1. Introduction
Telogen effluvium is a delayed consequence of a shift in the hair cycle phase away from anagen. Termination of anagen results in the onset of catagen and subsequently telogen. Telogen hair bulbs may remain anchored in the hair follicle until the onset of the next anagen phase at the end of telogen or be shed prematurely. The release of a telogen hair is called exogen. While some degree of telogen hair shedding is normal, excessive loss of telogen hairs will manifest as increased hair shedding or diffuse loss of hair volume [
1]. Increased hair shedding can be scored using validated visual analogue scales [
2]. Loss of hair volume can present with a reduction in the thickness of the pony tail when women have long hair, or as widening of the central part-line.
Important differential diagnoses include female pattern hair loss, anagen effluvium, loose anagen hair syndrome, diffuse alopecia areata, congenital atrichia, congenital hypotrichosis, and hair shaft fragility [
1,
3].
2. Physiology of Hair Shedding
In mammals, hair follicles undergo repetitive growth and resting phases. Hair follicle activity is cyclical. Each hair grows continuously at a rate of approximately 0.3 mm per day for the duration of anagen. At the end of anagen, the hair fibre is retained for a period of time without further hair growth until it is eventually shed and replaced. The duration of anagen varies greatly from region to region. On the moustache, anagen lasts about 4–14 weeks, on the arms 6–12 weeks, on the legs 19–26 weeks and on the scalp 3–5 years [
4]. The duration of anagen determines maximal hair length at each body site.
At the onset of catagen, apoptosis of keratinocytes in the hair bulb leads to involution of the transient portion of the follicle below the insertion of the arrector pili muscle. The process is complete within two weeks. Thereafter, the truncated follicle appears to be dormant for a further two months, before the next anagen cycle is initiated. Release of the dead hair from the follicle (exogen) occurs either late in telogen or early in anagen. Follicular activity within the anatomical region is synchronous in infancy. However, in humans, synchronous hair growth disappears in childhood [
1]. Thus, rather than all hairs being replaced at once in a single moult, normal human hair shedding occurs continuously with between 50 and 150 correct telogen hairs being shed each day [
5].
In the normal scalp, the total number of follicles remains constant throughout life. As the duration of telogen does not vary, anagen duration determines the proportion of follicles in the telogen. Trichograms of the normal scalp show 86% of hairs are in anagen, 1% is in catagen and 13% are in telogen [
6]. The biological clock that determines the end of anagen phase and the beginning of catagen/telogen phase is a complex phenomenon and can be influenced by various metabolic alterations such as pregnancy, malnutrition and other stressful conditions [
3].
The molecular basis is being revealed. Hair cycling is caused by rhythmic signal transducer changes in the bulge zone and dermal papilla region. The autonomous clock driving the hair follicle cycle resides in the hair follicle itself. It is controlled by a complex network of sequential activation of autocrine, paracrine and endocrine signalling pathways.
Hair cycle is regulated by signalling molecules of the Wnt correct family, Fibroblast Growth Factor (FGF), transforming growth factor β (TGF-β), and Hedgehog pathways.
Key inducers of anagen are Wnt family proteins, β-Catenin pathway, noggin, and the transcription factor Stat3. Furthermore, Sonic Hedgehog Proteins (Shh) correct and Hepatic Growth Factor (HGF) promotes anagen development. The duration of anagen phase prolong Insulin Growth Factor-1 (IGF-1), Vascular Endothelial Growth Factor (VEGF) and Thyrotropin-Releasing Hormone (TRH).
An important anagen prolongator/catagen inhibitor is polyamine-spermidine. Spermidine is a potent stimulator of human hair growth and a previously unknown modulator of human epithelial stem cell biology. Anagen is terminated by the concurrent decreasing of anagen upholding factors (IGF-1, HGF, FGF-5S) and increasing hair growth inhibitors like members of the transforming growth factor (TGF-β1, TGF-β2), and fibroblast growth factor. Dickkopf 1 (DKK-1) is involved in anagen-to-catagen transition in the hair cycle by regulating the activity of follicular keratinocytes.
