Menahydroquinone-4 Prodrug: A Promising Candidate Anti-Hepatocellular Carcinoma Agent
Abstract
:1. Introduction
2. MKH Delivery System in HCC Cells
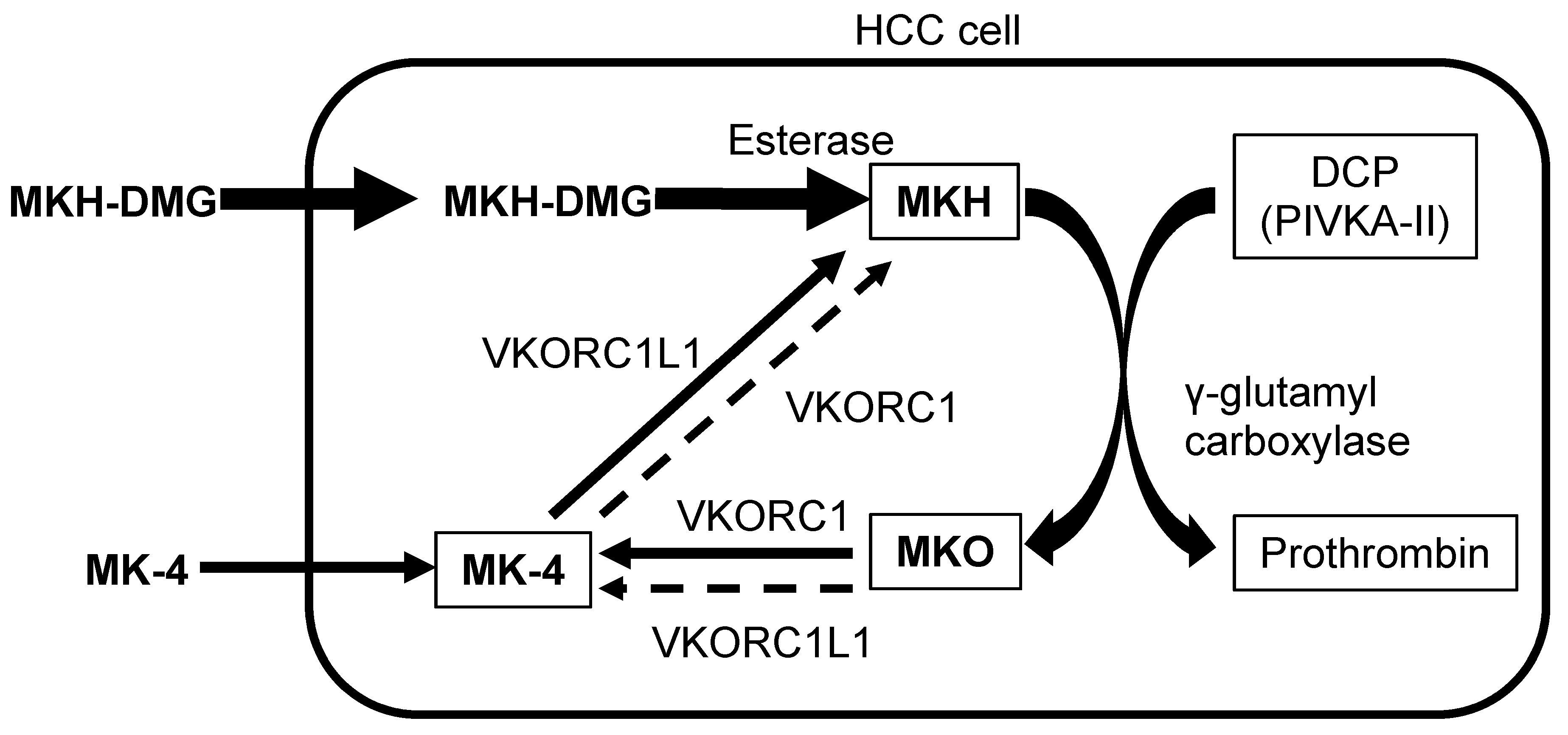
3. Inhibition of HCC Cell Proliferation by MKH-DMG
| Treatment | Inhibition of Cell Proliferation (in vitro) | MKH Delivery (in vitro) | Downregulation of DCP (in vitro) | HCC inhibition (in vivo) * |
|---|---|---|---|---|
| MKH-DMG (concentration) | obvious (20 μmol/L) | obvious (25 μmol/L) | obvious (10 μmol/L) | obvious (0.2 μmol/day) |
| MK-4 (concentration) | weak (40 μmol/L) | weak (25 μmol/L) | obvious (10 μmol/L) | - |
4. Effective MKH Delivery into HCC Cells by MKH-DMG
5. Downregulation of DCP by MKH-DMG
6. Induction of Cell-Cycle Arrest by MKH-DMG
7. Anti-Proliferation Effect of MKH-DMG in Vivo
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Huisse, M.G.; Leclercq, M.; Belghiti, J.; Flejou, J.F.; Suttie, J.W.; Bezeaud, A.; Stafford, D.W.; Guillin, M.C. Mechanism of the abnormal vitamin K-dependent gamma-carboxylation process in human hepatocellular carcinomas. Cancer 1994, 74, 1533–1541. [Google Scholar] [CrossRef]
- Li, Z.Q.; He, F.Y.; Stehle, C.J.; Wang, Z.; Kar, S.; Finn, F.M.; Carr, B.I. Vitamin K uptake in hepatocytes and hepatoma cells. Life Sci. 2002, 70, 2085–2100. [Google Scholar] [CrossRef]
- Orimo, H.; Shiraki, M.; Tomita, A.; Morii, H.; Fujita, T.; Ohata, M. Effects of menatetrenone on the bone and calcium metabolism in osteoporosis: A double-blind placebo-controlled study. J. Bone Miner. Metable 1998, 16, 106–112. [Google Scholar] [CrossRef]
- Shiraki, M.; Shiraki, Y.; Aoki, C.; Miura, M. Vitamin K2 (menatetrenone) effectively prevents fractures and sustains lumbar bone mineral density in osteoporosis. J. Bone Miner. Res. 2000, 15, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Knapen, M.H.; Schurgers, L.J.; Vermeer, C. Vitamin K2 supplementation improves hip bone geometry and bone strength indices in postmenopausal women. Osteoporos. Int. 2007, 18, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Fujita, T.; Kishimoto, H.; Makino, T.; Nakamura, T.; Nakamura, T.; Sato, T.; Yamazaki, K. Randomized controlled study on the prevention of osteoporotic fractures (OF study): A phase IV clinical study of 15-mg menatetrenone capsules. J. Bone Miner. Metable 2009, 27, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Bouzahzah, B.; Nishikawa, Y.; Simon, D.; Carr, B.I. Growth control and gene expression in a new hepatocellular carcinoma cell line, Hep40: Inhibitory actions of vitamin K. J. Cell. Physiol. 1995, 165, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, M.; Finn, F.; Carr, B.I. The growth inhibitory effects of vitamins K and their actions on gene expression. Hepatology 1995, 22, 876–882. [Google Scholar] [PubMed]
