New Biological Activities of Lythrum salicaria L.: Effects on Keratinocytes, Reconstructed Epidermis and Reconstructed Skins, Applications in Dermo-Cosmetic Sciences
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Hydro-Alcoholic Extraction
2.3. Characterization of Major Compounds
2.3.1. Isolation
2.3.2. ESI-Q-TOF-HRMS and MS2 Analysis
2.3.3. NMR Analysis
2.3.4. Content in Major Compounds
2.4. Biological Activity
2.4.1. Monolayer of Keratinocytes Culture and Treatments
2.4.2. Reconstructed Human Epidermis Culture and Treatments
2.4.3. Full Thickness Reconstructed Skins Culture and Treatments
2.4.4. Expression of Genes Monitored by RT-qPCR Array
2.4.5. Immunofluorescence
2.4.6. ELISA Tests
2.4.7. Histological Analysis
2.4.8. Statistical Analysis
3. Results
3.1. Extraction, Isolation, Structure Determination and Quantification of Major Compounds
3.2. Cytotoxicity
3.3. Pro-Differentiation Effect of Lythrum Salicaria Extract
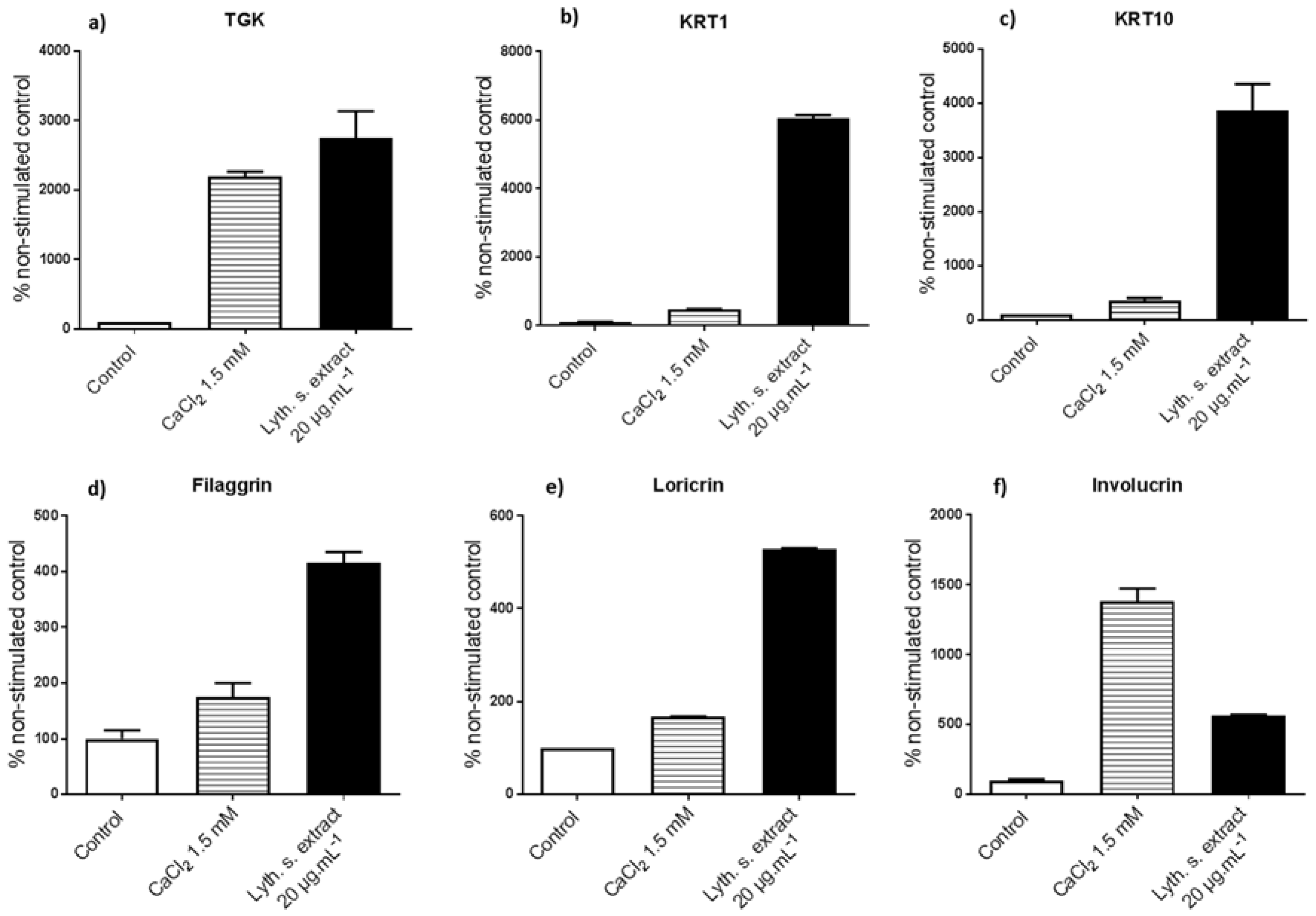
3.3.1. Levels of Expression of Genes Implicated in Differentiation
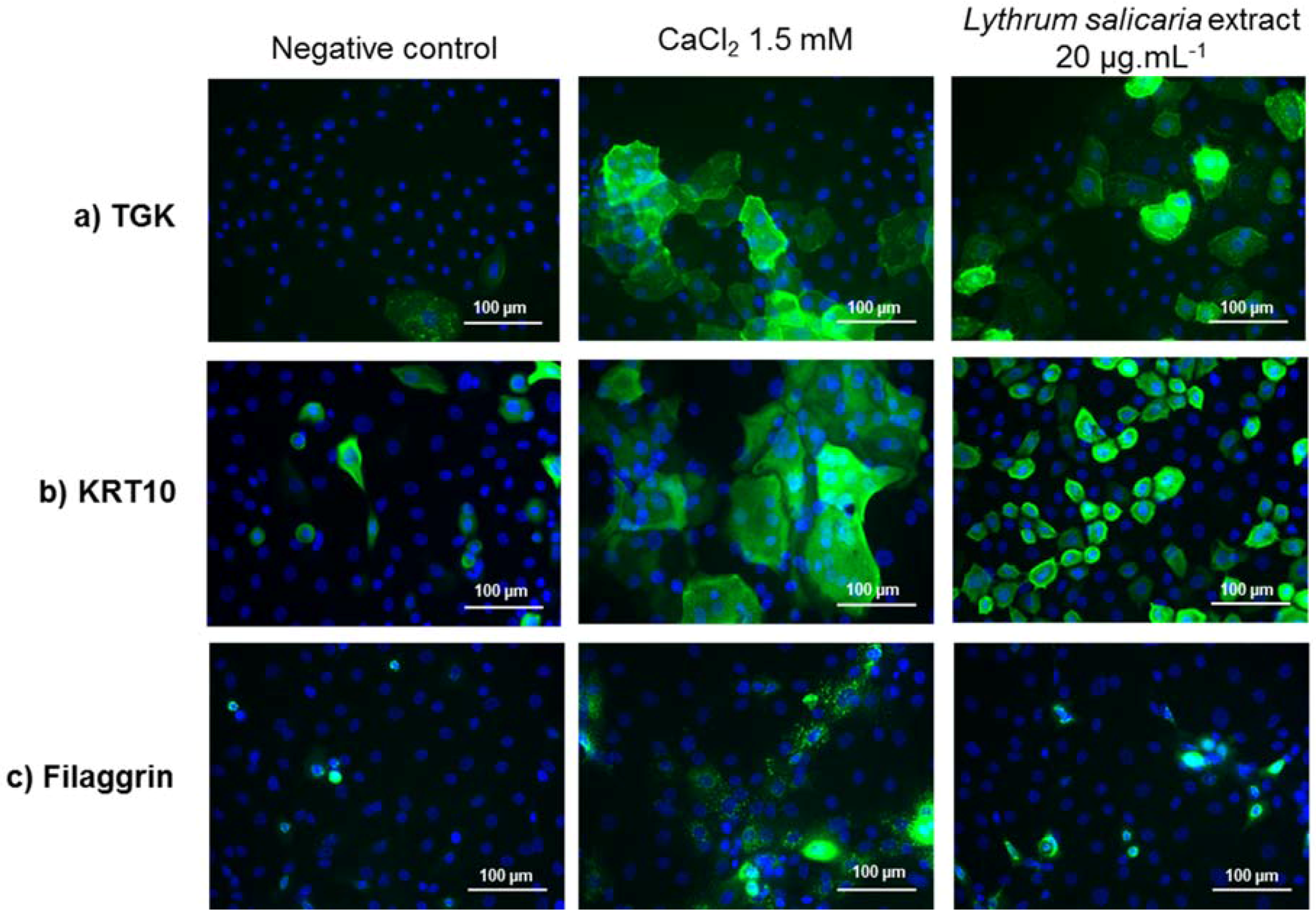
3.3.2. Expressions of Characteristic Proteins in Differentiated Keratinocytes
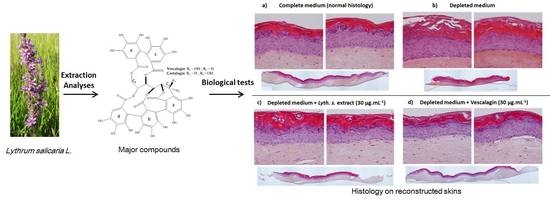
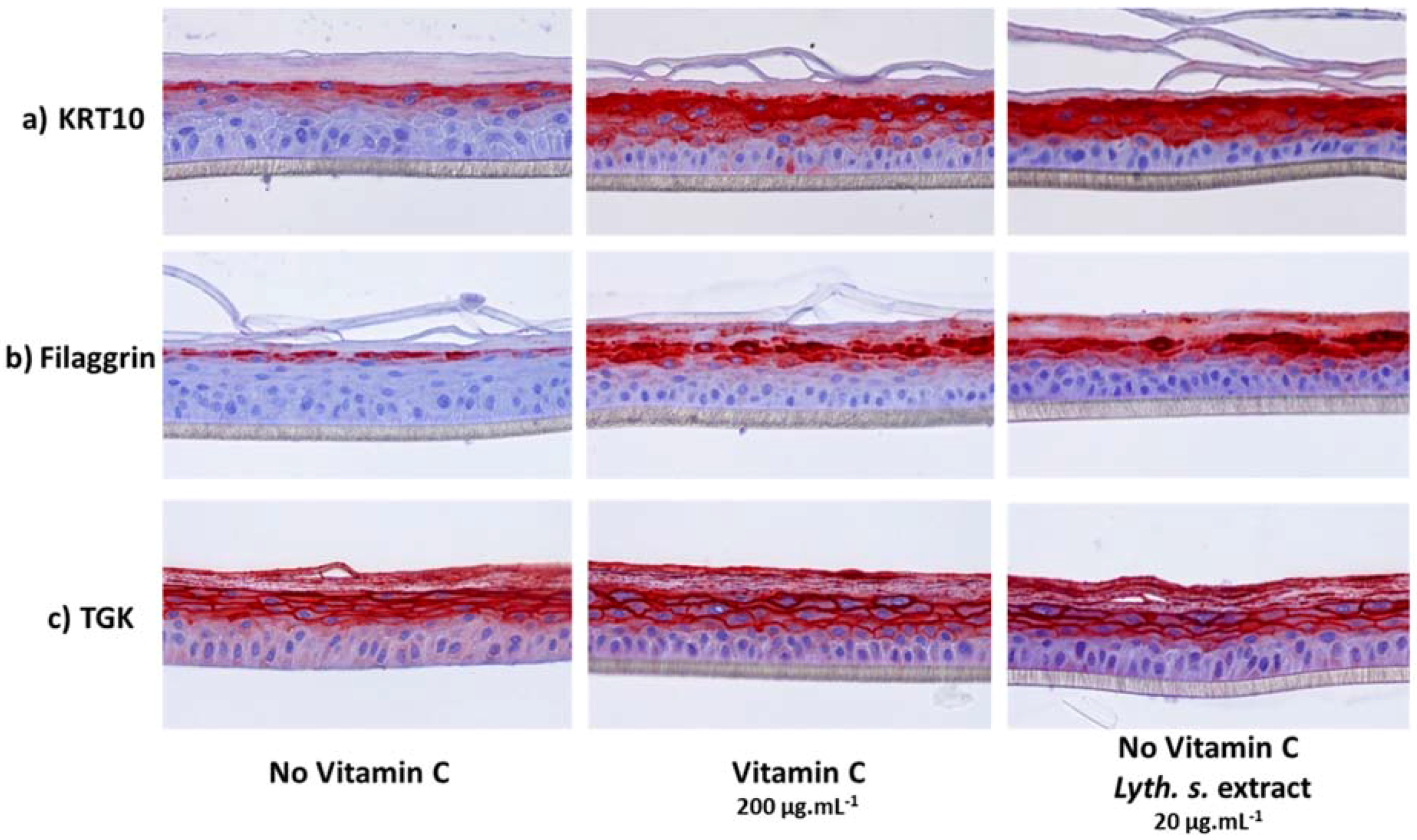
3.3.3. Histological Characterization of Reconstructed Human Epidermis (RHE)
3.4. Reinforcement of the Skin
