1. Introduction
Collagen fibrils, which comprise the bulk of the dermis, provide structural integrity and resilience in the skin. Collagen fibrils are primarily composed of type I collagen (COL1) [
1,
2]. Skin COL1 content declines with the passage of time (natural aging) and repetitive sun exposure (photoaging). Aging of sun-exposed skin such as the face and the forearms is the consequence of both natural aging and photoaging. Reduced COL1 content is a causal factor for elderly appearance, including loss of elasticity, wrinkling and thinning. Thin skin in the elderly is also prone to wounding and wound healing disorders.
The age-associated decline of skin COL1 content is in part due to reduced COL1 production. COL1 is produced as a precursor, type I procollagen (proCOL1), by fibroblasts in the dermis [
3]. After proCOL1 is secreted to the extracellular space, it is converted to mature COL1 by enzymatic removal of amino and carboxyl propeptides. COL1 mRNA levels in human skin in vivo can be quantified using reverse transcription followed by real-time PCR; proCOL1 protein levels can be quantified by enzyme immunoassay (EIA) and immunostaining, which utilize antibodies recognizing propeptides of proCOL1 [
4,
5].
Both COL1 mRNA and proCOL1 protein synthesis by fibroblasts are reduced in the skin of the elderly versus young adults [
4,
6,
7,
8]. Restoration of COL1 synthesis and ultimately COL1 content in aged skin holds promise to enhance the skin’s mechanical strength and its resistance to bruising. Accumulating evidence suggests that activities of skin fibroblasts in elderly individuals can be enhanced. We have found that COL1 production in the skin of the elderly can be enhanced within a few weeks by superficial skin wounding elicited by cosmetic procedures, such as microdermabrasion and skin resurfacing using laser ablation [
1,
8,
9,
10]. While the above cosmetic surgeries are effective in improving skin appearance and COL1 production, they can only be applied to a small area of skin. Topical agents that can safe and effectively enhance skin COL1 production in the elderly are desirable.
Vitamin A (all-trans-retinol, ROL) can be converted to retinal (RAL), which is further metabolized to all-trans-retinoic acid (RA) in epidermal keratinocytes. RA is the endogenous active form of ROL and RAL. RA exerts biological functions via binding to nuclear receptors and thus regulating gene expression [
11]. Clinical trials performed by us and other investigators have demonstrated that treatment with daily topical retinoids over six months can reduce the wrinkled appearance of the elderly [
12,
13]. A few trials quantified proCOL1 levels at the end of the treatment period, which ranged from six to 12 months. These studies found that proCOL1 levels are enhanced in RA/ROL-treated versus vehicle-treated sites. In addition, topical RA/ROL elevates basal keratinocyte proliferation and consequently the thickness of the epidermis, which is typically thinner in aged versus young adults [
14,
15].
The above evidence provides a foundation for carrying out investigations aimed at determining whether and to what extent COL1 synthesis in aged skin can be significantly enhanced by topical agents in a short period of time, such as a few weeks, which is an important question to address in terms of establishing a test to evaluate potential COL1-enhancing agents. Although countless skin care products have claimed to be capable of improving COL1 synthesis, most, if not all, of these claims are not substantiated by rigorous vehicle-controlled efficacy tests that directly measure COL1 mRNA and protein synthesis in human skin in vivo. Although cultured fibroblasts and skin equivalents are often used for evaluating potential COL1-enhancing agents, evaluations performed directly on human skin provide the most clinically relevant results [
16,
17,
18,
19]. For the purpose of testing efficacy, shorter treatment duration would be ideal because it is more cost-efficient and confounding factors such as compliance and sun exposure can be controlled better.
Herein, we investigated the effect of a four-week topical ROL (0.4%) treatment on the restoration of skin COL1 production in photoaged forearms in elderly individuals (>65 years of age).
2. Materials and Methods
2.1. Preparation of ROL Lotion
ROL was dissolved in vehicle (95% ethanol–propylene glycol (7:3, vol/vol)) and incorporated into a moisturizing lotion (Neutrogena; Neutrogena Corp, Los Angeles, CA, USA) to a final concentration of 0.4% (wt/vol). The same amount of vehicle without ROL was also incorporated into the moisturizing lotion to make control lotion.
