Equol’s Anti-Aging Effects Protect against Environmental Assaults by Increasing Skin Antioxidant Defense and ECM Proteins While Decreasing Oxidative Stress and Inflammation
Abstract
:1. Introduction
2. Perceptions of Sensitive Skin with Environmental Causes
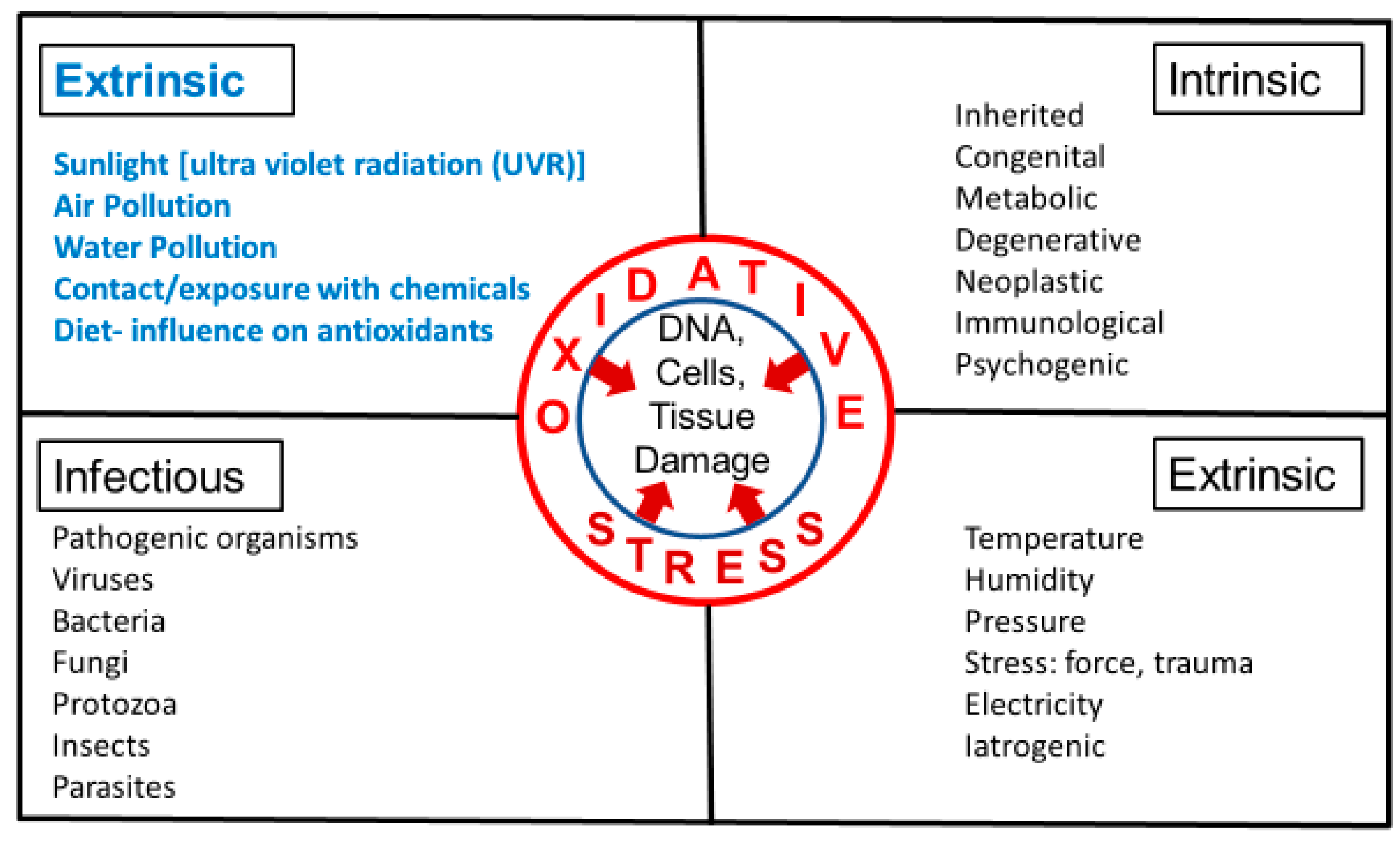
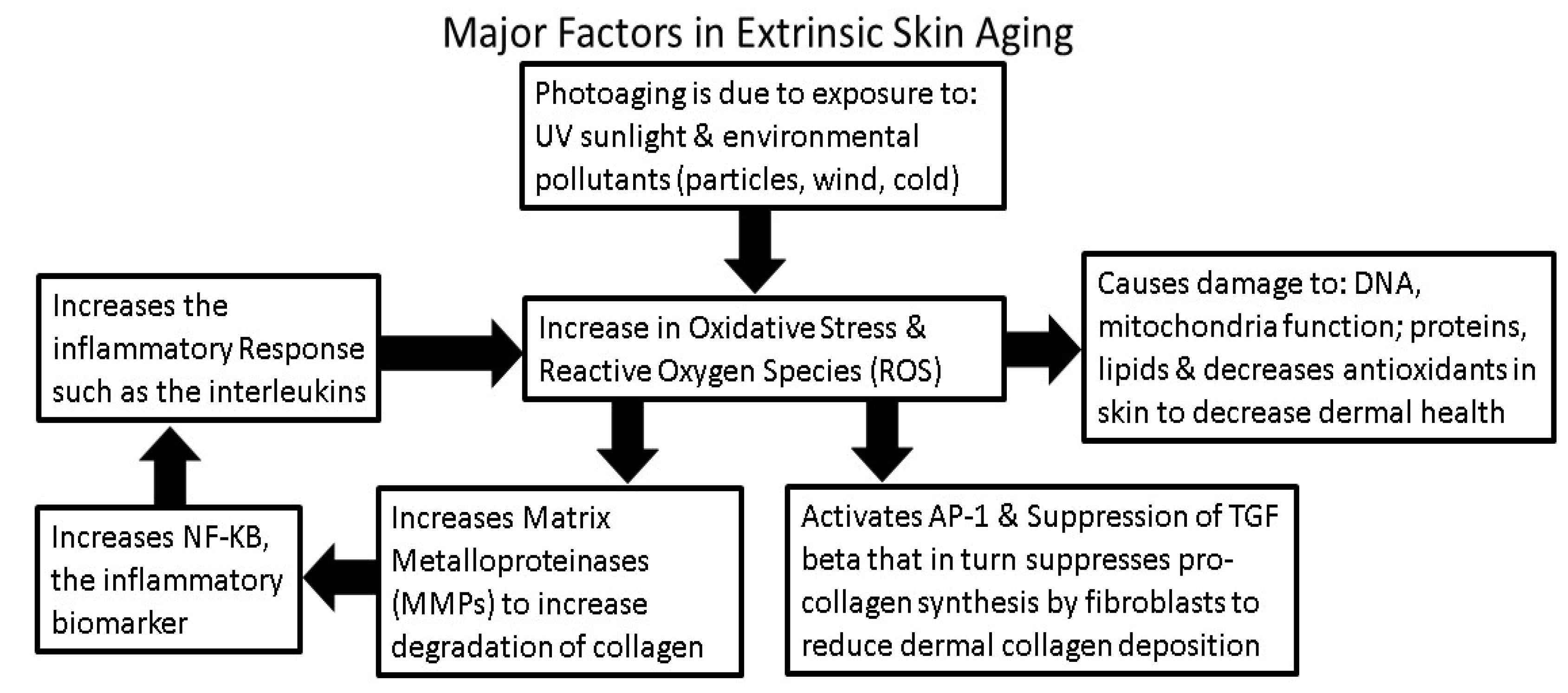
3. Intrinsic and Extrinsic Sources of Oxidative Stress
4. Environmental Factors that Influence Generation or Inhibition of ROS
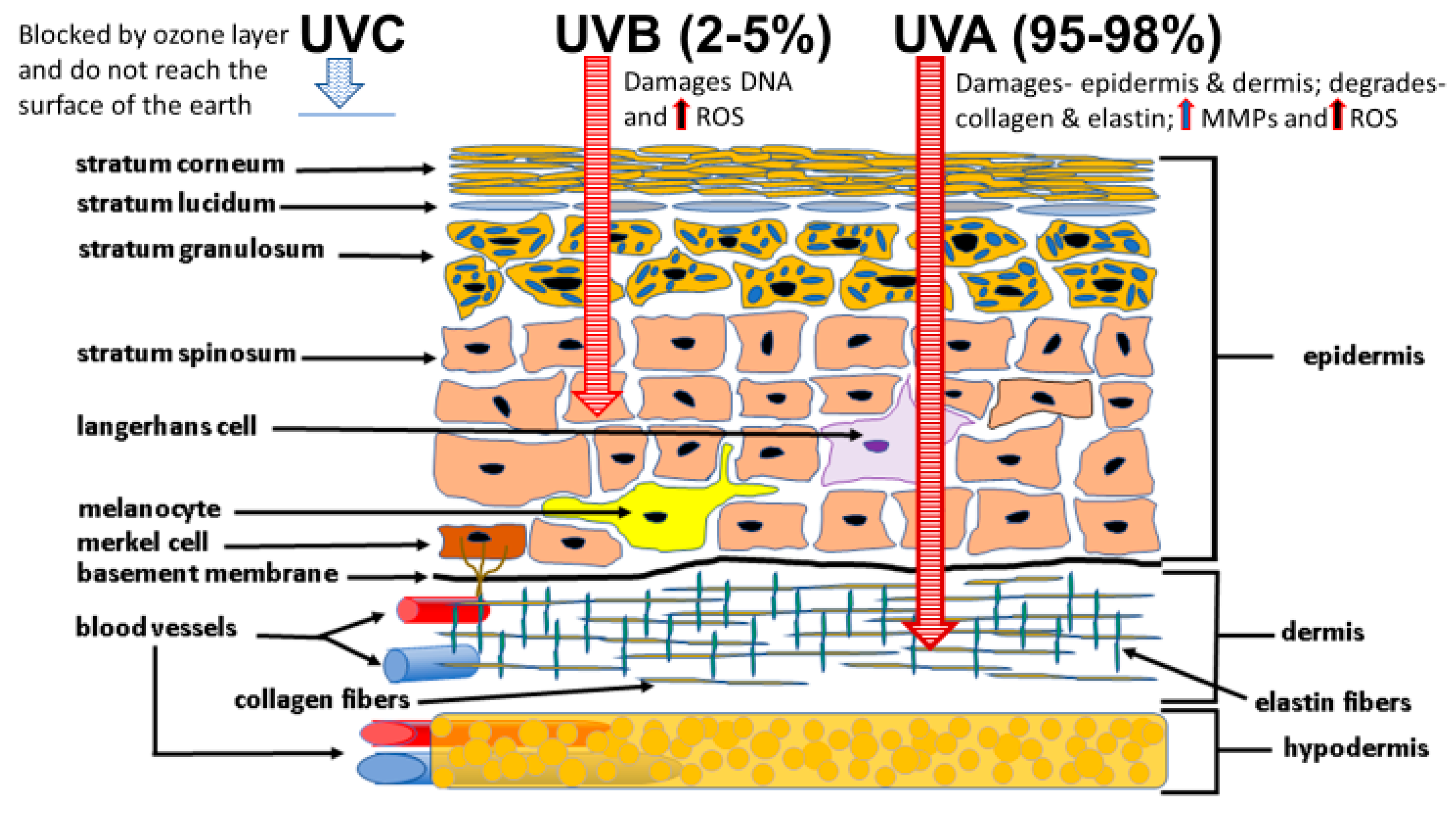
4.1. Sunlight Photoaging and the Generation of ROS
4.2. Cold Weather and the Generation of ROS
4.3. Air and Water Pollution and the Generation of ROS
4.4. Diet and the Influence of Antioxidants that Inhibit ROS
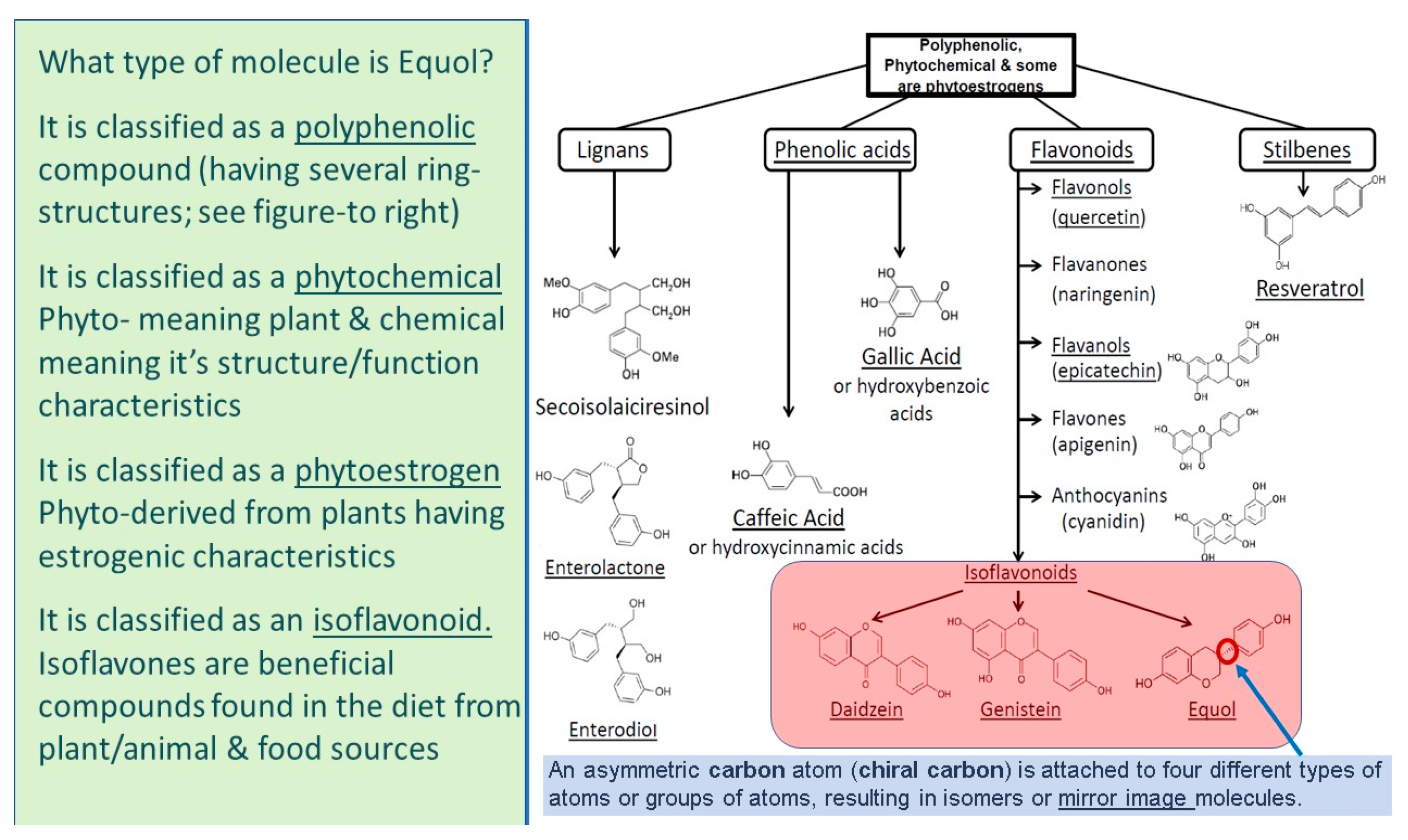
5. What Is Equol?
5.1. Equol Is a Polyphenolic Compound
5.2. Equol Is a Phytochemical
5.3. Equol Is a Phytoestrogen
5.4. Equol Is an Isoflavonoid
6. How Equol Protects Skin against Environmental Assaults
6.1. Equol Is Delivered into Human Skin via a Unique “Reservoir” Delivery Mechanism
6.2. Equol Increases Skin Antioxidant Defense and Stimulates Nrf2 Gene Expression
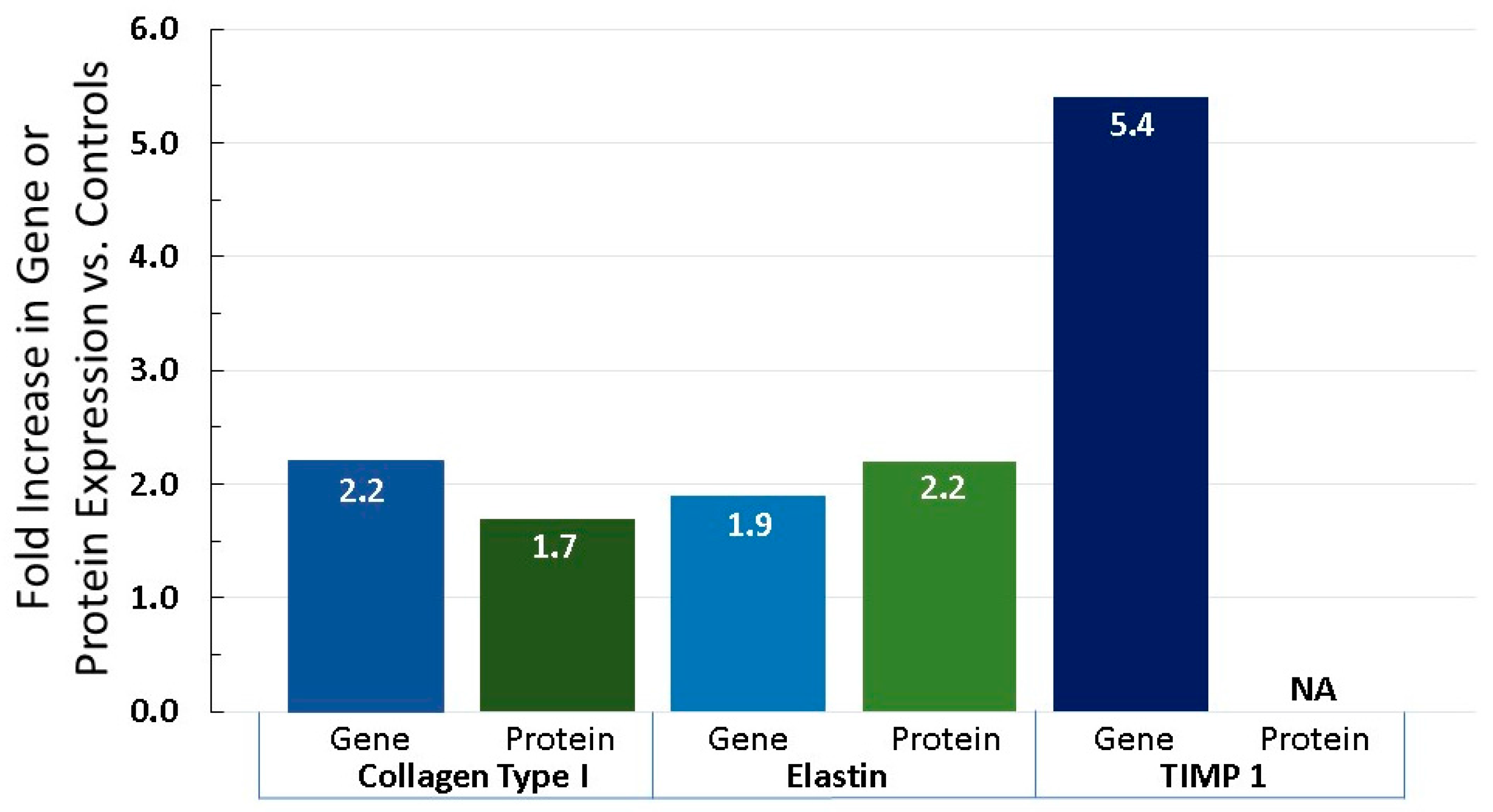
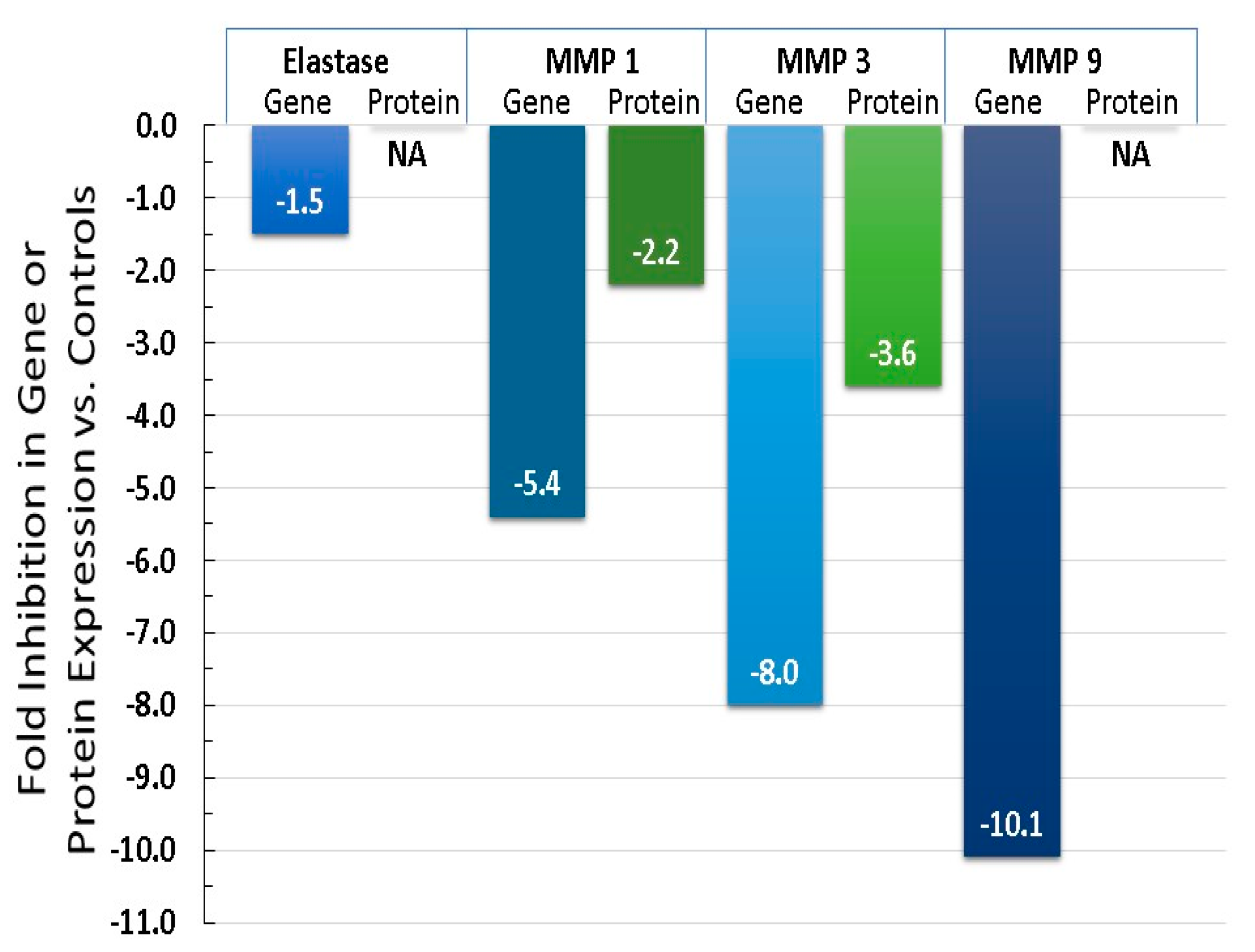
6.3. Equol Increases Extracellular Matrix Proteins, Like Collagen, Elastin, and TIMP 1
6.4. Equol Decreases Oxidative Stress (ROS) and Inflammation
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Intrinsic and extrinsic factors in skin ageing: A review. Int. J. Cosmet. Sci. 2008, 30, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Lephart, E.D. Skin aging and oxidative stress: Equol’s anti-aging effects via biochemical and molecular mechanisms. Ageing Res. Rev. 2016, 31, 36–54. [Google Scholar] [CrossRef] [PubMed]
- Katta, R.; Desai, S.P. Diet and dermatology, the role of dietary intervention in skin disease. J. Clin. Aesthet. Dermatol. 2014, 7, 46–51. [Google Scholar] [PubMed]
- Farage, M.A. Perceptions of sensitive skin: Changes in perceived severity and associations with environmental causes. Contact Dermat. 2008, 59, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Farage, M.A.; Miller, K.W.; Wippel, A.M.; Berardesca, E.; Misery, L.; Mailbach, H. Sensitive skin in the United States: Survey of regional differences. Family Med. Med. Sci. Res. 2013, 2, 3. [Google Scholar] [CrossRef]
- Misery, L. Neuropsychiatric factors in sensitive skin. Clin. Dermatol. 2017, 35, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.R. Cosmegizmoceuticals: The Physics and Chemistry of Looking Better. In Proceedings of the Society of Cosmetic Chemists Annual Meeting, New York, NY, USA, 6–7 December 2012; Volume 43. [Google Scholar]
- Kligman, A.M.; Koblenzer, C. Demographics and psychological implications for the aging population. Dermatol. Clin. 1997, 15, 549–553. [Google Scholar] [CrossRef]