3.4.1. Skin Barrier Function Enhancement on Full Thickness Reconstructed Skin Models
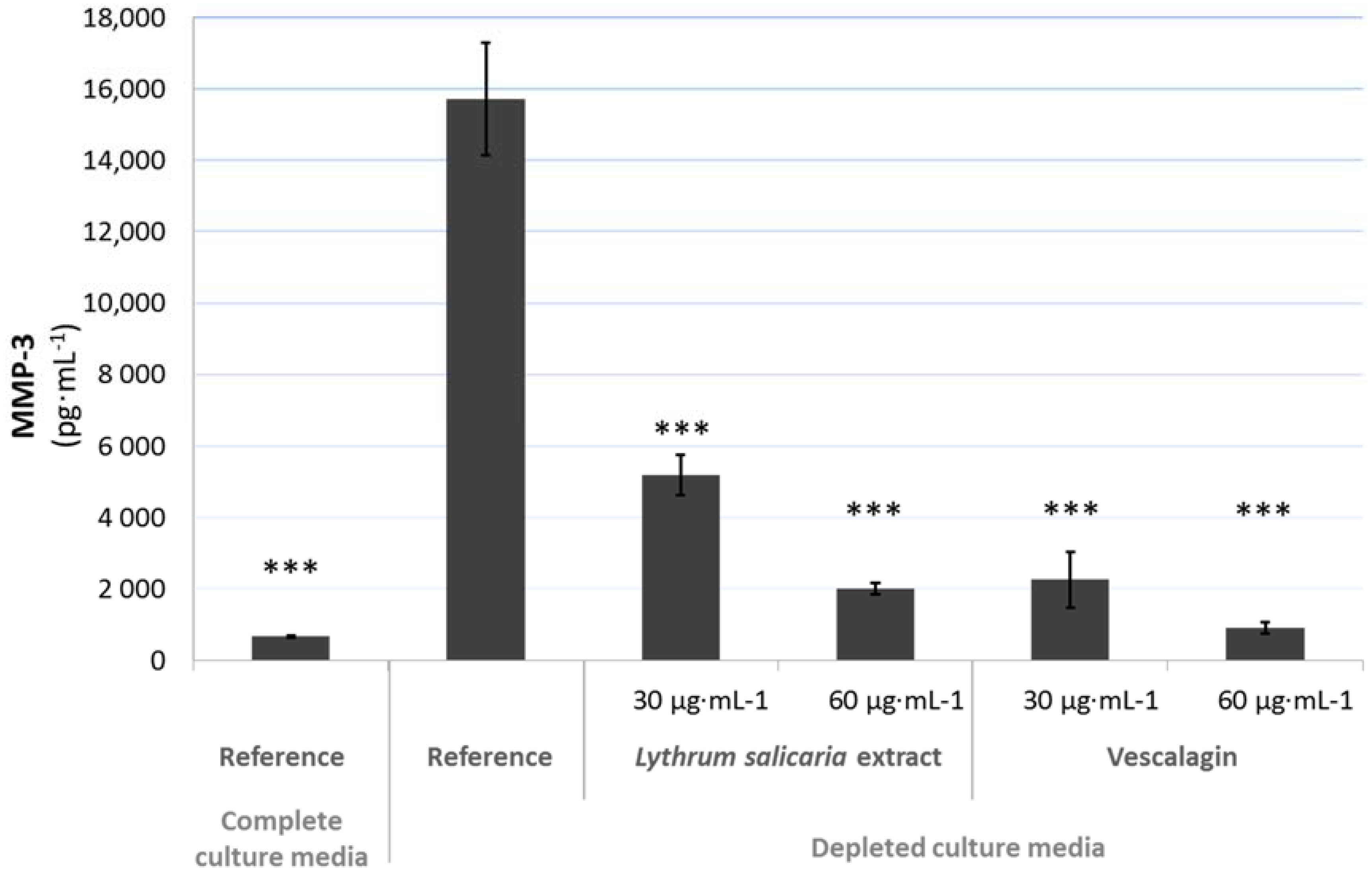
3.4.2. Beneficial Effects on Extracellular Matrix
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Piwowarski, J.P.; Granica, S.; Kiss, A.K. Lythrum salicaria L.—Underestimated medicinal plant from European traditional medicine. A review. J. Ethnopharmacol. 2015, 170, 226–250. [Google Scholar] [CrossRef] [PubMed]
- Manayi, A.; Khanavi, M.; Saiednia, S.; Hadjiakhoondi, A.; Azizi, E.; Mahmoodpour, M.; Vafi, F.; Siavashi, F.; Malmir, M. Biological activity and microscopic characterization of Lythrum salicaria L. Daru J. Pharm. Sci. 2013, 21, 61. [Google Scholar] [CrossRef] [PubMed]
- Piwowarski, J.P.; Kiss, A.K. C-glucosidic Ellagitannins from Lythri herba (European Pharmacopoeia): Chromatographic Profile and Structure Determination. Phytochem. Anal. 2013, 24, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Maity, N.; Nema, N.K.; Sarkar, B.K. Bioactive compounds from natural resources against skin aging. Phytomedicine 2011, 19, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Allemann, I.B.; Baumann, L. Botanicals in skin care products. Int. J. Dermatol. 2009, 48, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Simpson, C.L.; Patel, D.M.; Green, K.J. Deconstructing the skin: Cytoarchitectural determinants of epidermal morphogenesis. Nat. Rev. Mol. Cell Biol. 2011, 12, 565–580. [Google Scholar] [CrossRef] [PubMed]
