Protein Kinase C Epsilon Contributes to NADPH Oxidase Activation in a Pre-Eclampsia Lymphoblast Cell Model
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Cell Culture
2.3. Measurement of ROS Production
2.4. Cell Treatments, Lysis, Immunoprecipitation, Fractionation and SDS-PAGE
2.5. P47-Phox Kinase Assays
2.6. Fractionation of B-Lymphoblast Cytosol
2.7. Saponin Permeabilization and Inhibition of PKCε
2.8. Data Analysis
3. Results and Discussion
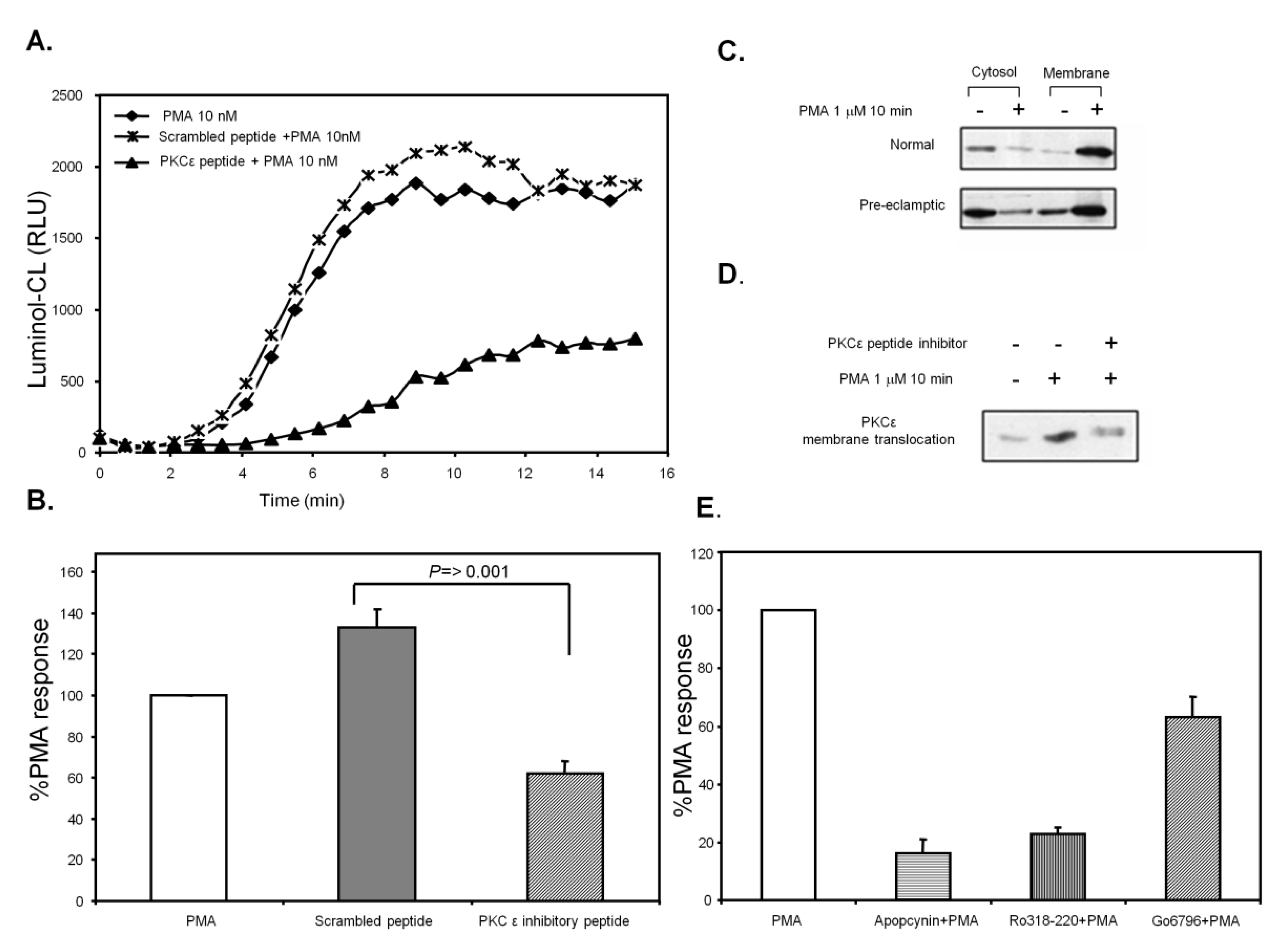
3.1. ROS Production is Increased in PMA-Stimulated Pre-Eclamptic B-LCLs
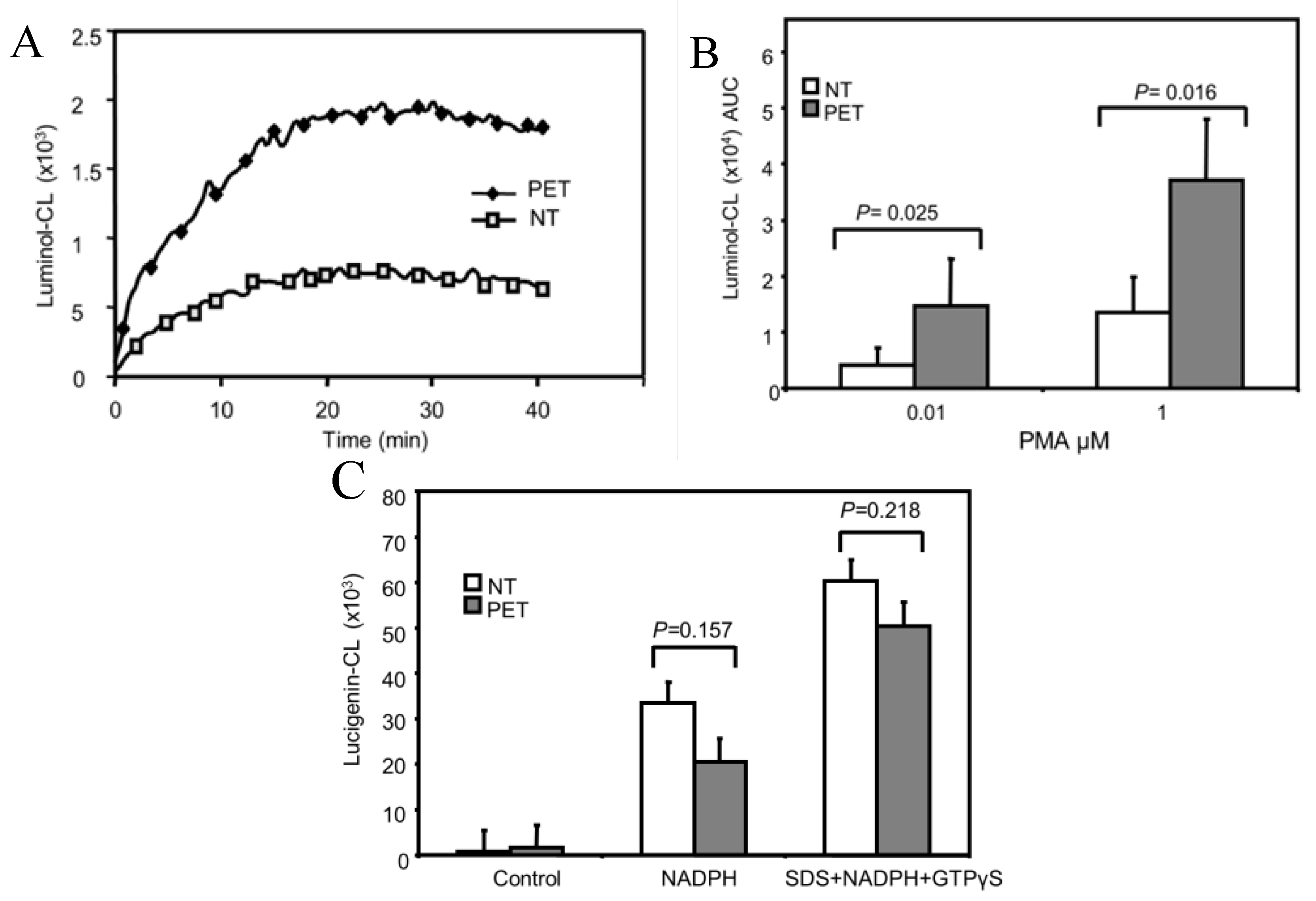
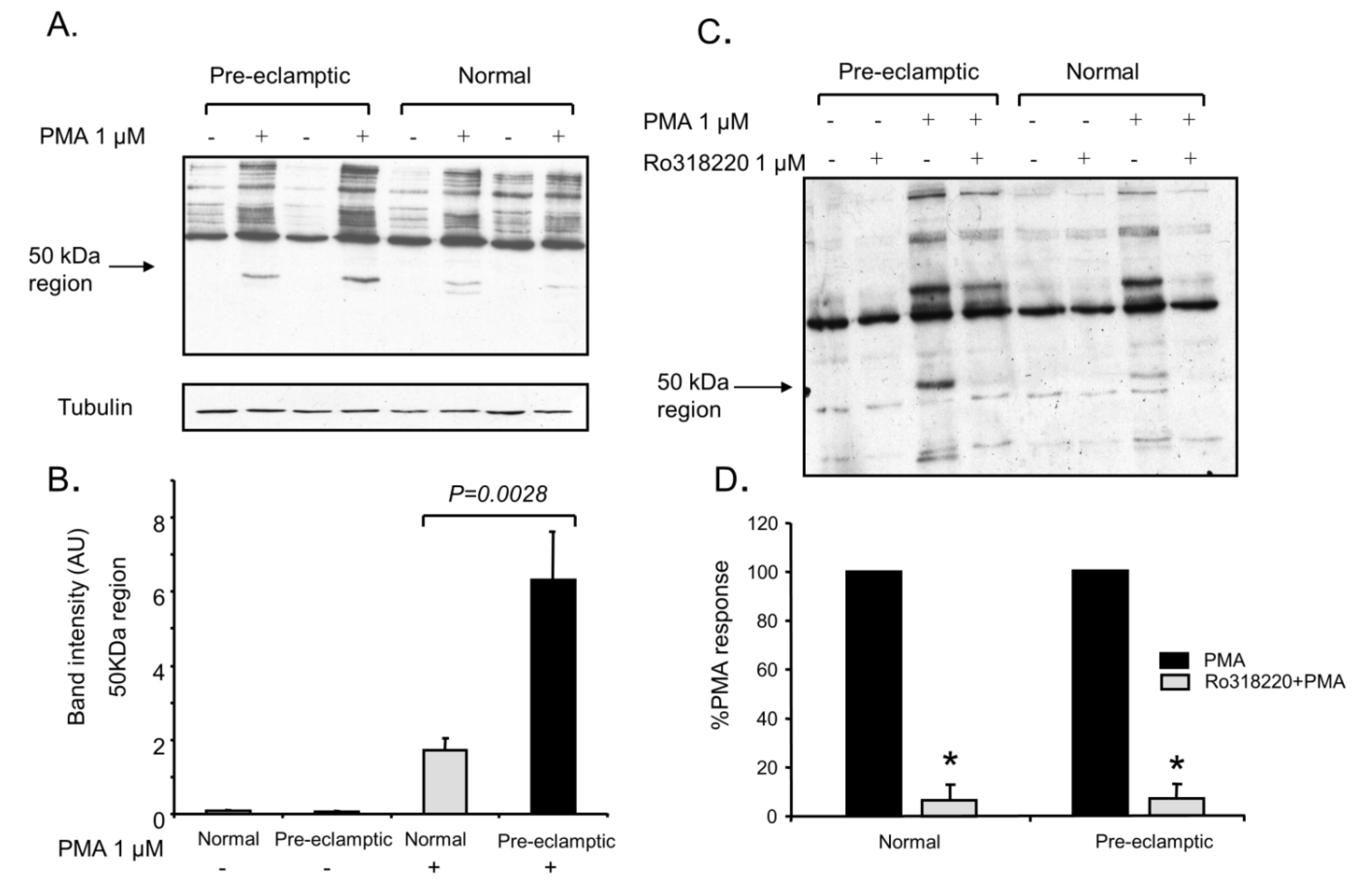
3.2. Increased Kinase Activity and P47-Phox Phosphorylation in B-LCLs
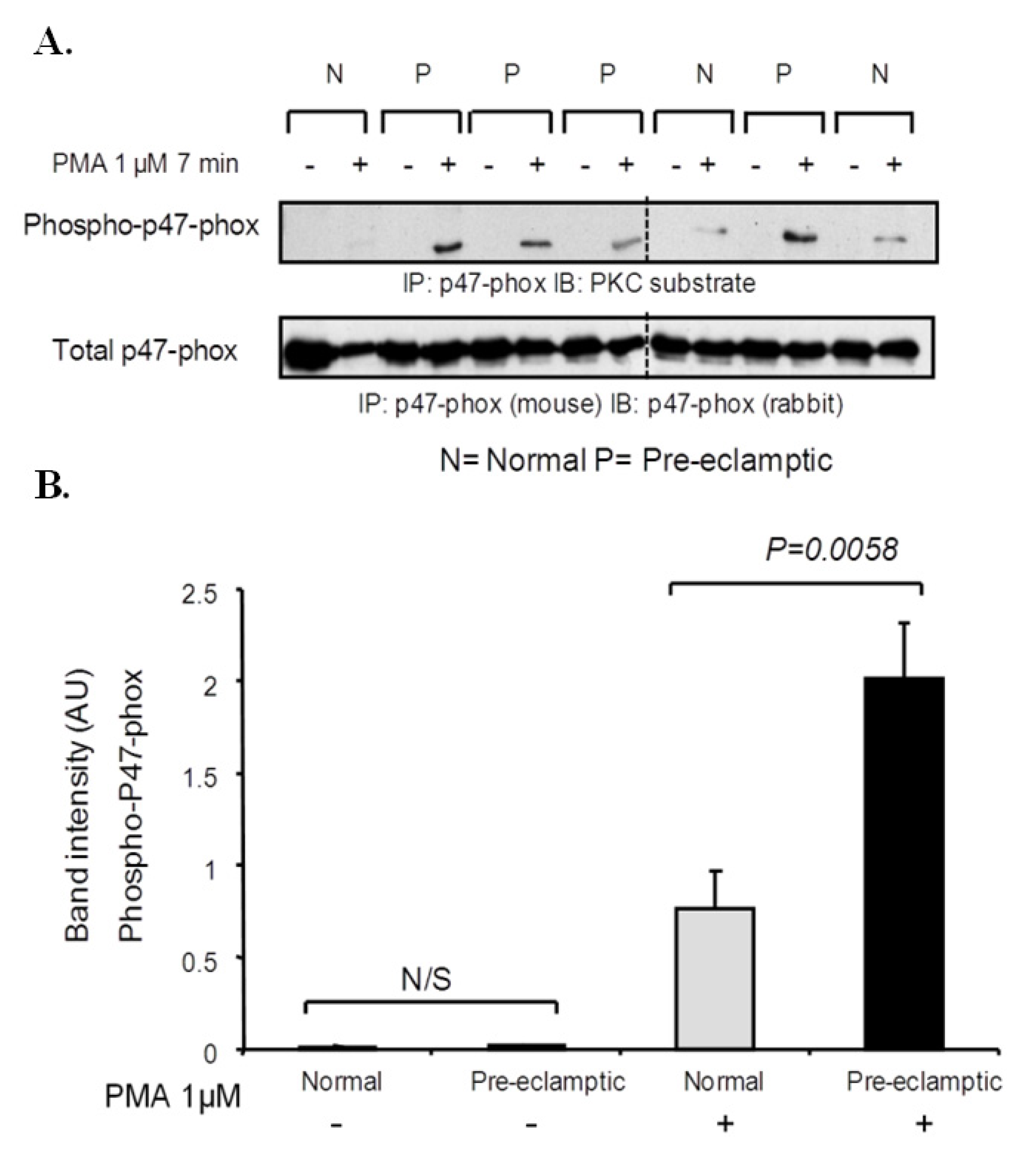
3.3. P47-Phox Phosphorylation Is Increased in Pre-Eclamptic B-LCLs
3.4. PKC Expression Is Unchanged between Normal and Pre-Eclamptic B-LCLs
3.5. P47-Phox Kinase Activity Is Increased in Pre-Eclamptic B-LCLs

3.6. Analysis of P47-Phox Kinase Activity in Pre-Eclamptic B-LCLs
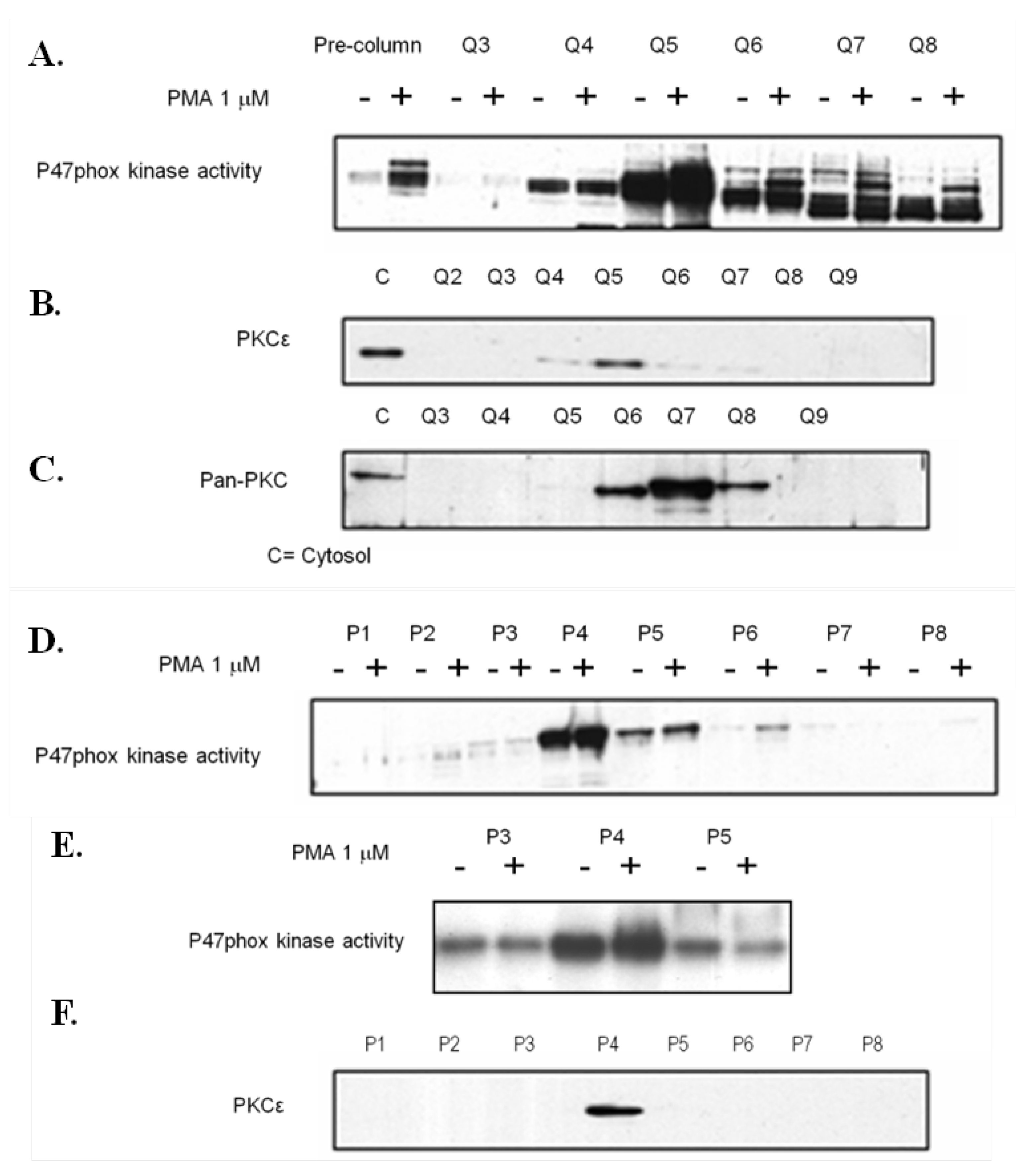
3.7. PKCε Activation in Pre-Eclamptic B-LCLs
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Roberts, J.M.; Gammill, H.S. Preeclampsia: Recent insights. Hypertension 2005, 46, 1243–1249. [Google Scholar] [CrossRef]
- Maynard, S.E.; Min, J.Y.; Merchan, J.; Lim, K.H.; Li, J.; Mondal, S.; Libermann, T.A.; Morgan, J.P.; Sellke, F.W.; Stillman, I.E.; et al. Excess placental soluble fms-like tyrosine kinase 1 (sflt1) may contribute to endothelial dysfunction, Hypertension, and proteinuria in preeclampsia. J. Clin. Invest. 2003, 111, 649–658. [Google Scholar]