- Al-Gubory, K.H. Environmental pollutants and lifestyle factors induce oxidative stress and poor prenatal development. Reprod. Biomed. Online 2014, 29, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Baynes, J.W. Role of oxidative stress in development of complications in diabetes. Diabetes 1991, 40, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M. Role of oxidative stress in infectious diseases. A review. Folia Microbiol. 2013, 58, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Kammeyer, A.; Luiten, R.M. Oxidative events and skin aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, V.T.; Ganju, P.; Ramkumar, A.; Grover, R.; Gokhale, R.S. Multifaceted pathways protect human skin from UV radiation. Nat. Chem. Biol. 2014, 10, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Shindo, Y.; Witt, E.; Han, D.; Epstein, W.; Packer, L. Enzymic and non-enzymic antioxidants in epidermis and dermis in human skin. J. Investig. Dermayol. 1994, 102, 122–124. [Google Scholar] [CrossRef]
- Krishnamurthy, P.; Wadhwani, A. Antioxidant Enzymes and Human Health, Chapter 1; El-Missiry, M.A., Ed.; Antioxidant Enzyme-InTech Science, Technology & Medicine: Vienna, Austria, 2012; pp. 4–18. [Google Scholar] [CrossRef]
- Pullar, J.M.; Carr, A.C.; Vissers, M.C.M. The role of vitamin C in skin health. Nutrients 2107, 9, 866. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.; Bowman, A.; Boulton, S.J.; Manning, P. A role for human mitochondrial complex II in the production of reactive oxygen species in human skin. Redox Biol. 2014, 2, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Meadows, C.; Moore, D.J.; Moore, D.M.; Draelos, Z.D.; Kern, D. Age-related NADH oxidase (arNOX)-catalyzed oxidative damage to skin protein. Arch. Dermatol. Res. 2014, 306, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Quan, C.; Cho, M.K.; Perry, D.; Quan, T. Age-associated reduction of cell spreading induces mitochondrial DNA common deletion by oxidative stress in human skin dermal fibroblasts: Implication for human skin connective tissue aging. J. Biomed. Sci. 2015, 22, 62. [Google Scholar] [CrossRef] [PubMed]
- Tulah, A.S.; Birch-Machin, M.A. Stressed out mitochondria: The role of mitochondria in ageing and cancer focusing on strategies and opportunities in human skin. Mitochondrion 2013, 13, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Valacchi, G.; Perorelli, A.; Cervellati, F.; Cervellati, C.; Maioli, F. Cutaneous responses to environmental stressors. Ann. N. Y. Acad. Sci. 2012, 1271, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.L.; Hawk, J.L.M.; Young, A.R. Acute and chronic effects of ultraviolet radiation on the skin. In Fitzpatrick’s Dermatology in General Medicine, 6th ed.; Freedberg, I.M., Eisen, A.Z., Wolff, K.F., Austen, L.A., Goldsmith, L.A., Katz, S.I., Eds.; McGraw-Hill: New York, NY, USA, 2003; pp. 1275–1282. [Google Scholar]
- Wlaschek, M.; Tantcheva-Poór, I.; Naderi, L.; Ma, W.; Schneider, L.A.; Razi-Wolf, Z.; Schüller, J.; Scharffetter-Kochanek, K. Solar UV irradiation and dermal photoaging. J. Photochem. Photobiol. B 2001, 63, 41–51. [Google Scholar] [CrossRef]
