clin. Neurophysiol. 1995, 94, 271–275. [Google Scholar] [CrossRef]
- Kwok, A.H.; Li, J.; Lai, T.Y.; Chan, W.M.; Bhede, P.; Lam, D.S. Multifocal electroretinographic and angiographic changes in preeclampsia. Br. J. Ophthalmol. 2001, 85, 111–112. [Google Scholar] [CrossRef] [PubMed]
- Citirik, M.; Tulay, S.; Zilelioglu, O. Bilateral permanent concentric visual field defect secondary to severe preeclampsia. Clin. Ophthalmol. 2008, 2, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Theodossiadis, P.G.; Kollia, A.K.; Gogas, P.; Panagiotidis, D.; Moschos, M.; Theodossiadis, G.P. Retinal disorders in preeclampsia studied with optical coherence tomography. Am. J. Ophthalmol. 2002, 133, 707–709. [Google Scholar] [CrossRef]
- Leitgeb, R.; Hitzenberger, C.K.; Fercher, A.F. Performance of fourier domain vs. time domain optical coherence tomography. Opt. Expess 2003, 11, 889–894. [Google Scholar] [CrossRef]
- Wang, Z.; Zou, Y.; Li, W.; Wang, X.; Zhang, M.; Wang, W. Application of optical coherence tomography and contrast sensitivity test for observing fundus changes of patients with pregnancy-induced hypertension syndrome. Medicine 2015, 94, e1641. [Google Scholar] [CrossRef] [PubMed]
- Fledelius, H.C. Ultrasound in ophthalmology. Ultrasound Med. Biol. 1997, 23, 365–375. [Google Scholar] [CrossRef]
- Sconfienza, L.M.; Lacelli, F.; Ardemagni, A.; Perrone, N.; Bertolotto, M.; Padolecchia, R.; Serafini, G. High-resolution, 3-D, and contrast-enhanced ultrasonographic findings in diseases of the eye. J. Ultrasound 2010, 13, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.P.; MacLean, C. Maternal cerebral vasospasm in eclampsia assessed by transcranial Doppler. Am. J. Perinatol. 1993, 10, 243–244. [Google Scholar] [PubMed]
- Riskin-Mashiah, S.; Belfort, M.A.; Saade, G.R.; Herd, J.A. Transcranial doppler measurement of cerebral velocity indices as a predictor of preeclampsia. Am. J. Obstet. Gynecol. 2002, 187, 1667–1672. [Google Scholar] [CrossRef] [PubMed]
- Waugh, J.; Bell, S.C.; Kilby, M.D.; Lambert, P.; Shennan, A.; Halligan, A. Urine protein estimation in hypertensive pregnancy: Which thresholds and laboratory assay best predict clinical outcome? Hypertens. Pregnancy 2005, 24, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.N., Jr.; May, W.L.; Magann, E.F.; Terrone, D.A.; Rinehart, B.K.; Blake, P.G. Early risk assessment of severe preeclampsia: Admission battery of symptoms and laboratory tests to predict likelihood of subsequent significant maternal morbidity. Am. J. Obstet. Gynecol. 1999, 180, 1407–1414. [Google Scholar] [CrossRef]
- Newman, M.G.; Robichaux, A.G.; Stedman, C.M.; Jaekle, R.K.; Fontenot, M.T.; Dotson, T.; Lewis, T.F. Perinatal outcomes in preeclampsia that is complicated by massive proteinuria. Am. J. Obstet. Gynecol. 2003, 188, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Melchiorre, K.; Thilaganathan, B. Maternal cardiac function in preeclampsia. Curr. Opin. Obstet. Gynecol. 2011, 23, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Salako, B.L.; Olayemi, O.; Odukogbe, A.T.; Adedapo, K.S.; Aimakhu, C.O.; Alu, F.E.; Ola, B. Microalbuminuria in pregnancy as a predictor of preeclampsia and eclampsia. West Afr. J. Med. 2004, 22, 295–300. [Google Scholar] [CrossRef]
- Voto, L.S.; Illia, R.; Darbon-Grosso, H.A.; Imaz, F.U.; Margulies, M. Uric acid levels: A useful index of the severity of preeclampsia and perinatal prognosis. J. Perinat. Med. 1988, 16, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, H.; Pryor-Koishi, K.; Suzuki, M.; Kato, T.; Sekiya, T.; Tada, S.; Kurahashi, H.; Udagawa, Y. Analysis of nitric oxide metabolism as a placental or maternal factor underlying the etiology of pre-eclampsia. Gynecol. Obstet. Investig. 2009, 68, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Fong, J.; Gurewitsch, E.D.; Volpe, L.; Wagner, W.E.; Gomillion, M.C.; August, P. Baseline serum and cerebrospinal fluid magnesium levels in normal pregnancy and preeclampsia. Obstet. Gynecol. 1995, 85, 444–448. [Google Scholar] [CrossRef]
- Koçyıgıt, Y.; Atamer, Y.; Atamer, A.; Tuzcu, A.; Akkus, Z. Changes in serum levels of leptin, cytokines and lipoprotein in preeclamptic and normotensive pregnant women. Gynecol. Endocrinol. 2004, 19, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Resnik, J.L.; Hong, C.; Resnik, R.; Kazanegra, R.; Beede, J.; Bhalla, V.; Maisel, A. Evaluation of B-type natriuretic peptide (BNP) levels in normal and preeclamptic women. Am. J. Obstet. Gynecol. 2005, 193, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Leeflang, M.M.; Cnossen, J.S.; Van der Post, J.A.M.; Mol, B.W.; Khan, K.S.; Ter Riet, G. Accuracy of fibronectin tests for the prediction of preeclampsia: A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2007, 133, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Mussey, R.D.; Mundell, B.J. Retinal examinations: A guide in the management of the toxic hypertensive syndrome of pregnancy. Am. J. Obstet. Gynecol. 1939, 37, 30–36. [Google Scholar]
- Perlmutter, R.; Friedland, S. Computer-generated holograms in biology and medicine. IEEE Comput. Graph. Appl. 1983, 5, 47–51. [Google Scholar] [CrossRef]
- Perednia, D.A.; Allen, A. Telemedicine technology and clinical applications. JAMA 1995, 273, 483–488. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakhda, R.N. Specialized Diagnostic Investigations to Assess Ocular Status in Hypertensive Diseases of Pregnancy. Diseases 2016, 4, 19. https://doi.org/10.3390/diseases4020019
Bakhda RN. Specialized Diagnostic Investigations to Assess Ocular Status in Hypertensive Diseases of Pregnancy. Diseases. 2016; 4(2):19. https://doi.org/10.3390/diseases4020019
Chicago/Turabian StyleBakhda, Rahul Navinchandra. 2016. "Specialized Diagnostic Investigations to Assess Ocular Status in Hypertensive Diseases of Pregnancy" Diseases 4, no. 2: 19. https://doi.org/10.3390/diseases4020019