- Candi, E.; Schmidt, R.; Melino, G. The cornified envelope: A model of cell death in the skin. Nat. Rev. Mol. Cell Biol. 2005, 6, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Holleran, W.M.; Takagi, Y.; Menon, G.K.; Legler, G.; Feingold, K.R.; Elias, P.M. Processing of epidermal glucosylceramides is required for optimal mammalian cutaneous permeability barrier function. J. Clin. Investig. 1993, 91, 1656–1664. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, A.V. Trends in stratum corneum research and the management of dry skin conditions. Int. J. Cosmet. Sci. 2003, 25, 63–95. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, E.; Raghavan, S. Getting under the skin of epidermal morphogenesis. Nat. Rev. Genet. 2002, 3, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, S.; Ponec, M. Intrinsic regulation of differentiation markers in human epidermis, hard palate and buccal mucosa. Arch. Oral Biol. 2000, 45, 149–158. [Google Scholar] [CrossRef]
- Marte, B.; Finkelstein, J.; Anson, L. Skin Biology. Nature 2007, 445, 833. [Google Scholar] [CrossRef]
- Rinnerthaler, M.; Streubel, M.K.; Bischof, J.; Richter, K. Skin aging, gene expression and calcium. Exp. Gerontol. 2015, 68, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Baumann, L.; Woolery-Lloyd, H.; Friedman, A. Natural Ingredients in Cosmetic Dermatology. J. Drugs Dermatol. 2009, 8, s5–s9. [Google Scholar] [PubMed]