- Birch-Machin, M.A.; Swalwell, H. How mitochondria record the effects of UV exposure and oxidative stress using human skin as a model tissue. Mutagenesis 2010, 25, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Lehmuskallio, E.; Hassi, J.; Kettunen, P. The skin in the cold. Int. J. Circumpolar Health 2002, 61, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Makinen, T.A.; Hassi, J. Health problems in cold work. Ind. Health 2009, 47, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Briggs, A.G.S.; Gillespie, T.J.; Brown, R.D. Measuring facial cooling in outdoor windy winter conditions: An exploratory study. Int. J. Biometeorol. 2017, 61, 1831–1835. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.C.; Askew, E.W.; Roberts, D.E.; Prior, R.L.; Ensign, W.Y., Jr.; Hesslink, R.E., Jr. Oxidative stress in humans training in a cold, moderate altitude environment and their response to a phytochemical antioxidant supplement. Wilderness Environ. Med. 2002, 13, 94–105. [Google Scholar] [CrossRef]
- Martarelli, D.; Cocchioni, M.; Scuri, S.; Spataro, A.; Pompei, P. Cold exposure increases exercise-induced oxidative stress. J. Sports Med. Phys. Fit. 2011, 51, 299–304. [Google Scholar]
- Naidoo, K.; Birch-Machin, M.A. Oxidative stress and ageing: The influence of environmental pollution, sunlight and diet on skin. Cosmetics 2017, 4, 4. [Google Scholar] [CrossRef]
- Hamer, M.A.; Pardo, L.M.; Jacobs, L.C.; Ikram, M.A.; Laven, J.S.; Kayser, M.; Hollestein, L.M.; Gunn, D.A.; Nijsten, T. Lifestyle and physiological factors associated with facial wrinkling in men and women. J. Investig. Dermatol. 2017, 137, 1692–1699. [Google Scholar] [CrossRef] [PubMed]
- Hodges, A.L.; Walker, D.K. Skin care for women. Nurs. Womens Health 2017, 20, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Vierkotter, A. Environmental pollution and skin aging. Hautarzt 2011, 62, 577–578. [Google Scholar] [PubMed]
- Kelly, F.J. Oxidative stress: Its role in air pollution and adverse health effects. Occup. Environ. Med. 2003, 60, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A.; Dockery, D.W.; Spengler, J.D.; Raizenne, M.E. Respiratory health and PM10 pollution—A daily time-series analysis. Am. Rev. Respir. Dis. 1991, 144, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Lodovici, M.; Bigagli, E. Oxidative stress and air pollution exposure. J. Toxicol. 2011, 2011, 487074. [Google Scholar] [CrossRef] [PubMed]
- Delfino, R.J.; Staimer, N.; Vaziri, N.D. Air pollution and circulating biomarkers of oxidative stress. Air Qual. Atmos. Health 2011, 4, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, M.; Nikic, D.; Arandjelovic, M. Air pollution and skin effects in children. J. Environ. Prot. Ecol. 2010, 11, 1215–1221. [Google Scholar]
- Krutmann, J.; Bouloc, A.; Sore, G.; Bernard, B.A.; Passeron, T. The skin aging exposome. J. Dermatol. Sci. 2017, 85, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Puri, P.; Nandar, S.K.; Kathuria, S.; Ramesh, V. Effects of air pollution on the skin. A review. Indian J. Dermatol. Venereol. Leprol. 2017, 83, 415–423. [Google Scholar] [PubMed]
- Lanuti, E.L.; Kirsner, R.S. Effects of pollution on skin aging. J. Investig. Dermatol. 2010, 130, 2696. [Google Scholar] [CrossRef] [PubMed]