- Redman, C.W.; Sacks, G.P.; Sargent, I.L. Preeclampsia: An excessive maternal inflammatory response to pregnancy. Am. J. Obstet. Gynecol. 1999, 180, 499–506. [Google Scholar] [CrossRef]
- Lee, V.M.; Quinn, P.A.; Jennings, S.C.; Ng, L.L. Neutrophil activation and production of reactive oxygen species in pre-eclampsia. J. Hypertens. 2003, 21, 395–402. [Google Scholar] [CrossRef]
- Young, B.C.; Levine, R.J.; Karumanchi, S.A. Pathogenesis of preeclampsia. Ann. Rev. Pathol. 2010, 5, 173–192. [Google Scholar] [CrossRef]
- Dang, P.M.; Dewas, C.; Gaudry, M.; Fay, M.; Pedruzzi, E.; Gougerot-Pocidalo, M.A.; El Benna, J. Priming of human neutrophil respiratory burst by granulocyte/macrophage colony-stimulating factor (gm-csf) involves partial phosphorylation of p47-phox. J. Biol. Chem. 1999, 274, 20704–20708. [Google Scholar]
- Dang, P.M.; Morel, F.; Gougerot-Pocidalo, M.A.; Benna, J.E. Phosphorylation of the nadph oxidase component p67-phox by erk2 and p38mapk: Selectivity of phosphorylated sites and existence of an intramolecular regulatory domain in the tetratricopeptide-rich region. Biochemistry 2003, 42, 4520–4526. [Google Scholar] [CrossRef]
- Reeves, E.P.; Dekker, L.V.; Forbes, L.V.; Wientjes, F.B.; Grogan, A.; Pappin, D.J.; Segal, A.W. Direct interaction between p47phox and protein kinase C: Evidence for targeting of protein kinase C by p47phox in neutrophils. Biochem. J. 1999, 344, 859–866. [Google Scholar] [CrossRef]
