The Effects of Vitamin D Supplementation on Pulmonary Function of Chronic Obstructive Pulmonary Disease Patients, before and after Clinical Trial
Abstract
:1. Introduction
2. Method and Materials
3. Statistical Analysis
4. Results
| Characteristic | Value |
|---|---|
| Men, n(%) | 20 (83) |
| Mean age (SD), y | 64 (11) |
| GOLD stage, n(%) | |
| I | 1 |
| II | 8 |
| III | 13 |
| IV | 1 |
| Mean 25(OH) vit D | 13 (4) |
| level (SD), ng/mL |
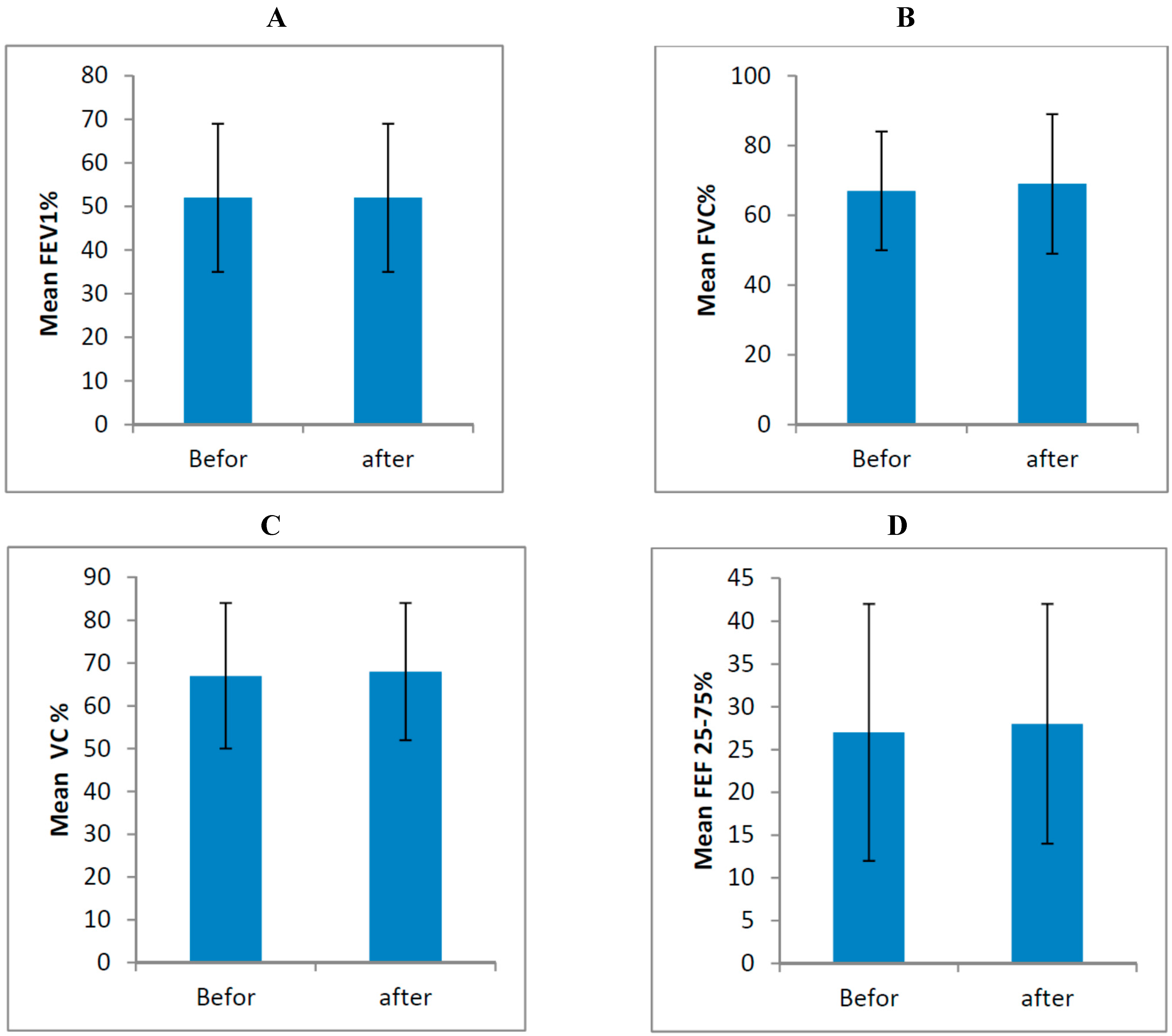
| Variable | Mean ± SD (Before) | Mean ± SD (After) | p-Value |
|---|---|---|---|
| FEV1 | 52% ± 17% | 52% ± 17% | 0.866 |
| FVC | 67% ± 17% | 69% ± 20% | 0.475 |
| VC | 67% ± 17% | 68% ± 16% | 0.452 |
| FEF 25%–75% | 27% ± 15% | 28% ± 14% | 0.555 |
| 6MWT, m | 350 | 360 | 0.175 |
| O2 Sat | 95% ± 1% | 95% ± 1% | 0.635 |
5. Discussion
Acknowledgment
Author Contributions
Conflicts of Interest
References
- Hogg, J.C.; Chu, F.; Utokaparch, S.; Woods, R.; Elliott, W.M.; Buzatu, L.; Cherniack, R.M.; Rogers, R.M.; Sciurba, F.C.; Coxson, H.O.; et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N. Engl. J. Med. 2004, 350, 2645–2653. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, G.C.; Seemungal, T.A.; Bhowmik, A.; Wedzicha, J.A. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002, 57, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Anthonisen, N.R.; Skeans, M.A.; Wise, R.A.; Manfreda, J.; Kanner, R.E.; Connett, J.E.; Lung Health Study Research Group. The effects of a smoking cessation intervention on 14.5-year mortality, a randomized clinical trial. Ann. Intern. Med. 2005, 142, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, C.R.; Arum, S.M.; Smith, C.M. Vitamin D deficiency and chronic lung disease. Can. Respir. J. 2009, 16, 75–80. [Google Scholar] [PubMed]
- Van Etten, E.; Mathieu, M.C. Immunoregulation by 1,25-dihydroxyvitamin D3: Basic concepts. J. Steroid Biochem. Mol. Biol. 2005, 97, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Baeke, F.; van Etten, E.; Gysemans, C.; Overbergh, L.; Mathieu, C. Vitamin D signaling in immune-mediated disorders: Evolving insights and therapeutic opportunities. Mol. Aspects Med. 2008, 29, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Ferrari, H.A.; Dawson-Hughes, B.; Willett, W.C.; Staehelin, H.B.; Bazemore, M.G.; Zee, R.Y.; Wong, J.B. Effect of Vitamin D on falls: A meta-analysis. JAMA 2004, 291, 1999–2006. [Google Scholar] [CrossRef] [PubMed]