- Vierkötter, A.; Schikowski, T.; Ranft, U.; Sugiri, D.; Matsui, M.; Krämer, U.; Krutmann, J. Airborne particle exposure and extrinsic skin aging. J. Investig. Dermatol. 2010, 130, 2719–2726. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, P.K.; Klingelhutz, A.J.; Jacobus, J.A.; Lehmler, H.; Robertson, L.W.; Ludewig, G. Airborne polychlorinated biphenyls (PBCs) reduce telomerase activity and shorten telomere length in immortal human skin keratinocytes (HaCat). Toxicol. Lett. 2011, 204, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Poljšak, B.; Dahmane, R. Free radicals and extrinsic aging. Dermatol. Res. Pract. 2012, 2012, 135206. [Google Scholar] [CrossRef] [PubMed]
- Pedala, P.; Boccellino, M.; La Porta, R.; Napolitano, M.; Minutolo, P.; Sgro, L.A.; Zei, F.; Sannolo, N.; Quagliuolo, L. Interaction between combustion-generated organic nanoparticles and biological systems: In vitro study of cell toxicity and apoptosis in human keratinocytes. Nanotoxicology 2012, 6, 338–352. [Google Scholar]
- Bakke, J.V.; Wieslander, G.; Norback, D.; Moen, B.E. Eczema increases susceptibility to PM10 in office indoor environments. Arch. Environ. Occup. Health 2012, 67, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Kamide, R.; Misery, L.; Perez-Cullell, N.; Sibaud, V.; Taieb, C. Sensitive skin evaluation in the Japanese population. J. Dermatol. 2013, 40, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K. The role of air pollutants in atopic dermatitis. J. Allergy Clin. Immunol. 2014, 134, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J.; Liu, W.; Li, L.; Pan, X.; Crawford, M.; Sore, G.; Seite, S. Pollution and skin: From epidermiological and mechanistic studies to clinical implications. J. Dermatol. Sci. 2014, 76, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Vierkötter, A.; Schikowski, T.; Hüls, A.; Matsul, M.S.; Deng, B.; Ma, C.; Ren, A.; Zhang, J.; Tan, J.; et al. Epidemiological evidence that indoor air pollution from cooking with solid fuels accelerates skin aging in Chinese women. J. Dermatol. Sci. 2015, 79, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-K.; Kim, H.; Lim, D.-H.; Lee, Y.-K.; Kim, J.H. Effects of indoor air pollutants on atopic dermatitis. Int. J. Environ. Res. Public Health 2016, 13, 1220. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.L.; Wang, P.W.; Aljuffali, A.; Huang, C.T.; Lee, C.W.; Fang, J.Y. The impact of urban particulate pollution on skin barrier function and the subsequent drug absorption. J. Dermatol. Sci. 2015, 78, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.E. Pollution as a risk factor for the development of melasma and other skin disorders of facial hyperpigmentation-is there a case to be made? J. Drugs Dermatol. 2015, 14, 337–341. [Google Scholar] [PubMed]
- Kim, K.E.; Cho, D.; Park, H.J. Air pollution and skin diseases: Adverse effects of airborne particulate matter on various skin diseases. Life Sci. 2016, 152, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Morita, A.; Seitè, S.; Haarmann-Stemmann, T.; Grether-Beck, S.; Krutmann, J. Environment-induced lentigines: Formation of solar lentigines beyond ultraviolet radiation. Exp. Dermatol. 2015, 24, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Xue, C.H.; Hwang, S.K.; Li, W.H.; Chen, Z.; Zhang, J.Z. Exposure to fine particulate matter associated with senile lentingo in Chinese women: A cross-sectional study. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Li, Q.; Du, H.-Y.; Wang, Q.-W.; Huang, Y.; Liu, W. Airborne polycyclic aromatic hydrocarbons trigger human skin cells aging through aryl hydrocarbon receptor. Biochem. Biophys. Res. Commun. 2017, 488, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Bae, I.-H.; Son, E.D.; Park, J.; Cha, N.; Na, H.-W.; Jung, C.; Go, Y.-S.; Kim, D.-Y.; Lee, T.R.; Shin, D.W. Transcriptome analysis of airborne PM 2.5-induced detrimental effects on human keratinocytes. Toxicol. Lett. 2017, 273, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Mancebo, S.E.; Wang, S.Q. Recognizing the impact of ambient air pollution on skin health. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 2326–2332. [Google Scholar] [CrossRef] [PubMed]
- Koohgoli, R.; Hudson, L.; Naidoo, K.; Wilkinson, S.; Chavan, B.; Birch-Machin, M.A. Bad air gets under your skin. Exp. Dermatol. 2017, 26, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Valavanidis, A.; Vlahogianni, T.; Dassenakis, M.; Scoullos, M. Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicol. Environ. Saf. 2006, 64, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I. Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol. 2011, 101, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Draelos, Z.D. Aging Skin: The role of diet: Facts and controversies. Clin. Dermatol. 2013, 31, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Rona, C.; Berardesca, E. Aging skin and food supplements: The myth and the truth. Clin. Dermatol. 2008, 26, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Koo, N.; Min, D.B. Reactive oxygen species, aging and antioxidative nutraceuticals. Compr. Rev. Food Sci. Food Saf. 2004, 3, 21–33. [Google Scholar] [CrossRef]
- Heinrich, U. Anti-aging by nutricosmetics. Aktuelle Dermatol. 2017, 43, 427–430. [Google Scholar]
- Valacchi, G.; Sticozzi, C.; Belmonte, G.; Cervellati, F.; Demaude, J.; Chen, N.; Krol, Y.; Oresajo, C. Vitamin C compound mixtures prevent ozone-induced oxidative damage in human keratinocytes as initial assessment of pollution protection. PLoS ONE 2015, 10, e0131097. [Google Scholar] [CrossRef] [PubMed]
- Lacher, S.E.; Lee, J.S.; Wang, X.; Campbell, M.R.; Bell, D.A. Beyond antioxidant genes in the ancient Nrf2 regulatory network. Free Radic. Biol. Med. 2015, 88, 452–465. [Google Scholar] [CrossRef] [PubMed]
- Beyer, T.A.; Keller, U.; Braun, S.; Schafer, M.; Werner, S. Roles and mechanisms of action of the Nrf2 transcription factor in skin morphogenesis, wound repair and skin cancer. Cell Death Differ. 2007, 14, 1250–1554. [Google Scholar] [CrossRef] [PubMed]
- Greenwald, M.B.Y.; Frusic-Zlotkin, M.; Soroka, Y.; Sasson, S.B.; Bianco-Peled, H.; Bitton, R.; Kohen, R. Nitroxide delivery system for Nrf2 activation and skin protection. Eur. J. Pharm. Biopharm. 2015, 94, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Sekhar, K.R.; Freeman, M.L. Nrf2 promotes survival following exposure to ionizing radiation. Free Radic. Biol. Med. 2015, 88, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liang, X.Y.; Shi, L.Y.; Wang, L.; Chen, J.L.; Kang, C.; Zhu, J.D.; Mi, M.T. Estrogen receptor and PI3K/Akt signaling pathway involvement in S-equol-induced activation of Nrf2/ARE in endothelia cells. PLoS ONE 2013, 8, e79075. [Google Scholar] [CrossRef]