- Hoyal, C.R.; Gutierrez, A.; Young, B.M.; Catz, S.D.; Lin, J.H.; Tsichlis, P.N.; Babior, B.M. Modulation of p47phox activity by site-specific phosphorylation: Akt-dependent activation of the nadph oxidase. Proc. Natl. Acad. Sci. USA 2003, 100, 5130–5135. [Google Scholar] [CrossRef]
- Lee, V.M.; Quinn, P.A.; Jennings, S.C.; Ng, L.L. Nadph oxidase activity in preeclampsia with immortalized lymphoblasts used as models. Hypertension 2003, 41, 925–931. [Google Scholar] [CrossRef]
- Poolman, T.M.; Ng, L.L.; Farmer, P.B.; Manson, M.M. Inhibition of the respiratory burst by resveratrol in human monocytes: Correlation with inhibition of pi3k signaling. Free Radic. Biol. Med. 2005, 39, 118–132. [Google Scholar] [CrossRef]
- Kitada, M.; Koya, D.; Sugimoto, T.; Isono, M.; Araki, S.; Kashiwagi, A.; Haneda, M. Translocation of glomerular p47phox and p67phox by protein kinase C-beta activation is required for oxidative stress in diabetic nephropathy. Diabetes 2003, 52, 2603–2614. [Google Scholar] [CrossRef]
- Bohlen, P.; Stein, S.; Dairman, W.; Udenfriend, S. Fluorometric assay of proteins in the nanogram range. Arch. Biochem. Biophys. 1973, 155, 213–220. [Google Scholar] [CrossRef]
- Cachia, O.; Benna, J.E.; Pedruzzi, E.; Descomps, B.; Gougerot-Pocidalo, M.A.; Leger, C.L. Alpha-tocopherol inhibits the respiratory burst in human monocytes. Attenuation of p47-phox membrane translocation and phosphorylation. J. Biol. Chem. 1998, 273, 32801–32805. [Google Scholar]