2.2. Topical ROL Treatment and Skin Tissue Procurement
All procedures involving human subjects were approved by the University of Michigan Institutional Review Board, and were carried out according to the principles of the Declaration of Helsinki. Written informed consent was obtained from each subject. Aged (>65 years old) volunteers with moderate to severe photo-damage on the forearms were recruited. Young (23 to 33 years old) volunteers without photo-damage on the forearms were recruited as controls. Photoaged forearm skin (one square inch) was treated with 35 μL moisturizing lotion containing ROL (0.4%) or control lotion. Photo-damage between the left and right forearm in the same person may differ. To avoid this potential variation, treatments were applied on the same arm. Treated sites were separated by at least one inch. Control lotion was also applied on the forearm of young individuals in the same manner. The control moisturizing lotion alone does not affect COL1 synthesis or induce histologically noticeable changes when used in various treatment regimens (authors’ unpublished observations).
In the case of occlusion, treated sites were occluded under plastic wrap secured by surgical tape and covered by a Tegaderm patch for 24 h. In some experiments, one-day occlusion was applied once and skin samples (4 mm in diameter) were obtained by punch biopsies immediately after one-day occlusion or three days later. In some experiments, one-day occlusion was applied once per week for four weeks. Skin samples were obtained one week after the last application. Skin samples were placed in OCT (optimum cutting temperature), snap-frozen and stored at −80 °C until processing.
2.3. RNA Extraction and Real-Time RT-PCR
Total RNA was extracted from skin sections obtained from OCT embedded specimens using an RNA extraction kit (Qiagen, Chatsworth, CA, USA). Reverse transcription followed by real-time polymerase chain reaction (real time RT-PCR) was performed using 100 ng of total RNA as described previously [
4]. PCR results were normalized to the level of housekeeping gene 36B4.
2.4. Immunofluorescent Staining
Frozen skin sections (7 μm thick) were fixed in 4% paraformaldehyde and stained with antibodies which recognize Ki67 (Biogenex, Fremont, CA, USA), amino-terminal and carboxyl-terminal proCOL1 propeptide (both from Millipore, Billerica, MA, USA), respectively. Staining per unit area was quantified using computerized image analysis.
2.5. proCOL1 Enzyme Immunoassay (EIA)
Two 50-μm-thick frozen skin sections were prepared from OCT-embedded specimens and placed on membrane slides (Leica Microsystems Inc., Buffalo Grove, IL, USA). Dermal areas of sections were measured with Image ProPlus software (Media Cybernetics, Bethesda, MD, USA) and used to calculate total dermal volume of sections. The dermis was collected using laser capture microdissection (Leica ASLMD System; Leica Microsystems). Soluble proteins were extracted from collected dermis using extraction buffer (50 mM Tris hydrochloride, pH 7.4; 0.15 M sodium chloride; 1% Triton X-100; protease inhibitors (Complete Mini; Roche Diagnostics, Indianapolis, IN, USA)). proCOL1 levels in protein extracts were determined using an EIA kit (Takara Bio, Otsu Shiga, Japan) and normalized to volumes of corresponding specimens.
2.6. Assessment of Skin Erythema
Skin erythema was assessed by objective measurement using a chromameter (Minolta CR200; Minolta, Osaka, Japan) and subjective visual evaluation by an experienced investigator. Results obtained by both methods were similar. The erythema level measured by chromameter is shown in
Supplemental Materials.
2.7. Statistical Analysis
The significances of differences between groups of samples were determined by one-way ANOVA followed by Tukey-Kramer honestly significance difference test. Differences were considered significant when p was less than 0.05. Data are shown as mean ± standard error of mean (SEM). N numbers indicate sample size of each group. * p < 0.05, ** p < 0.01. Linear regression was used to determine the correlation between ROL induced erythema and proCOL1 protein expression.
3. Results
We initially investigated whether occlusion was necessary to induce a rapid, robust skin response to topical ROL. We found that in comparison with ROL without occlusion, one-day-occluded ROL (0.4%) induced an approximately 10-fold higher mRNA expression of cellular retinoic acid binding protein II (CRABPII), which is a well-characterized marker for retinoid activity (
Supplementary Materials Figure S1) [
11]. Thus, occlusion substantially enhances the short-term effect of ROL treatment. The expression of CRABPII did not differ between occlusion versus no occlusion on vehicle-treated skin, indicating that occlusion per se does not stimulate the action of ROL. We, therefore, utilized occluded ROL in the rest of our studies.