- Gruber, F.; Ornelas, C.M.; Karner, S.; Narzt, M.S.; Nagelreiter, M.I.; Gschwandtner, M.; Bochkov, V.; Tschachler, E. Nrf2 deficiency causes lipid oxidation, inflammation, and matrix-protease expression in DHA-supplemented and UVA-irradiated skin fibroblasts. Free Radic. Biol. Med. 2015, 88, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Park, G.; Oh, M.S. Acceleration of heat shock-induced collagen breakdown in human dermal fibroblasts with knockdown of NF-E2-related factor 2. BMB Rep. 2015, 48, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Greenwald, M.B.-Y.; Ben-Sasson, S.; Blanco-Peled, H.; Kohen, R. Skin redox balance maintenance: The need for a Nrf2-activator delivery system. Cosmetics 2016, 3, 1. [Google Scholar] [CrossRef]
- Park, E.-J.; Pezzuto, J.M. The pharmacology of resveratrol in animals and humans. Biochim. Biophys. Acta 2015, 1852, 1071–1113. [Google Scholar] [CrossRef] [PubMed]
- Lephart, E.D. Resveratrol, 4′ Acetoxy Resveratrol, R-Equol, Racemic Equol or S-Equol as Cosmeceuticals to Improve Dermal Health. Int. J. Mol. Sci. 2017, 18, 1193. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.R.; Clerici, C. Equol: History, chemistry, and formation. J. Nutr. 2010, 140, 1355S–1362S. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.R.; Clerici, C.; Lephart, E.D.; Cole, S.J.; Heenan, C.; Castellani, D.; Wolfe, B.E.; Nechemias-Zimmer, L.; Brown, N.M.; Lund, T.D.; et al. S-equol, a potent ligand for estrogen receptor β, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora. Am. J. Clin. Nutr. 2005, 81, 1072–1079. [Google Scholar] [PubMed]
- Krahn-Bertil, E.; Bolzinger, M.A.; Andre, V.; Orly, I.; Kanitakis, J.; Rousselle, P.; Damour, O. Expression of estrogen-related receptor gamma (ERRgamma) in human skin. Eur. J. Dermatol. 2008, 18, 427–432. [Google Scholar] [PubMed]
- Hirvonen, J.; Rajalin, A.M.; Wohlfart, G.; Adlercreutz, H.; Wähälä, K.; Aarnisalo, P. Transcriptional activation of estrogen-related receptor gamma (ERRgamma) is stimulated by the phytoestrogen equol. J. Steroid Biochem. Mol. Biol. 2010, 123, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Liliana, M.; Rusu, T.; Wang, T.Y.; Park, J.B.; Clapa, D. Soy-derived microbial metabolite equol in cancer prevention. Agric. Sci. Pract. 2017, 1–2, 5–20. [Google Scholar]
- Gopaul, R.; Knaggs, H.; Lephart, E.D. Biochemical investigation and gene analysis of equol: A plant and soy-derived isoflavonoid with anti-aging and antioxidant properties with potential human skin applications. Biofactors 2012, 38, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Lephart, E.D. Protective effects of equol and their polyphenolic isomers against dermal aging: Microarray/protein evidence with clinical implications and unique delivery into human skin. Pharm. Biol. 2013, 51, 1393–1400. [Google Scholar] [CrossRef] [PubMed]