- Otsuka, M.; Kato, N.; Shao, R.X.; Hoshida, Y.; Ijichi, H.; Koike, Y.; Taniguchi, H.; Moriyama, M.; Shiratori, Y.; Kawabe, T.; et al. Vitamin K2 inhibits the growth and invasiveness of hepatocellular carcinoma cells via protein kinase A activation. Hepatology 2004, 40, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, I.; Zhang, H.; Mizuta, T.; Ide, Y.; Eguchi, Y.; Yasutake, T.; Sakamaki, T.; Pestell, R.G.; Yamamoto, K. Menatetrenone, a vitamin K2 analogue, inhibits hepatocellular carcinoma cell growth by suppressing cyclin D1 expression through inhibition of nuclear factor kappaB activation. Clin. Cancer Res. 2007, 13, 2236–2245. [Google Scholar] [CrossRef] [PubMed]
- Kaneda, M.; Zhang, D.; Bhattacharjee, R.; Nakahama, K.; Arii, S.; Morita, I. Vitamin K2 suppresses malignancy of HuH7 hepatoma cells via inhibition of connexin 43. Cancer Lett. 2008, 263, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Hitomi, M.; Yokoyama, F.; Kita, Y.; Nonomura, T.; Masaki, T.; Yoshiji, H.; Inoue, H.; Kinekawa, F.; Kurokohchi, K.; Uchida, N.; et al. Antitumor effects of vitamins K1, K2 and K3 on hepatocellular carcinoma in vitro and in vivo. Int. J. Oncol. 2005, 26, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Habu, D.; Shiomi, S.; Tamori, A.; Takeda, T.; Tanaka, T.; Kubo, S.; Nishiguchi, S. Role of vitamin K2 in the development of hepatocellular carcinoma in women with viral cirrhosis of the liver. Jama 2004, 292, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Mizuta, T.; Ozaki, I.; Eguchi, Y.; Yasutake, T.; Kawazoe, S.; Fujimoto, K.; Yamamoto, K. The effect of menatetrenone, a vitamin K2 analog, on disease recurrence and survival in patients with hepatocellular carcinoma after curative treatment: A pilot study. Cancer 2006, 106, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Shiratori, Y.; Kudo, M.; Shiina, S.; Mizuta, T.; Kojiro, M.; Yamamoto, K.; Koike, Y.; Saito, K.; Koyanagi, N.; et al. Effect of vitamin K2 on the recurrence of hepatocellular carcinoma. Hepatology 2011, 54, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Riaz, I.B.; Riaz, H.; Riaz, T.; Rahman, S.; Amir, M.; Badshah, M.B.; Kazi, A.N. Role of vitamin K2 in preventing the recurrence of hepatocellular carcinoma after curative treatment: A meta-analysis of randomized controlled trials. BMC Gastroenterol. 2012, 12. [Google Scholar] [CrossRef] [PubMed]
- Takata, J.; Karube, Y.; Hanada, M.; Matsunaga, K.; Matsushima, Y.; Sendo, T.; Aoyama, T. Vitamin K prodrugs: 1. Synthesis of amino acid esters of menahydroquinone-4 and enzymatic reconversion to an active form. Pharm. Res. 1995, 12, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Takata, J.; Karube, Y.; Hanada, M.; Matsunaga, K.; Matsushima, Y.; Sendo, T.; Oishi, R. Vitamin K prodrugs: 2. water-soluble prodrugs of menahydroquinone-4 for systemic site-specific delivery. Pharm. Res. 1995, 12, 1973–1979. [Google Scholar] [CrossRef] [PubMed]