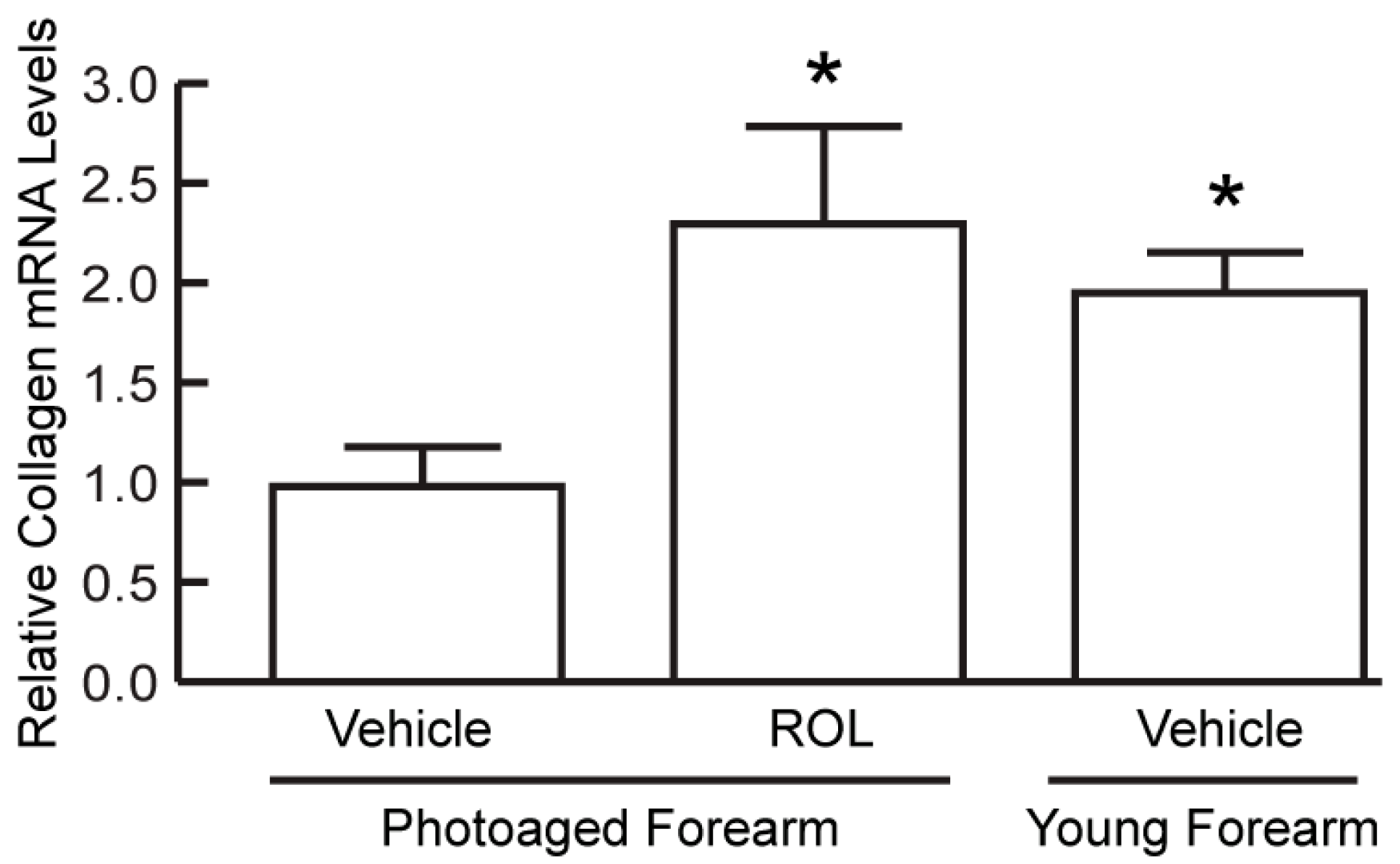
We found that a single ROL treatment under four-day or seven-day continuous occlusion did not reproducibly enhance COL1 synthesis, indicating that longer ROL treatment is required. We, thus, investigated a four-week regimen involving one-day occlusion of ROL or vehicle on photoaged forearm skin once a week for four weeks. Young (23–33 years old) forearm skin was treated with vehicle alone in the same manner. Skin samples were obtained seven days after the last treatment and analyzed for COL1 mRNA and type I procollagen (proCOL1) protein levels. COL1 mRNA levels were approximately 50% (
n = 6–9,
p < 0.05) lower in photoaged versus young forearm skin (
Figure 1) as determined by RT-PCR. ROL enhanced COL1 mRNA expression by 230% (
n = 9,
p < 0.05) to a level comparable to that of young skin.
