The Diabetes Assistant: A Smartphone-Based System for Real-Time Control of Blood Glucose
Abstract
:1. Introduction
- Readily available at low-cost;
- Suitable for ambulatory use and computationally capable of running closed-loop control algorithms;
- Wirelessly connectable to CGM devices and insulin pumps; and
- Capable of broadband communication with a central location for data collection, remote monitoring and safety supervision.
2. The DiAs AP System: A Mobile Medical Network

2.1. Graphical User Interface
- Bluetooth status
- System operating mode (Pump, Closed Loop, Safety, Sensor, Stopped)
- Most recent CGM value and trend
- CGM device status
- Pump device status
- Connectivity with cloud monitoring services
- Network data link status
- Smartphone battery level
- System time
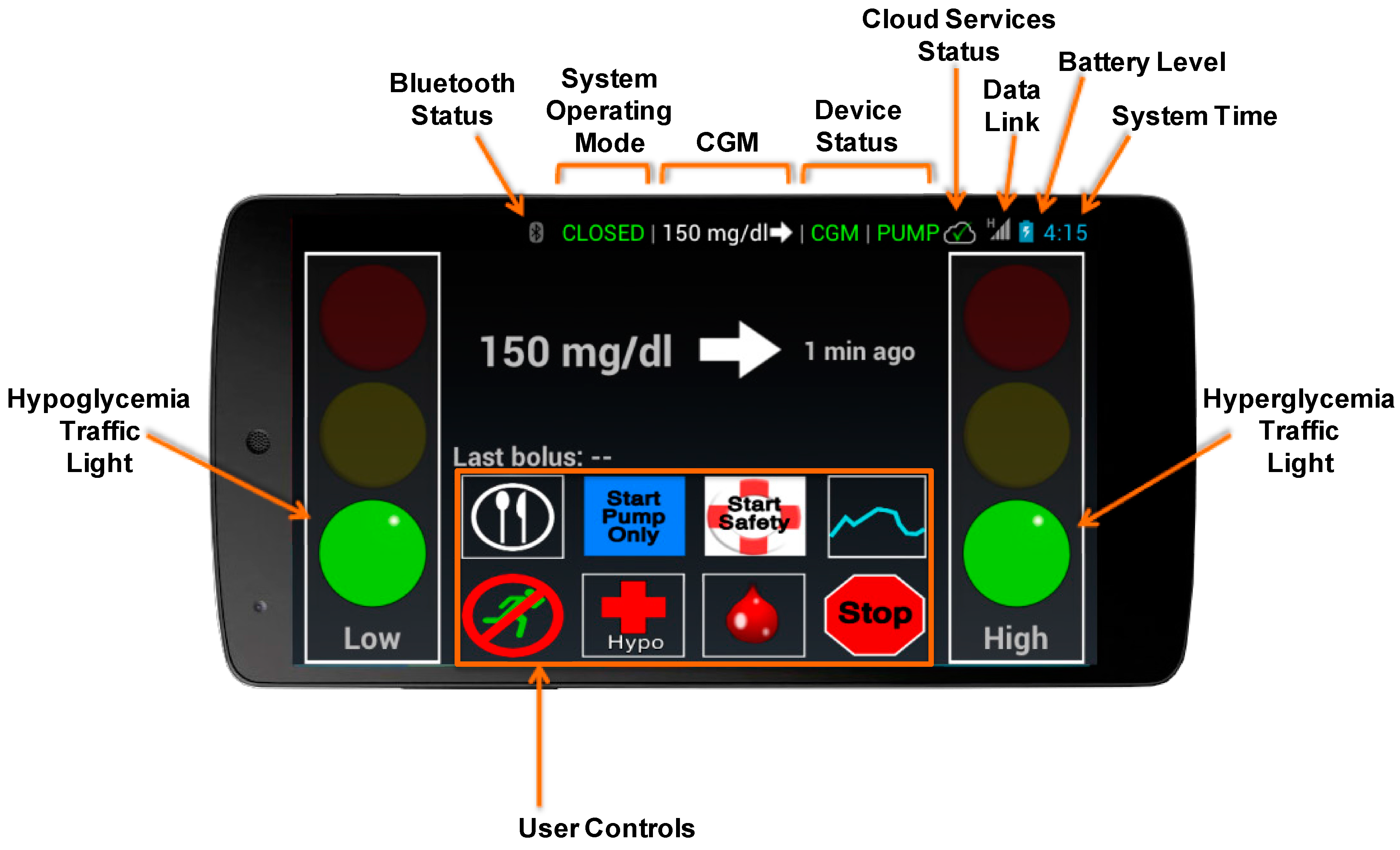
- Green—No predicted risk of hypo/hyperglycemia, no user action required.