- Uhlinger, D.J.; Inge, K.L.; Kreck, M.L.; Tyagi, S.R.; Neckelmann, N.; Lambeth, J.D. Reconstitution and characterization of the human neutrophil respiratory burst oxidase using recombinant p47-phox, p67-phox and plasma membrane. Biochem. Biophys. Res. Commun. 1992, 186, 509–516. [Google Scholar] [CrossRef]
- Palmer, R.H.; Dekker, L.V.; Woscholski, R.; Le Good, J.A.; Gigg, R.; Parker, P.J. Activation of prk1 by phosphatidylinositol 4,5-bisphosphate and phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 1995, 270, 22412–22416. [Google Scholar]
- Ng, L.L.; O'Brien, R.J.; Demme, B.; Jennings, S. Non-competitive immunochemiluminometric assay for cardiotrophin-1 detects elevated plasma levels in human heart failure. Clin. Sci. 2002, 102, 411–416. [Google Scholar] [CrossRef]
- Ohira, T.; Zhan, Q.; Ge, Q.; VanDyke, T.; Badwey, J.A. Protein phosphorylation in neutrophils monitored with phosphospecific antibodies. J. Immunol. Methods 2003, 281, 79–94. [Google Scholar] [CrossRef]
- Martiny-Baron, G.; Kazanietz, M.G.; Mischak, H.; Blumberg, P.M.; Kochs, G.; Hug, H.; Marme, D.; Schachtele, C. Selective inhibition of protein kinase C isozymes by the indolocarbazole go 6976. J. Biol. Chem. 1993, 268, 9194–9197. [Google Scholar]
- Mellembakken, J.R.; Aukrust, P.; Olafsen, M.K.; Ueland, T.; Hestdal, K.; Videm, V. Activation of leukocytes during the uteroplacental passage in preeclampsia. Hypertension 2002, 39, 155–160. [Google Scholar] [CrossRef]
- Schmid-Schonbein, G.W. The damaging potential of leukocyte activation in the microcirculation. Angiology 1993, 44, 45–56. [Google Scholar] [CrossRef]