- Ginde, A.A.; Mansbach, J.M.; Camargo, C.A., Jr. Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch. Intern. Med. 2009, 169, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Kriegel, M.A.; Manson, J.E.; Costenbader, K.H. Does vitamin D affect risk of developing autoimmune disease?: A systematic review. Semin. Arthritis Rheum. 2011, 40, 512–531. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.V.; Trump, D.L.; Johnson, C.S.; Feldman, D. The role of vitamin D in cancer prevention and treatment. Endocrinol. Metab. Clin. North Am. 2010, 39, 401–418. [Google Scholar] [CrossRef] [PubMed]
- Nnoaham, K.E.; Clarke, A. Low serum vitamin D levels and tuberculosis, a systematic review and meta-analysis. Int. J. Epidemiol. 2008, 37, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Pencina, M.J.; Booth, S.L.; Jacques, P.F.; Ingelsson, E.; Lanier, K.; Benjamin, E.J.; D’Agostino, R.B.; Wolf, M.; Vasan, R.S. Vitamin D deficiency and risk of cardiovascular disease. Circulation 2008, 117, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Skaaby, T.; Husemoen, L.L.; Pisinger, C.; Jørgensen, T.; Thuesen, B.H.; Fenger, M.; Linneberg, A. Vitamin D status and chronic obstructive pulmonary disease: A prospective general population study. PLoS ONE 2014, 9, e90654. [Google Scholar] [CrossRef] [PubMed]
- Skaaby, T.; Husemoen, L.L.; Pisinger, C.; Jørgensen, T.; Thuesen, B.H.; Fenger, M.; Linneberg, A. Vitamin D status and cause-specific mortality: A general population study. PLoS ONE 2012, 7, e52423. [Google Scholar] [CrossRef] [PubMed]
- Janssens, W.; Bouillon, R.; Claes, B.; Carremans, C.; Lehouck, A.; Buysschaert, I.; Coolen, J.; Mathieu, C.; Decramer, M.; Lambrechts, D. Vitamin D deficiency is highly prevalent in COPD and correlates withvariants in the vitamin D-binding gene. Thorax 2010, 65, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Black, P.N.; Scragg, R. Relationship between serum 25-Hydroxyvitamin D and pulmonary function in the Third National Health and Nutrition Examination Survey. Chest 2005, 128, 3792–3798. [Google Scholar] [CrossRef] [PubMed]
- Pearce, S.H.; Cheetham, T.D. Diagnosis and management of vitamin D deficiency. BMJ 2010, 340. [Google Scholar] [CrossRef] [PubMed]