- Yellow—Some risk of hypo/hyperglycemia, DiAs taking action if in Closed Loop or Safety mode, no user action required.
- Red—Immediate risk of hypo/hyperglycemia, immediate user action required.
2.2. Software Architecture
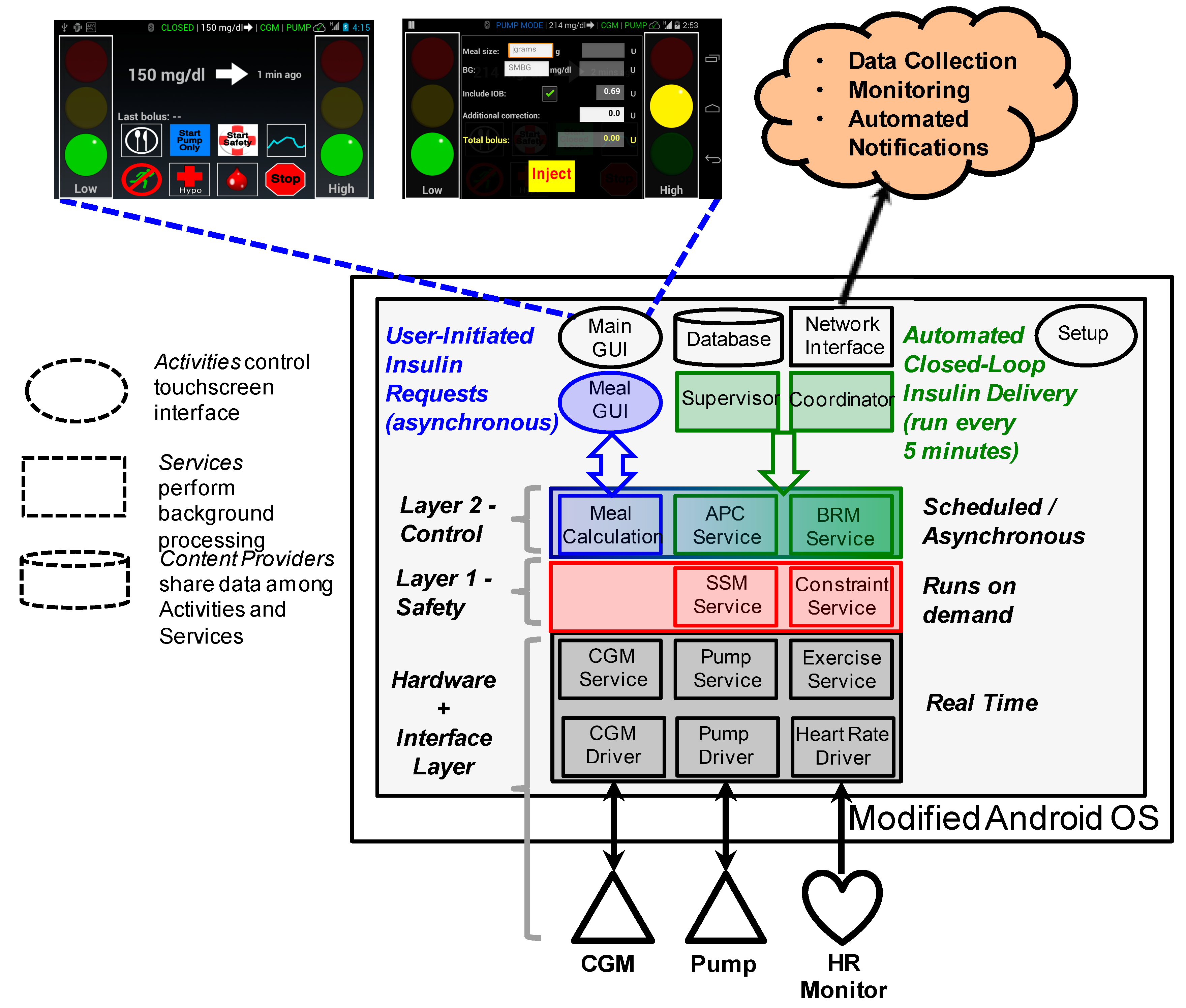
2.3. Application Programming Interfaces

- The Supervisor module emits a broadcast Intent every five minutes, which is received by a broadcast listener within the Coordinator module.