- Tsukimori, K.; Maeda, H.; Ishida, K.; Nagata, H.; Koyanagi, T.; Nakano, H. The superoxide generation of neutrophils in normal and preeclamptic pregnancies. Obstet. Gynecol. 1993, 81, 536–540. [Google Scholar]
- Tsukimori, K.; Fukushima, K.; Tsushima, A.; Nakano, H. Generation of reactive oxygen species by neutrophils and endothelial cell injury in normal and preeclamptic pregnancies. Hypertension 2005, 46, 696–700. [Google Scholar] [CrossRef]
- Ferreira, J.C.; Brum, P.C.; Mochly-Rosen, D. Βiipkc and εpkc isozymes as potential pharmacological targets in cardiac hypertrophy and heart failure. J. Mol. Cell. Cardiol. 2011, 51, 479–484. [Google Scholar] [CrossRef]
- Takeishi, Y.; Ping, P.; Bolli, R.; Kirkpatrick, D.L.; Hoit, B.D.; Walsh, R.A. Transgenic overexpression of constitutively active protein kinase C epsilon causes concentric cardiac hypertrophy. Circ. Res. 2000, 86, 1218–1223. [Google Scholar] [CrossRef]
- Inagaki, K.; Koyanagi, T.; Berry, N.C.; Sun, L.; Mochly-Rosen, D. Pharmacological inhibition of epsilon-protein kinase C attenuates cardiac fibrosis and dysfunction in hypertension-induced heart failure. Hypertension 2008, 51, 1565–1569. [Google Scholar] [CrossRef]
- Melchiorre, K.; Sutherland, G.R.; Liberati, M.; Thilaganathan, B. Preeclampsia is associated with persistent postpartum cardiovascular impairment. Hypertension 2011, 58, 709–715. [Google Scholar] [CrossRef]