- Rabeony, H.; Petit-Paris, I.; Garnier, J.; Barrault, C.; Pedretti, N.; Guilloteau, K.; Jegou, J.-F.; Guillet, G.; Huguier, V.; Lecron, J.-C.; et al. Inhibition of Keratinocyte Differentiation by the Synergistic Effect of IL-17A, IL-22, IL-1α, TNFα and Oncostatin M. PLoS ONE 2014, 9, e101937. [Google Scholar] [CrossRef] [PubMed]
- Guenou, H.; Nissan, X.; Larcher, F.; Feteira, J.; Lemaitre, G.; Saidani, M.; Del Rio, M.; Barrault, C.C.; Bernard, F.-X.; Peschanski, M.; et al. Human embryonic stem-cell derivatives for full reconstruction of the pluristratified epidermis: A preclinical study. Lancet 2009, 374, 1745–1753. [Google Scholar] [CrossRef]
- Rauha, J.-P.; Wolfender, J.-L.; Vuorela, H. Characterization of the polyphenolic composition of purple loosestrife (Lythrum salicaria). Z. Für Naturforschung C 2000, 56c, 13–20. [Google Scholar] [CrossRef]
- Nonaka, G.; Nishioka, I. Tannins and Related Compounds. XCVII. Structure revision of C-glycosidic ellagitannins, Castalagin, Vescalagin, Casuarinin and Stachyurin, and related hydrolyzable tannins. Chem. Pharm. Bull. (Tokyo) 1990, 38, 2151–2156. [Google Scholar] [CrossRef]
- Tanaka, T.; Kouno, I. Four new C-glycosidic ellagitannins, Castacrenins D-G, from Japanese Chesnut Wood (Castanea crenata SIEB. et ZUCC.). Chem. Pharm. Bull. (Tokyo) 1997, 45, 1751–1755. [Google Scholar] [CrossRef]