- Setoguchi, S.; Watase, D.; Matsunaga, K.; Matsubara, M.; Kubo, Y.; Kusuda, M.; Nagata-Akaho, N.; Enjoji, M.; Nakashima, M.; Takeshita, M.; et al. Enhanced antitumor effects of novel intracellular delivery of an active form of menaquinone-4, menahydroquinone-4, into hepatocellular carcinoma. Cancer Prev. Res. 2015, 8, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Koike, Y.; Shiratori, Y.; Sato, S.; Obi, S.; Teratani, T.; Imamura, M.; Yoshida, H.; Shiina, S.; Omata, M. Des-gamma-carboxy prothrombin as a useful predisposing factor for the development of portal venous invasion in patients with hepatocellular carcinoma: A prospective analysis of 227 patients. Cancer 2001, 91, 561–569. [Google Scholar] [CrossRef]
- Weitz, I.C.; Liebman, H.A. Des-gamma-carboxy (abnormal) prothrombin and hepatocellular carcinoma: A critical review. Hepatology 1993, 18, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, Y.; Tang, W.; Makuuchi, M.; Hasegawa, K.; Sugawara, Y.; Kokudo, N. Clinical and molecular insights into the hepatocellular carcinoma tumour marker des-gamma-carboxyprothrombin. Liver Int. 2011, 31, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Shiraha, H.; Fujikawa, T.; Takaoka, N.; Ueda, N.; Nakanishi, Y.; Koike, K.; Takaki, A.; Shiratori, Y. Des-gamma-carboxy prothrombin is a potential autologous growth factor for hepatocellular carcinoma. J. Biol. Chem. 2005, 280, 6409–6415. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Qu, X.J.; Mu, G.Y.; Chen, M.H.; Cheng, Y.N.; Kokudo, N.; Tang, W.; Cui, S.X. Vitamin K2 inhibits the growth of hepatocellular carcinoma via decrease of des-gamma-carboxy prothrombin. Chemotherapy 2009, 55, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Yue, P.; Gao, Z.H.; Xue, X.; Cui, S.X.; Zhao, C.R.; Yuan, Y.; Yin, Z.; Inagaki, Y.; Kokudo, N.; Tang, W.; et al. Des-gamma-carboxyl prothrombin induces matrix metalloproteinase activity in hepatocellular carcinoma cells by involving the ERK1/2 MAPK signalling pathway. Eur. J. Cancer 2011, 47, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, M.; Shiraha, H.; Kataoka, J.; Iwamuro, M.; Horiguchi, S.; Nishina, S.; Takaoka, N.; Uemura, M.; Takaki, A.; Nakamura, S.; et al. Des-gamma-carboxyl prothrombin is associated with tumor angiogenesis in hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2012, 27, 1602–1608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.J.; Feng, X.B.; Inagaki, Y.; Song, P.P.; Kokudo, N.; Hasegawa, K.; Sugawara, Y.; Tang, W. Des-gamma-carboxy prothrombin and c-Met were concurrently and extensively expressed in hepatocellular carcinoma and associated with tumor recurrence. Biosci. Trends 2012, 6, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Okuda, H.; Obata, H.; Nakanishi, T.; Furukawa, R.; Hashimoto, E. Production of abnormal prothrombin (des-gamma-carboxy prothrombin) by hepatocellular carcinoma. A clinical and experimental study. J. Hepatol. 1987, 4, 357–363. [Google Scholar]
- Furukawa, M.; Nakanishi, T.; Okuda, H.; Ishida, S.; Obata, H. Changes of plasma des-gamma-carboxy prothrombin levels in patients with hepatocellular carcinoma in response to vitamin K. Cancer 1992, 69, 31–38. [Google Scholar] [CrossRef]