- Lephart, E.D. Human Skin Gene Expression Doesn’t Correlate with Protein Expression? Unless Both Parameters Are Quantified. J. Cosmet. Dermatol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Lademann, J.; Vergou, T.; Darvin, M.; Patzelt, A.; Meinke, M.; Voit, C.; Papakostas, D.; Zastrow, L.; Sterry, W.; Doucet, O. Influence of topical, systematic and combined application of antioxidants on barrier properties of the human skin. Skin Pharm. Physiol. 2016, 29, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.; Nair, M.G.; Strasburg, G.M. Antioxidant activities of isoflavones and their biological metabolites in a liposomal system. Arch. Biochem. Biophys. 1988, 356, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.H.; Gardner, P.T.; McPhail, D.B.; Morrice, P.C.; Collins, A.R.; Duthie, G.C. Antioxidant efficacy of phytoestrogens in chemical and biological model systems. Arch. Biochem. Biophys. 1998, 360, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Rufer, C.E.; Kulling, S.E. Antioxidant activity of isoflavones and their major metabolites using different in vitro assays. J. Agric. Food Chem. 2006, 54, 2926–2931. [Google Scholar] [CrossRef] [PubMed]
- Avantaggiato, A.; Bertuzzi, G.; Vitiello, U.; Iannucci, G.; Pasin, M.; Cervelli, V.; Carinci, F. Role of antioxidants in dermal aging: An in vitro study by q-RT-PCR. Aesthet. Plast. Surg. 2014, 38, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Pandel, R.; Poljsak, B.; Godic, A.; Dahmane, R. Skin photoaging and the role of antioxidants in its prevention. ISRN Dermatol. 2013, 2013, 930164. [Google Scholar] [CrossRef] [PubMed]
- Velarde, M.C.; Flynn, J.M.; Day, N.U.; Melvo, S.; Campisi, J. Mitochondrial oxidative stress caused by SOD deficiency promotes cellular senescene and aging phenotypes in the skin. Aging 2012, 4, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Widyarini, S.; Domanski, D.; Painter, N.; Reeve, V.E. Photoimmune protective effect of the phytoestrogenic isoflavonoid equol is partially due to its antioxidant activities. Photochem. Photobiol. Sci. 2012, 11, 1186–1192. [Google Scholar] [CrossRef] [PubMed]
- Mann, G.E.; Bonacasa, B.; Ishii, T.; Siow, R.C.M. Targeting the redox sensitive Nrf2-Keep1 defense pathway in cardiovascular disease: Protection afforded by dietary isoflavones. Curr. Opin. Pharmacol. 2009, 9, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Widyarini, S.; Allanson, M.; Gallagher, N.L.; Pedley, J.; Boyle, G.M.; Parsons, P.G.; Whiteman, D.C.; Walker, C.; Reeve, V.E. Isoflavonoid photoprotection in mouse and human skin is dependent on metallothionein. J. Investig. Dermatol. 2006, 126, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.L.; Greiwe, J.S.; Schwen, R.J. S-equol, an antioxidant metabolite of soy dadizein, and oxidative stress in aging: A focus on skin and the cardiovascular system. In Aging: Oxidative Stress and Dietary Antioxidants; Elsevier Inc.: London, UK, 2014; pp. 145–155. [Google Scholar]