- Lee, W. J.; Park, K. H.; Cha, H. W.; Sohn, M. Y.; Park, K. D.; Lee, S.-J.; Kim, D. W. The Expression of Involucrin, Loricrin, and Filaggrin in Cultured Sebocytes. Ann. Dermatol. 2014, 26, 134. [Google Scholar] [CrossRef] [PubMed]
- Vičanová, J.; Boelsma, E.; Mommaas, A.M.; Kempenaar, J.A.; Forslind, B.; Pallon, J.; Egelrud, T.; Koerten, H.K.; Ponec, M. Normalization of epidermal calcium distribution profile in reconstructed human epidermis is related to improvement of terminal differentiation and stratum corneum barrier formation. J. Investig. Dermatol. 1998, 111, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Lyga, J. Inflammaging in Skin and other Tissues - The Roles of Complement System and Macrophage. Inflamm. Allergy - Drug Targets 2014, 13, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Philips, N.; Auler, S.; Hugo, R.; Gonzalez, S. Beneficial Regulation of Matrix Metalloproteinases for Skin Health. Enzyme Res. 2011, 2011, 1–4. [Google Scholar] [CrossRef] [PubMed]




| Compound | m/z + | Molecular Formula | Error (ppm) | MS2 | Molecular Formula | m/z − | Molecular Formula | Error (ppm) | MS2 | Molecular Formula |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 935.0783 (100) [M+H]+ 952.1050 (32) [M+NH4]+ | C41H27O26 C41H30O26N | 0.3 0.1 | 935.0784 (71) [M+H]+ 917.0671 (18) [M-H2O+H]+ 873.0776 (9) [M-CO2-H2O+H]+ 633.0719 (100) [M-HHDP+H]+ 615.0616 (75) [M-HHDP-H2O+H]+ 597.0511 (24) [M-HHDP-2H2O+H]+ 553.0608 (33) [M-HHDP-2H2O-CO2+H]+ 535.0506 (66) [M-HHDP-3H2O-CO2+H]+ 523.0507 (17) 517.0400 (23) [M-HHDP-4H2O-CO2+H]+ 505.0400 (50) 495.0193 (43) 469.0399 (42) 303.0137 (14) [HHDP+H]+ 277.0343 (16) | C41H27O26 C41H25O25 C40H25O23 C27H21O18 C27H19O17 C27H17O16 C26H17O14 C26H15O13 C25H15O13 C26H13O12 C25H13O12 C23H11O13 C22H13O12 C14H7O8 C13H9O7 | 933.0630 (47) [M-H]− 477.0189 (10) [M-3H+Na]2− 466.0282 (100) [M-2H]2− 300.9989 (8) [HHDP-H]− | C41H25O26 C41H23NaO26 C41H24O26 C14H5O8 | 1.0 0.9 0.4 0.3 | 933.0635 (57) [M-H]− 915.0521 (27) [M-H2O-H]− 897.0449 (5) [M-2H2O-H]− 889.0733 (9) [M-CO2-H]− 871.0653 (13) [M-CO2-H2O-H]− 613.0471 (16) [M-HHDP-H2O-H]− 569.0574 (34) [M-HHDP-CO2-H2O-H]− 493.0052 (92) 489.0463 (32) 467.0254 (89) 425.0150 (26) 300.9992 (100) [HHDP-H]− 275.0192 (40) 249.0404 (54) | C41H25O26 C41H23O25 C41H21O24 C40H25O24 C40H23O23 C27H17O17 C26H17O15 C23H9O13 C25H13O11 C22H11O12 C20H9O11 C14H5O8 C13H7O7 C12H9O6 |
| 2 | 935.0783 (100) [M+H]+ 952.1049 (40) [M+NH4]+ | C41H27O26 C41H30O26N | 0.3 0.2 | 935.0781 (34) [M+H]+ 917.0677 (7) [M-H2O+H]+ 873.0781 (10) [M-CO2-H2O+H]+ 633.0721 (68) [M-HHDP+H]+ 615.0615 (40) [M-HHDP-H2O+H]+ 597.0511 (20) [M-HHDP-2H2O+H]+ 553.0612 (26) [M-HHDP-2H2O-CO2+H]+ 535.0506 (34) [M-HHDP-3H2O-CO2+H]+ 523.0503 (12) 517.0402 (10) [M-HHDP-4H2O-CO2+H]+ 505.0400 (16) 495.0195 (81) 469.0402 (100) 439.0295 (14) 303.0136 (8) [HHDP+H]+ 277.0343 (10) | C41H27O26 C41H25O25 C40H25O23 C27H21O18 C27H19O17 C27H17O16 C26H17O14 C26H15O13 C25H15O13 C26H13O12 C25H13O12 C23H11O13 C22H13O12 C21H11O11 C14H7O8 C13H9O7 | 933.0633 (27) [M-H]− 477.0192 (18) [M-3H+Na]−2 466.0282 (100) [M-2H]2− | C41H25O26 C41H23NaO26 C41H24O26 | 0.6 0.2 0.3 | 933.0634 (100) [M-H]− 915.0514 (17) [M-H2O-H]− 889.0757 (12) [M-CO2-H]− 871.0619 (9) [M-CO2-H2O-H]− 631.0574 (64) [M-HHDP-H]− 569.0576 (41) [M-HHDP-CO2-H2O-H]− 467.0257 (32) 425.0150 (74) 300.9990 (99) [HHDP-H]− 275.0199 (33) 249.0407 (28) | C41H25O26 C41H23O25 C40H25O24 C40H23O23 C27H19O18 C26H17O15 C22H11O12 C20H9O11 C14H5O8 C13H7O7 C12H9O6 |
| Type of Protons and/or Carbons | Position | Compound 1 | Compound 2 | ||
|---|---|---|---|---|---|
| δC | δH | δC | δH | ||
| Glycosyl | 1 | 65.37 | 4.87 (d; 2.00) | 65.62 | 5.71 (d,4.40) |
| 2 | 77.61 | 5.19–5.23 (m; na) | 73.36 | 5.03–5.05 (m; na) | |
| 3 | 68.29 | 4.56 (dd; 0.80/6.80) | 66.38 | 5.03–5.05 (m; na) | |
| 4 | 69.17 | 5.19–5.23 (m; na) | 68.51 | 5.25 (t; 7.20) | |
| 5 | 71.03 | 5.65 (d; 6.40) | 70.50 | 5.62 (dd; 1.60/8.00) | |
| 6 | 77.61 | 5.08 (dd; 2.80) | 64.65 | 5.11 (dd; 2.40/12.80) | |
| 6’ | 4.01 (d; 12.80) | 4.00 (d; 12.80) | |||