- Murata, K.; Suzuki, H.; Okano, H.; Oyamada, T.; Yasuda, Y.; Sakamoto, A. Cytoskeletal changes during epithelial-to-fibroblastoid conversion as a crucial mechanism of des-gamma-carboxy prothrombin production in hepatocellular carcinoma. Int. J. Oncol. 2009, 35, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Binkley, N.C.; Suttie, J.W. Vitamin K nutrition and osteoporosis. J. Nutr. 1995, 125, 1812–1821. [Google Scholar] [PubMed]
- Furie, B.; Bouchard, B.A.; Furie, B.C. Vitamin K-dependent biosynthesis of gamma-carboxyglutamic acid. Blood 1999, 93, 1798–1808. [Google Scholar] [PubMed]
- Stafford, D.W. The vitamin K cycle. J. Thromb. Haemost. 2005, 3, 1873–1878. [Google Scholar] [CrossRef] [PubMed]
- Hallgren, K.W.; Qian, W.; Yakubenko, A.V.; Runge, K.W.; Berkner, K.L. r-VKORC1 expression in factor IX BHK cells increases the extent of factor IX carboxylation but is limited by saturation of another carboxylation component or by a shift in the rate-limiting step. Biochemistry 2006, 45, 5587–5598. [Google Scholar] [CrossRef] [PubMed]
- Westhofen, P.; Watzka, M.; Marinova, M.; Hass, M.; Kirfel, G.; Müller, J.; Bevans, C.G.; Müller, C.R.; Oldenburg, J. Human vitamin K 2,3-epoxide reductase complex subunit 1-like 1 (VKORC1L1) mediates vitamin K-dependent intracellular antioxidant function. J. Biol. Chem. 2011, 286, 15085–15094. [Google Scholar] [CrossRef] [PubMed]
- Ishii, M.; Shimomura, M.; Hasegawa, J.; Asano, Y.; Yamato, T.; Yamano, Y.; Kayano, M.; Kanemaru, M.; Nakashima, M. Multiple Dose Pharmacokinetic Study of Soft Gelatin Capsule of Menatetrenone (Ea-0167) in Elderly and Young Volunteers. Jpn. Pharmacol. Ther. 1995, 23, 2637–2642. [Google Scholar]
- Sano, Y.; Tadano, K.; Kaneko, K.; Kikuchi, K.; Yuzuriha, T. Metabolic Fate of Menatetrenone in Rats: Absorption, Distribution, Metabolism and Excretion after a Single Oral Administration. Jpn. Pharmacol. Ther. 1995, 23, 2659–2667. [Google Scholar]
- Karin, M.; Lin, A. NF-kappaB at the crossroads of life and death. Nat. Immunol. 2002, 3, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Hawke, N.; Baldwin, A.S. NF-kappaB and IKK as therapeutic targets in cancer. Cell Death Differ. 2006, 13, 738–47. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, C.; Prendergast, G.C.; Pestell, R.G. Cyclin D1 functions in cell migration. Cell Cycle 2006, 5, 2440–2442. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enjoji, M.; Watase, D.; Matsunaga, K.; Kusuda, M.; Nagata-Akaho, N.; Karube, Y.; Takata, J. Menahydroquinone-4 Prodrug: A Promising Candidate Anti-Hepatocellular Carcinoma Agent. Diseases 2015, 3, 150-158. https://doi.org/10.3390/diseases3030150
Enjoji M, Watase D, Matsunaga K, Kusuda M, Nagata-Akaho N, Karube Y, Takata J. Menahydroquinone-4 Prodrug: A Promising Candidate Anti-Hepatocellular Carcinoma Agent. Diseases. 2015; 3(3):150-158. https://doi.org/10.3390/diseases3030150
Chicago/Turabian StyleEnjoji, Munechika, Daisuke Watase, Kazuhisa Matsunaga, Mariko Kusuda, Nami Nagata-Akaho, Yoshiharu Karube, and Jiro Takata. 2015. "Menahydroquinone-4 Prodrug: A Promising Candidate Anti-Hepatocellular Carcinoma Agent" Diseases 3, no. 3: 150-158. https://doi.org/10.3390/diseases3030150