- Froyen, E.B.; Steinberg, F.M. Soy isoflavones increase quinone reductase in heap-1c1c7 cells via estrogen receptor beta and nuclear factor erythroid 2-related factor 2 binding to the antioxidant response element. J. Nutr. Biochem. 2011, 22, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Rabe, J.H.; Mamelak, A.J.; McElgunn, P.J.; Morison, W.L.; Sauder, D.N. Photoaging: Mechanisms and repair. J. Am. Acad. Dermatol. 2006, 55, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Datta, S.C.; Taylor, H.S. Molecular basis of sun induced premature skin aging and retionoid antagonism. Nature 1996, 379, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.J.; Lee, K.W.; Rogozin, E.A.; Cho, Y.-Y.; Heo, Y.-S.; Bode, A.M.; Lee, H.J.; Dong, Z. Equol inhibits neoplastic cell transformation by targeting the MEK/ERK/p90RSK/ activator protein—1 pathway. J. Biol. Chem. 2007, 45, 32856–32866. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.-L.; Allanson, M.; Domanski, D.; Arun, S.J.; Reeve, V.E. Oestrogen receptor-beta signaling protects against transplanted skin tumor growth in the mouse. Photochem. Photobiol. Sci. 2010, 9, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Schmid, P.C.; Ma, W.Y.; Schmidt, H.H.; Dong, Z. Phosphatidylinositol-3 kinase is necessary for 12-O-tetradecanoylphorbol 13-acetate-induced cell transformation and activated protein 1 activation. J. Biol. Chem. 1997, 272, 4187–4194. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Sundaram, C.; Reuter, S.; Aggarwal, B.B. Inhibiting NFkappaB activation by small molecules as a therapeutic strategy. Biochim. Biophys. Acta 2010, 1799, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. NF-kB, the first quarter-century: Remarkable progress and outstanding questions. Genes Dev. 2012, 26, 203–234. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.S.; Yoon, Y.D.; Han, M.H.; Han, S.B.; Lee, K.; Kang, M.R.; Moon, E.Y.; Jeon, Y.J.; Park, S.K.; Kim, H.M. Estrogen receptor-independent inhibition of tumor necrosis factor-alpha gene expression by phytoestrogen equol is mediated by blocking nuclear factor-kappa B activation in mouse macrophages. Biochem. Pharm. 2005, 71, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.S.; Yoon, Y.D.; Han, M.H.; Han, S.D.; Lee, K.; Park, S.K.; Kim, H.M. Equol inhibits nitric oxide production and inducible nitric oxide synthase gene expression through down-regulating the activation of Akt. Int. Immunopharmacol. 2007, 7, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Lephart, E.D. A review of the role of estrogen in dermal aging and facial attractiveness in women. J. Cosmet. Dermatol. 2018, in press. [Google Scholar]
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lephart, E.D. Equol’s Anti-Aging Effects Protect against Environmental Assaults by Increasing Skin Antioxidant Defense and ECM Proteins While Decreasing Oxidative Stress and Inflammation. Cosmetics 2018, 5, 16. https://doi.org/10.3390/cosmetics5010016
Lephart ED. Equol’s Anti-Aging Effects Protect against Environmental Assaults by Increasing Skin Antioxidant Defense and ECM Proteins While Decreasing Oxidative Stress and Inflammation. Cosmetics. 2018; 5(1):16. https://doi.org/10.3390/cosmetics5010016
Chicago/Turabian StyleLephart, Edwin D. 2018. "Equol’s Anti-Aging Effects Protect against Environmental Assaults by Increasing Skin Antioxidant Defense and ECM Proteins While Decreasing Oxidative Stress and Inflammation" Cosmetics 5, no. 1: 16. https://doi.org/10.3390/cosmetics5010016