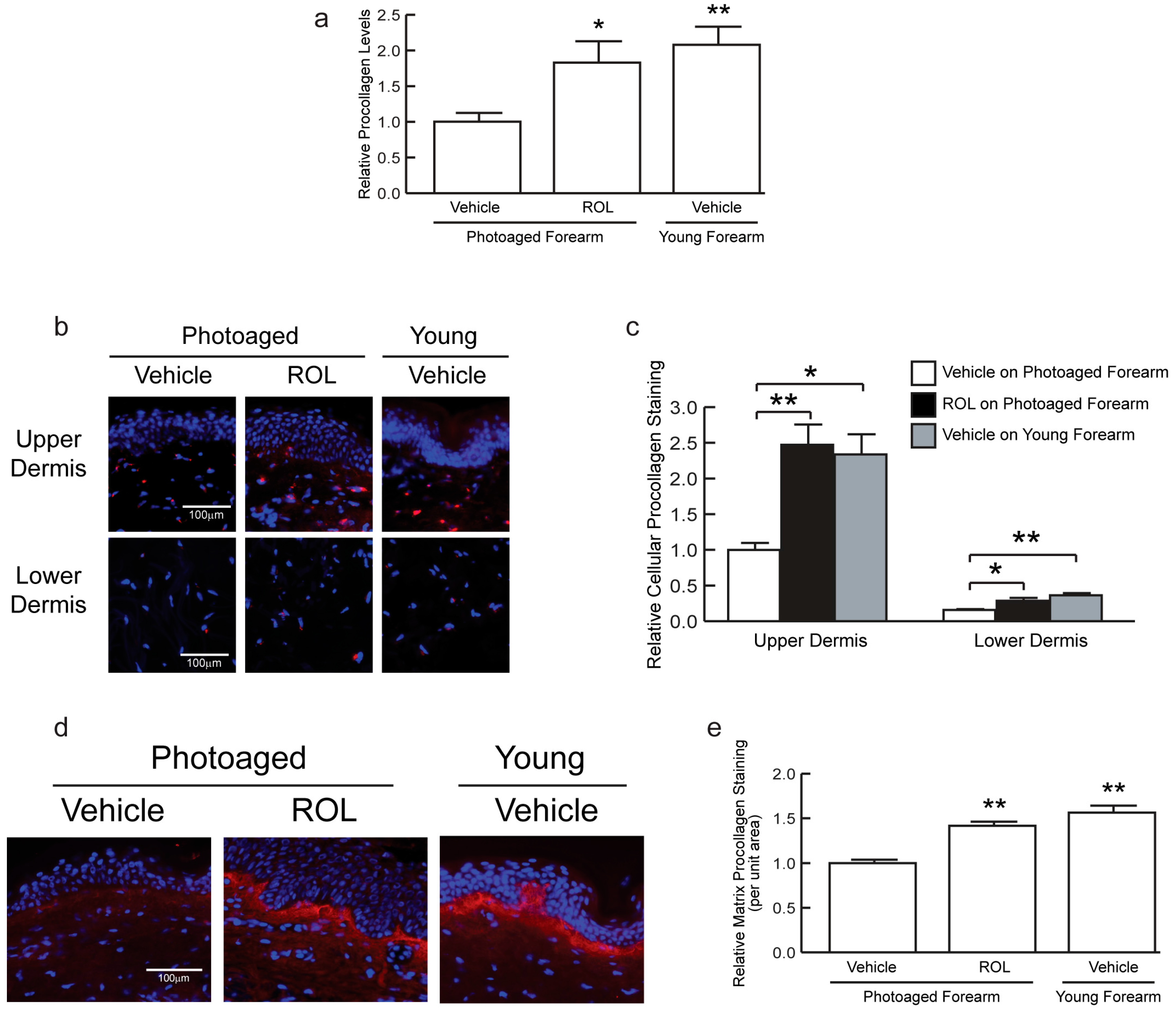
We determined proCOL1 levels by enzyme immunoassay (EIA) and immunostaining [
3,
6,
9]. The EIA results demonstrated that proCOL1 levels were approximately 56% (
n = 6–9, *
p < 0.05) lower in photoaged versus young forearm skin (
Figure 2a). ROL enhanced proCOL1 by 180% (
n = 9,
p < 0.05) to levels comparable to that of young skin.
proCOL1 was also examined by immunofluorescent staining (
Figure 2b–e). It has been shown that antibodies targeting the N- or C-terminal proCOL1 propeptide differentially reveal procollagen localized inside fibroblasts or the dermal extracellular matrix [
6]. Consistent with previously reported staining patterns, immunostaining using antibody targeting the C-terminal propeptide primarily revealed procollagen protein in fibroblasts (red staining associated with blue nuclei) throughout the dermis (
Figure 2b). proCOL1 staining was more pronounced in the upper than the lower dermis. ROL enhanced the staining in the upper dermis by 40% (
n = 6–7, **
p < 0.01) and the lower dermis by 90% (
n = 6–7, *
p < 0.05) compared to vehicle-treated sites in photoaged forearm skin (
Figure 2b,c). The results indicate that ROL enhances COL1 production throughout the whole dermis, which is consistent with the fact that topical ROL can penetrate into the lower dermis.