| Aromatic | Har (c) | 6.77 (s; na) | 6.81 (s; na) | ||
| Har (d) | 6.76 (s; na) | 6.78 (s; na) | |||
| Har (e) | 6.61 (s; na) | 6.63 (s; na) | |||
| C=O (a) | 165.37 | 164.04 | |||
| C=O (b) | 165.49 | 164.98 | |||
| C=O (c) | 166.50 | 165.99 | |||
| C=O (d) | 167.23 | 166.55 | |||
| C=O (e) | 169.27 | 168.58 | |||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jouravel, G.; Guénin, S.; Bernard, F.-X.; Elfakir, C.; Bernard, P.; Himbert, F. New Biological Activities of Lythrum salicaria L.: Effects on Keratinocytes, Reconstructed Epidermis and Reconstructed Skins, Applications in Dermo-Cosmetic Sciences. Cosmetics 2017, 4, 52. https://doi.org/10.3390/cosmetics4040052
Jouravel G, Guénin S, Bernard F-X, Elfakir C, Bernard P, Himbert F. New Biological Activities of Lythrum salicaria L.: Effects on Keratinocytes, Reconstructed Epidermis and Reconstructed Skins, Applications in Dermo-Cosmetic Sciences. Cosmetics. 2017; 4(4):52. https://doi.org/10.3390/cosmetics4040052
Chicago/Turabian StyleJouravel, Glorianne, Samuel Guénin, François-Xavier Bernard, Claire Elfakir, Philippe Bernard, and Franck Himbert. 2017. "New Biological Activities of Lythrum salicaria L.: Effects on Keratinocytes, Reconstructed Epidermis and Reconstructed Skins, Applications in Dermo-Cosmetic Sciences" Cosmetics 4, no. 4: 52. https://doi.org/10.3390/cosmetics4040052