- The Coordinator broadcast listener handler sends the “calculate bolus” command to the APC Service module using an Intent message through a bound connection.
- APC Service queries the database for recent time-stamped CGM, SMBG, calibration and delivered insulin data. It may also request data regarding meals, physical activity and previously calculated quantities.
- After verifying that APC Service has appropriate read permissions the Database returns the requested information.
- APC Service calculates the appropriate amount of insulin to deliver and sends a “calculation result” message to the Coordinator using an Intent through a bound connection. Attached to this Intent is the amount of insulin, in Units, which should be immediately delivered to the subject as well as the “differential basal rate” in Units/hour, which indicates how much the current profile basal rate should be increased or decreased.
- Coordinator sends an Intent to SSM Service notifying it of the size of the bolus and differential basal rate which should be delivered to the insulin pump.
2.4. Smartphones and Peripheral Devices
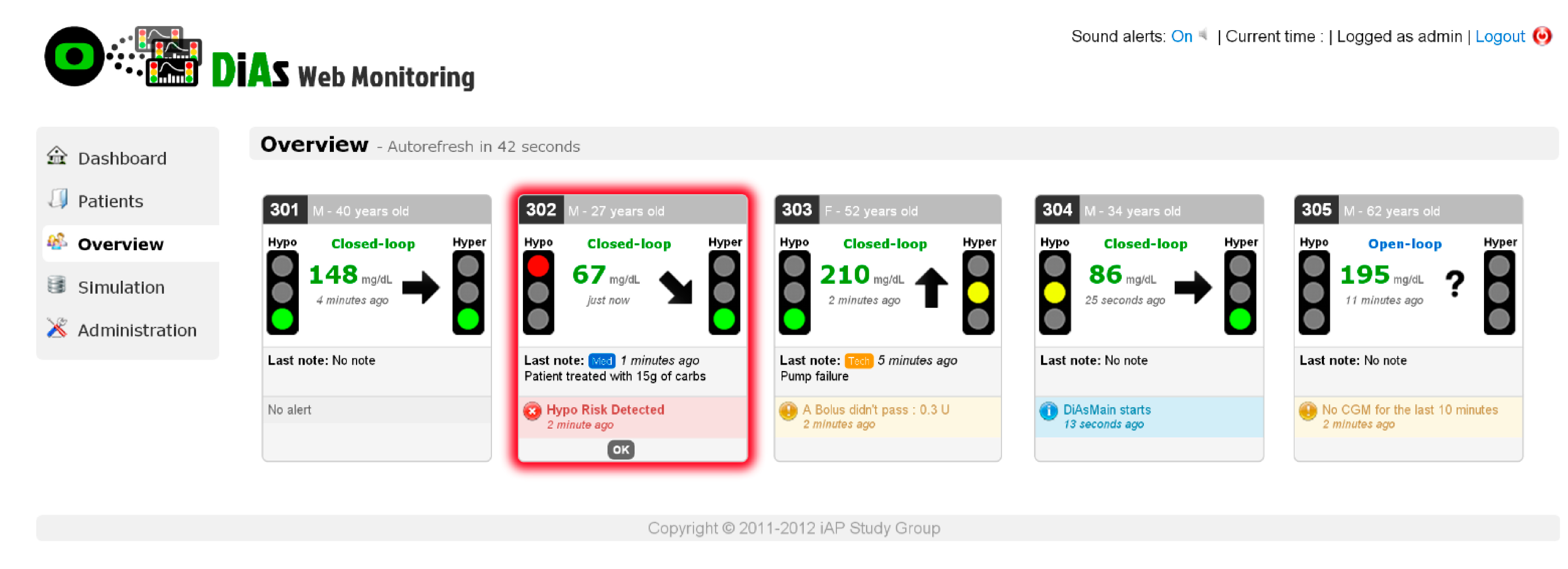
2.5. Cloud Services
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Albisser, A.M.; Leibel, B.S.; Ewart, T.G.; Davidovac, Z.; Botz, C.K.; Zinggg, W. An artificial endocrine pancreas. Diabetes 1974, 23, 389–396. [Google Scholar] [PubMed]
- Clemens, A.H.; Chang, P.H.; Myers, R.W. The development of Biostator, a glucose-controlled insulin infusion system. Horm. Metab. Res. Suppl. 1977, 7, 23–33. [Google Scholar]
- Marliss, E.B.; Murray, F.T.; Stokes, E.F.; Zinman, B.; Nakhooda, A.F.; Denoga, A.; Leibel, B.S.; Albisser, A.M. Normalization of glycemia in diabetics during meals with insulin and glucagon delivery by the artificial pancreas. Diabetes 1977, 26, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, E.F.; Thum, C.H.; Clemens, A.H. The artificial beta cell—A continuous control of blood sugar by external regulation of insulin infusion (glucose controlled insulin infusion system). Horm Metab. Res. 1974, 487, 339–342. [Google Scholar] [CrossRef]
- Santiago, J.V.; Clemens, A.H.; Clarke, W.L.; Kipnis, D.M. Closed-loop and open-loop devices for blood glucose control in normal and diabetic subjects. Diabetes 1979, 28, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Broekhuyse, H.M.; Nelson, J.D.; Zinman, B.; Albisser, A.M. Comparison of algorithms for the closed-loop control of blood glucose using the artificial beta cell. IEEE Trans. Biomed. Eng. 1981, 28, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Clemens, A.H. Feedback control dynamics for glucose controlled insulin infusion system. Med. Prog. Technol. 1979, 6, 91–98. [Google Scholar] [PubMed]