- Karima, M.; Kantarci, A.; Ohira, T.; Hasturk, H.; Jones, V.L.; Nam, B.-H.; Malabanan, A.; Trackman, P.C.; Badwey, J.A.; van Dyke, T.E. Enhanced superoxide release and elevated protein kinase C activity in neutrophils from diabetic patients: Association with periodontitis. J. Leukocyte Biol. 2005, 78, 862–870. [Google Scholar] [CrossRef]
- El Benna, J.; Hayem, G.; Dang, P.M.; Fay, M.; Chollet-Martin, S.; Elbim, C.; Meyer, O.; Gougerot-Pocidalo, M.A. Nadph oxidase priming and p47phox phosphorylation in neutrophils from synovial fluid of patients with rheumatoid arthritis and spondylarthropathy. Inflammation 2002, 26, 273–278. [Google Scholar] [CrossRef]
- Dang, P.M.; Stensballe, A.; Boussetta, T.; Raad, H.; Dewas, C.; Kroviarski, Y.; Hayem, G.; Jensen, O.N.; Gougerot-Pocidalo, M.A.; El-Benna, J. A specific p47phox -serine phosphorylated by convergent mapks mediates neutrophil nadph oxidase priming at inflammatory sites. J. Clin. Invest. 2006, 116, 2033–2043. [Google Scholar] [CrossRef]
- Faust, L.R.; el Benna, J.; Babior, B.M.; Chanock, S.J. The phosphorylation targets of p47phox, a subunit of the respiratory burst oxidase. Functions of the individual target serines as evaluated by site-directed mutagenesis. J. Clin. Invest. 1995, 96, 1499–1505. [Google Scholar] [CrossRef]
- Inanami, O.; Johnson, J.L.; McAdara, J.K.; Benna, J.E.; Faust, L.R.; Newburger, P.E.; Babior, B.M. Activation of the leukocyte nadph oxidase by phorbol ester requires the phosphorylation of p47phox on serine 303 or 304. J. Biol. Chem. 1998, 273, 9539–9543. [Google Scholar]