Immunostaining using anti-N-terminal propeptide antibody primarily revealed proCOL1 in the extracellular matrix (
Figure 2d), with the most pronounced staining displayed below the basement membrane. Staining was 42% (
n = 6–7, **
p < 0.01) greater in ROL-treated versus vehicle-treated skin in photoaged forearm skin (
Figure 2e). In addition to increasing COL1 expression, ROL treatment enhanced the epidermal thickness in photoaged skin as a consequence of enhanced basal keratinocyte proliferation (
Figure S2). It is worth noting that skin erythema, which typically appears after consecutive daily ROL application [
15], was observed in only two out of nine subjects (
Figure S3), indicating that the effect of ROL on enhancing the proCOL1 levels is not dependent on erythema. A low incidence of erythema also indicates that the intermittent ROL regimen reduces unwanted side effects while retaining the COL1-enhancing capacity. Thus, this regimen may have a superior therapeutic index than that of daily ROL application when used for anti-aging purposes.
4. Discussion
Age-dependent skin COL1 deficiency contributes to both clinical signs of aging and impairments of skin function [
1,
2]. To this end, boosting the COL1 content in aged skin has drawn much interest. Considerable effort has been spent on screening agents that can promote COL1 synthesis using preclinical approaches [
20]. Establishment of a short-term in vivo protocol to evaluate the COL1-enhancing activity of candidate agents would be desirable.
Retinoids, particularly RA and ROL, are considered the gold standard of anti-aging topical treatment because of their well-demonstrated efficacy in improving skin appearance [
12,
13]. We investigated ROL rather than RA and RAL because pharmaceutical grade ROL was readily available at the time when we performed this study. It was also shown that RA/ROL is able to induce molecular and cellular changes in the skin within a few days. We previously found that a single four-day continuous occlusion of RA/ROL enhanced the mRNA expression of CRABPII and basal keratinocyte proliferation in human skin [
21]. The present study found that a single one-day occlusion enhanced epidermal keratinocyte proliferation and epidermal thickness, but did not consistently enhance proCOL1 levels.
In contrast to single one-day occlusion, a once-a-week treatment for four weeks enhanced COL1 mRNA expression and proCOL1 protein levels to that of young skin. Enhanced COL1 synthesis was observed in fibroblasts throughout the dermis, which is consistent with our previous study, which has shown that topical ROL was able to penetrate into cells throughout the skin, as enhanced CRABPII mRNA levels revealed by in situ hybridization were observed throughout the skin [
21]. The above data indicates a prolonged topical ROL treatment can uniformly elevate COL1 synthesis and consequently COL1 content in the whole dermis.
Increased proCOL1 protein levels were observed in all subjects examined, suggesting that enhancement of proCOL1 is highly reproducible after one-day occlusion for four consecutive weeks. The results demonstrate we have established a reliable short-term regimen for evaluating the ability of anti-aging topical agents to increase COL1 synthesis in human skin. Our study also supports the notion that age-associated reduction of COL1 production by skin fibroblasts is reversible, which provides a scientific foundation for developing anti–skin aging methods/agents aimed at restoring fibroblast function to improve the dermal extracellular matrix.
Topical RA/ROL often causes skin irritation, which has led to a speculation that irritation is necessary for the beneficial effect of RA/ROL [
13]. However, our previous and present studies have shown no correlation between irritation and the beneficial effect. We previously compared a long-term (48 weeks) topical treatment of 0.1% versus 0.025% RA [
22]. We found that the efficacy of these two concentrations in improving photoaged skin at both clinical and histological levels was similar, while 0.1% RA caused significantly greater side effects versus 0.025% RA. The present study has shown that a pronounced increase of COL1 synthesis occurred with the absence of visible skin irritation in most (seven out of nine) subjects, suggesting that irritation can be separated from the efficacy and the restoration of COL1 synthesis may be achieved without irritation by an intermittent treatment regimen.