- Cobelli, C.; Ruggeri, A. Evaluation of portal/peripheral route and of algorithms for insulin delivery in the closed-loop control of glucose in diabetes. A modeling study. IEEE Trans. Biomed. Eng. 1983, 30, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Kraegen, E.W.; Campbell, L.V.; Chia, Y.O.; Meler, H.; Lazarus, L. Control of blood glucose in diabetics using an artificial pancreas. Aust. N. Z. J. Med. 1977, 7, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Fischer, U.; Jutzi, E.; Freyse, E.-J.; Salzsieder, E. Derivation and experimental proof of a new algorithm for the artificial beta-cell based on the individual analysis of the physiological insulin- glucose relationship. Endokrinologie 1978, 71, 65–75. [Google Scholar] [PubMed]
- Salzsieder, E.; Albrecht, G.; Fischer, U.; Freyse, E.-J. Kinetic modeling of the gluco-regulatory system to improve insulin therapy. IEEE Trans. Biomed. Eng. 1985, 32, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, P.; Cobelli, C.; Cruciani, P.; Fabietti, P.G.; Filippucci, F.; Santeusanio, F. A simulation study on a self-tuning portable controller of blood glucose. Int. J. Artif. Organs 1993, 16, 51–57. [Google Scholar] [PubMed]
- Fischer, U.; Schenk, W.; Salzsieder, E.; Albrecht, G.; Abel, P.; Freyse, E.-J. Does physiological blood glucose control require an adaptive strategy? IEEE Trans. Biomed. Eng. 1987, 34, 575–582. [Google Scholar]
- Sorensen, J.T. A Physiologic Model of Glucose Metabolism in Man and Its Use to Design and Assess Improved Insulin Therapies for Diabetes. Ph.D. Thesis, Dept. Chemical Engineering, MIT , Cambridge, MA, USA, 1985. [Google Scholar]
- Parker, R.S.; Doyle, F.J., 3rd; Peppas, N.A. A model-based algorithm for blood glucose control in Type I diabetic patients. IEEE Trans. Biomed. Eng. 1999, 48, 148–157. [Google Scholar] [CrossRef]
- Parker, R.S.; Doyle, F.J., 3rd; Peppas, N.A. The intravenous route to blood glucose control. IEEE Eng. Med. Biol. 2001, 20, 65–73. [Google Scholar] [CrossRef]
- Leblanc, H.; Chauvet, D.; Lombrail, P.; Robert, J.J. Glycemic control with closed-loop intraperitoneal insulin in type I diabetes. Diabetes Care 1986, 9, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Renard, E. Implantable closed-loop glucose-sensing and insulin delivery: The future for insulin pump therapy. Curr. Opin. Pharmacol. 2002, 2, 708–716. [Google Scholar] [PubMed]
- Bellazzi, R.; Nucci, G.; Cobelli, C. The subcutaneous route to insulin-dependent diabetes therapy: Closed-loop and partially closed-loop control strategies for insulin delivery and measuring glucose concentration. IEEE Eng. Med. Biol. 2001, 20, 54–64. [Google Scholar] [CrossRef]
- Hovorka, R.; Chassin, L.J.; Wilinska, M.E.; Canonico, V.; Akwi, J.A.; Federici, M.O.; Massi-Benedetti, M.; Hutzli, I.; Zaugg, C.; Kaufmann, H.; et al. Closing the loop: The ADICOL experience. Diabetes Technol. Ther. 2004, 6, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Steil, G.M.; Rebrin, K.; Darwin, C.; Hariri, F.; Saad, M.F. Feasibility of automating insulin delivery for the treatment of type 1 diabetes. Diabetes 2006, 55, 3344–3350. [Google Scholar] [CrossRef] [PubMed]
- Clarke, W.L.; Kovatchev, B.P. The Artificial Pancreas: How Close We Are to Closing the Loop? Endocrinol. Rev. 2007, 4, 314–316. [Google Scholar]
- The JDRF e-Newsletter: Emerging Technologies in Diabetes Research, September 2006.