- Johnson, J.L.; Park, J.W.; Benna, J.E.; Faust, L.P.; Inanami, O.; Babior, B.M. Activation of p47-phox, a cytosolic subunit of the leukocyte nadph oxidase. Phosphorylation of ser-359 or ser-370 precedes phosphorylation at other sites and is required for activity. J. Biol. Chem. 1998, 273, 35147–35152. [Google Scholar]
- Fontayne, A.; Dang, P.M.; Gougerot-Pocidalo, M.A.; El-Benna, J. Phosphorylation of p47phox sites by pkc alpha, beta ii, delta, and zeta: Effect on binding to p22phox and on nadph oxidase activation. Biochemistry 2002, 41, 7743–7750. [Google Scholar] [CrossRef]
- Bey, E.A.; Xu, B.; Bhattacharjee, A.; Oldfield, C.M.; Zhao, X.; Li, Q.; Subbulakshmi, V.; Feldman, G.M.; Wientjes, F.B.; Cathcart, M.K. Protein kinase C delta is required for p47phox phosphorylation and translocation in activated human monocytes. J. Immunol. 2004, 173, 5730–5738. [Google Scholar]
- Nishikawa, K.; Toker, A.; Johannes, F.J.; Songyang, Z.; Cantley, L.C. Determination of the specific substrate sequence motifs of protein kinase C isozymes. J. Biol. Chem. 1997, 272, 952–960. [Google Scholar] [CrossRef]
- Johnson, J.A.; Gray, M.O.; Chen, C.H.; Mochly-Rosen, D. A protein kinase C translocation inhibitor as an isozyme-selective antagonist of cardiac function. J. Biol. Chem. 1996, 271, 24962–24966. [Google Scholar] [CrossRef]
- Palaniyandi, S.S.; Inagaki, K.; Mochly-Rosen, D. Mast cells and epsilonpkc: A role in cardiac remodeling in hypertension-induced heart failure. J. Mol. Cell. Cardiol. 2008, 45, 779–786. [Google Scholar] [CrossRef]