- Vieth, R.; Bischoff-Ferrari, H.; Boucher, B.J.; Dawson-Hughes, B.; Garland, C.F.; Heaney, R.P.; Holick, M.F.; Hollis, B.W.; Lamberg-Allardt, C.; McGrath, J.J.; et al. The urgent need to recommend an intake of vitamin D that is effective. Am. J. Clin. Nutr. 2007, 85, 649–650. [Google Scholar] [PubMed]
- Vieth, R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am. J. Nutr. 1999, 69, 842–856. [Google Scholar]
- Lehouck, A.; Mathieu, C.; Carremans, C.; Baeke, F.; Verhaegen, J.; Van Eldere, J.; Decallonne, B.; Bouillon, R.; Decramer, M.; Janssens, W. High doses vitamin D to reduce exacerbations in chronic obstructive pulmonary disease. Ann. Intern. Med. 2012, 156, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Anzueto, A. Impact of exacerbations on COPD. Eur. Respir. Rev. 2010, 19, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Ferrari, H.A.; Giovannucci, E.; Willett, W.C.; Dietrich, T.; Dawson-Hughes, B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am. J. Clin. Nutr. 2006, 84, 18–28. [Google Scholar] [PubMed]
- Vieth, R. Vitamin D toxicity, policy, and science. J Bone Miner. Res. 2007, 22, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Kunisaki, K.M.; Niewoehner, D.E.; Singh, R.J.; Connett, J.E. Vitamin D status and longitudinal lung function decline in the Lung Health Study. Eur. Respir. J. 2011, 37, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Witham, M.D.; Crighton, L.J.; Gillespie, N.D.; Struthers, A.D.; McMurdo, M.E. The effects of vitamin D supplementation on physical function and quality of life in older patients with heart failure: A randomized controlled trial. Circ. Heart Fail. 2010, 3, 195–201. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moosavi, S.A.J.; Shoushtari, M.H. The Effects of Vitamin D Supplementation on Pulmonary Function of Chronic Obstructive Pulmonary Disease Patients, before and after Clinical Trial. Diseases 2015, 3, 253-259. https://doi.org/10.3390/diseases3040253
Moosavi SAJ, Shoushtari MH. The Effects of Vitamin D Supplementation on Pulmonary Function of Chronic Obstructive Pulmonary Disease Patients, before and after Clinical Trial. Diseases. 2015; 3(4):253-259. https://doi.org/10.3390/diseases3040253
Chicago/Turabian StyleMoosavi, Seyed Ali Javad, and Maryam Haddadzadeh Shoushtari. 2015. "The Effects of Vitamin D Supplementation on Pulmonary Function of Chronic Obstructive Pulmonary Disease Patients, before and after Clinical Trial" Diseases 3, no. 4: 253-259. https://doi.org/10.3390/diseases3040253