- Weinzimer, S.A.; Steil, G.M.; Swan, K.L.; Dziura, J.; Kurtz, N.; Tamborlane, W.V. Fully automated closed-loop insulin delivery versus semi-automated hybrid control in pediatric patients with type 1 diabetes using an artificial pancreas. Diabetes Care 2008, 31, 934–939. [Google Scholar] [CrossRef]
- Hovorka, R.; Allen, J.M.; Elleri, D.; Chassin, L.J.; Harris, J.; Xing, D.; Kollman, C.; Hovorka, T.; Larsen, A.M.; Nodale, M.; et al. Manual closed-loop insulin delivery in children and adolescents with type 1 diabetes: A phase 2 randomised crossover trial. Lancet 2010, 375, 743–751. [Google Scholar] [CrossRef] [PubMed]
- El-Khatib, F.H.; Russell, S.J.; Nathan, D.M.; Sutherlin, R.G.; Damiano, E.R. A Bihormonal Closed-Loop Artificial Pancreas for Type 1 Diabetes. Sci. Transl. Med. 2010, 2, 27ra27. [Google Scholar] [CrossRef] [PubMed]
- Kovatchev, B.P.; Cobelli, C.; Renard, E.; Anderson, S.; Breton, M.; Patek, S.; Clarke, W.; Bruttomesso, D.; Maran, A.; Costa, S.; et al. Multi-National Study of Subcutaneous Model-Predictive Closed-Loop Control in Type 1 Diabetes: Summary of the Results. J. Diabetes Sci. Technol. 2010, 4, 1374–1381. [Google Scholar] [CrossRef] [PubMed]
- Breton, M.D.; Farret, A.; Bruttomesso, D.; Anderson, S.M.; Magni, L.; Patek, S.; Dalla Man, C.; Place, J.; Demartini, S.; del Favero, S.; on behalf of The International Artificial Pancreas (iAP) Study Group; et al. Fully-integrated artificial pancreas in type 1 diabetes: Modular closed-loop glucose control maintains near-normoglycemia. Diabetes 2012, 61, 2230–2237. [Google Scholar] [CrossRef]
- Cobelli, C.; Renard, E.; Kovatchev, B.P. Perspectives in Diabetes: Artificial Pancreas: Past, Present, Future. Diabetes 2011, 60, 2672–2682. [Google Scholar] [CrossRef]
- Mobile Medical Applications—Guidance for Industry and Food and Drug Administration Staff, FDA. Available online: http://www.fda.gov/downloads/MedicalDevices/.../UCM263366.pdf (accessed on 25 September 2013).
- Russell, S.J.; El-Khatib, F.H.; Sinha, M.; Magyar, K.L.; McKeon, K.; Goergen, L.; Balliro, C.; Hilliard, M.A.; Nathan, D.M.; Damiano, E.R. Outpatient Glycemic Control with a Bionic Pancreas in Type 1 Diabetes. N. Engl. J. Med. 2014, 371, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Leelarathna, L.; Thabit, H.; Allen, J.M.; Nodale, M.; Wilinska, M.E.; Powell, K.; Lane, S.; Evans, M.; Hovorka, R. Evaluating the Performance of a Novel Embedded Closed-loop System. J. Diabetes Sci. Technol. 2014, 8, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Peyser, T.; Dassau, E.; Breton, M.; Skyler, J. The artificial pancreas: Current status and future prospects in the management of diabetes. Ann. N Y Acad. Sci. 2014, 1311, 102–123. [Google Scholar] [CrossRef] [PubMed]
- Patek, S.D.; Chen, S.; Keith-Hynes, P.; Lee, I. Distributed aspects of the artificial pancreas. In Proceedings of the 2013 51st Annual Allerton Conference on Communication, Control, and Computing (Allerton), Monticello, IL, USA, 2–4 October 2013; pp. 543–550.