- Murriel, C.L.; Mochly-Rosen, D. Opposing roles of [delta] and [var epsilon]pkc in cardiac ischemia and reperfusion: Targeting the apoptotic machinery. Arch. Biochem. Biophys. 2003, 420, 246–254. [Google Scholar] [CrossRef]
- Ping, P.; Song, C.; Zhang, J.; Guo, Y.; Cao, X.; Li, R.C.; Wu, W.; Vondriska, T.M.; Pass, J.M.; Tang, X.L.; et al. Formation of protein kinase C(epsilon)-lck signaling modules confers cardioprotection. J. Clin. Invest. 2002, 109, 499–507. [Google Scholar]
- Haller, H.; Hempel, A.; Homuth, V.; Mandelkow, A.; Busjahn, A.; Maasch, C.; Drab, M.; Lindschau, C.; Jupner, A.; Vetter, K. Endothelial-cell permeability and protein kinase C in pre-eclampsia. The Lancet 1998, 351, 945–949. [Google Scholar]
- Rask-Madsen, C.; King, G.L. Differential regulation of vegf signaling by pkc-alpha and pkc-epsilon in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 919–924. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Poolman, T.M.; Quinn, P.A.; Ng, L. Protein Kinase C Epsilon Contributes to NADPH Oxidase Activation in a Pre-Eclampsia Lymphoblast Cell Model. Diseases 2013, 1, 1-17. https://doi.org/10.3390/diseases1010001
Poolman TM, Quinn PA, Ng L. Protein Kinase C Epsilon Contributes to NADPH Oxidase Activation in a Pre-Eclampsia Lymphoblast Cell Model. Diseases. 2013; 1(1):1-17. https://doi.org/10.3390/diseases1010001
Chicago/Turabian StylePoolman, Toryn M., Paulene A. Quinn, and Leong Ng. 2013. "Protein Kinase C Epsilon Contributes to NADPH Oxidase Activation in a Pre-Eclampsia Lymphoblast Cell Model" Diseases 1, no. 1: 1-17. https://doi.org/10.3390/diseases1010001