- Keith-Hynes, P.; Guerlain, S.; Mize, B.; Hughes-Karvetski, C.; Khan, M.; McElwee-Malloy, M.; Kovatchev, B.P. DiAs User Interface: A Patient-Centric Interface for Mobile Artificial Pancreas Systems. J. Diabetes Sci. Technol. 2013, 7, 1416–1426. [Google Scholar] [PubMed]
- Kovatchev, B.P.; Patek, S.D.; Dassau, E.; Doyle, F.J., III; Magni, L.; de Nicolao, G.; Cobelli, C. Control-to-range for diabetes: Functionality and modular architecture. J. Diabetes Sci. Technol. 2009, 3, 1058–1065. [Google Scholar] [CrossRef] [PubMed]
- Patek, S.D.; Magni, L.; Dassau, E.; Hughes-Karvetski, C.; Toffanin, C.; de Nicolao, G.; del Favero, S.; Breton, M.; Man, C.D.; Renard, E.; et al. Modular closed loop control of diabetes. IEEE Trans. Biomed. Eng. 2012, 59, 2986–2999. [Google Scholar] [CrossRef] [PubMed]
- Place, J.; Robert, A.; Ben Brahim, N.; Keith-Hynes, P.; Farret, A.; Pelletier, M.-J.; Buckingham, B.; Breton, M.; Kovatchev, B.; Renard, E. DiAs Web Monitoring: A Real-Time Remote Monitoring System Designed for Artificial Pancreas Outpatient Trials. J. Diabetes Sci. Technol. 2013, 7, 1427–1435. [Google Scholar] [PubMed]
- Kovatchev, B.P.; Renard, E.; Cobelli, C.; Zisser, H.; Keith-Hynes, P.; Anderson, S.M.; Brown, S.A.; Chernavvsky, D.R.; Breton, M.D.; Farret, A.; et al. Feasibility of outpatient fully integrated closed-loop control: First studies of wearable artificial pancreas. Diabetes Care 2013, 36, 1851–1858. [Google Scholar] [CrossRef] [PubMed]
- Kovatchev, B.P.; Cobelli, C.; Renard, E.; Zisser, H.; International Artificial Pancreas Study Group. Efficacy of Outpatient Closed-Loop Control (CLC); 73rd ADA Scientific Sessions: Chicago, IL, USA, 2013. [Google Scholar]
- DeSalvo, D.J.; Keith-Hynes, P.; Peyser, T.; Place, J.; Caswell, K.; Wilson, D.M.; Harris, B.; Clinton, P.; Kovatchev, B.; Buckingham, B.A. Remote Glucose Monitoring in Camp Setting Reduces the Risk of Prolonged Nocturnal Hypoglycemia. In Diabetes Technol. Ther.; 2014; Volume 16, pp. 1–7. [Google Scholar] [CrossRef]
- Ly, T.T.; Breton, M.D.; Keith-Hynes, P.; de Salvo, D.; Clinton, P.; Benassi, K.; Mize, B.; Chernavvsky, D.; Place, J.; Wilson, D.M; et al. Overnight Glucose Control With an Automated, Unified Safety System in Children and Adolescents With Type 1 Diabetes at Diabetes Camp. In Diabetes Care; 2014; Volume 37, pp. 2310–2316. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keith-Hynes, P.; Mize, B.; Robert, A.; Place, J. The Diabetes Assistant: A Smartphone-Based System for Real-Time Control of Blood Glucose. Electronics 2014, 3, 609-623. https://doi.org/10.3390/electronics3040609
Keith-Hynes P, Mize B, Robert A, Place J. The Diabetes Assistant: A Smartphone-Based System for Real-Time Control of Blood Glucose. Electronics. 2014; 3(4):609-623. https://doi.org/10.3390/electronics3040609
Chicago/Turabian StyleKeith-Hynes, Patrick, Benton Mize, Antoine Robert, and Jérôme Place. 2014. "The Diabetes Assistant: A Smartphone-Based System for Real-Time Control of Blood Glucose" Electronics 3, no. 4: 609-623. https://doi.org/10.3390/electronics3040609