Other involved controlling anagen-catagen transformation molecules are neurotrophins NT-3, NT-4, prolactin and retinoids. Prolactin (PRL) participates in the regulation of anagen and telogen initiation, and is produced by the follicle itself.
The signalling that controls hair cycle resting phase is only partly understood. On the contrary, telogen probably represents a key stage in hair cycle control. Bone Morphogenetic Protein 4 (BMP4) arrest hair follicle in the telogen phase. The hair cycle resting phase is regulated also by cyclic epithelial Fibroblast Growth Factor (FGF18). Exogen has its own control mechanisms, and it is presumed that its regulators are protease cathepsin L and Msx-2 [
7].
3. Pathogenesis of Telogen Effluvium
Headington described five functional alterations in the hair cycle that could lead to increased telogen hair shedding. These are immediate anagen release, delayed anagen release, short anagen syndrome, immediate telogen release, and delayed telogen release [
8].
Immediate anagen release is a short onset effluvium where a trigger terminates anagen prematurely. Follicles enter catagen and then telogen follicles and increased hair shedding occurs at the end of the telogen approximately 2–3 months later. It is common after physiological stress such as severe illness and with drug induced hair loss. Reversal is associated with resumption of normal cycle.
Immediate anagen release can also be associated with a premature exogen. This is seen in alopecia areata when telogen hairs may be shed from the edge of an enlarging patch within a few weeks of the patch first appearing.
Delayed anagen release is the cause of post-partum hair loss. During pregnancy, hairs remain in prolonged anagen rather than cycling into telogen. If large number of follicles are involved postpartum no comma telogen conversion will be accompanied by increased shedding some months later.
Short anagen syndrome is due to an idiopathic shortening of the duration of anagen and can cause a persistent telogen hair shedding in some individuals. This also occurs in loose anagen syndrome.
Immediate telogen release results from a shortening of normal telogen with release of club hairs as the follicles are stimulated to re-enter anagen. It is not known precisely how long a telogen hair remains in the follicle, but it is believed that club hairs are released 4–6 weeks after the onset of anagen. Drugs such as minoxidil can precipitate immediate telogen release.
Delayed telogen release occurs after prolonged telogen followed by transition to anagen. It occurs in animals with synchronous hair cycle during shedding of their winter coats. It may occur in some humans seasonally.
4. Clinical Definition of Increased Hair Shedding
4.1. Acute Telogen Effluvium
Telogen effluvium was first described as excessive scalp hair shedding disorder commencing 2–3 months after a triggering event such as high fever, surgical trauma, sudden starvation, haemorrhage, or initiation of a new drug treatment [
9]. In about 33% of cases of telogen effluvium, no trigger could be identified [
8]. Emotional stress is commonly identified as a cause of acute telogen effluvium, but the evidence for this is weak and there is no evidence that suggests the stresses of everyday life are sufficient to induce diffuse hair loss [
1]. The functional mechanism of the shedding is immediate anagen release [
10,
11]. How these events trigger hair shedding at a molecular level is not known.
Seasonal fluctuations in hair shedding are a frequent observation by women with long hair. However, it has not been reliably evaluated, and it is not known whether these fluctuations can produce hair shedding of sufficient severity for women to present complaining of hair loss [
10].
Telogen gravid arum occurs because the high circulating placental hormones prolong anagen and lead to a very full head of hair during pregnancy. The withdrawal of these trophic hormones at delivery causes all the overdue anagen hairs to simultaneously enter catagen. Telogen hairs are then shed a few months later [
4]. It is not known what hormone specifically is trophic to hair follicles. Oestrogen therapy does not prevent or arrest post-partum hair shedding.
Diagnosis and Management
In acute telogen effluvium, the hair pull test is strongly positive, with clumps of telogen hairs being extracted with ease from both the vertex and margin of the scalp (
Figure 1) [
12]. Inspection with the naked eye can distinguish anagen from telogen hairs. Telogen hairs have depigmented bulbs and absence of an inner root sheaths, whereas anagen hairs have inner root sheaths [
4]. Beau’s line of the nail may coexist.
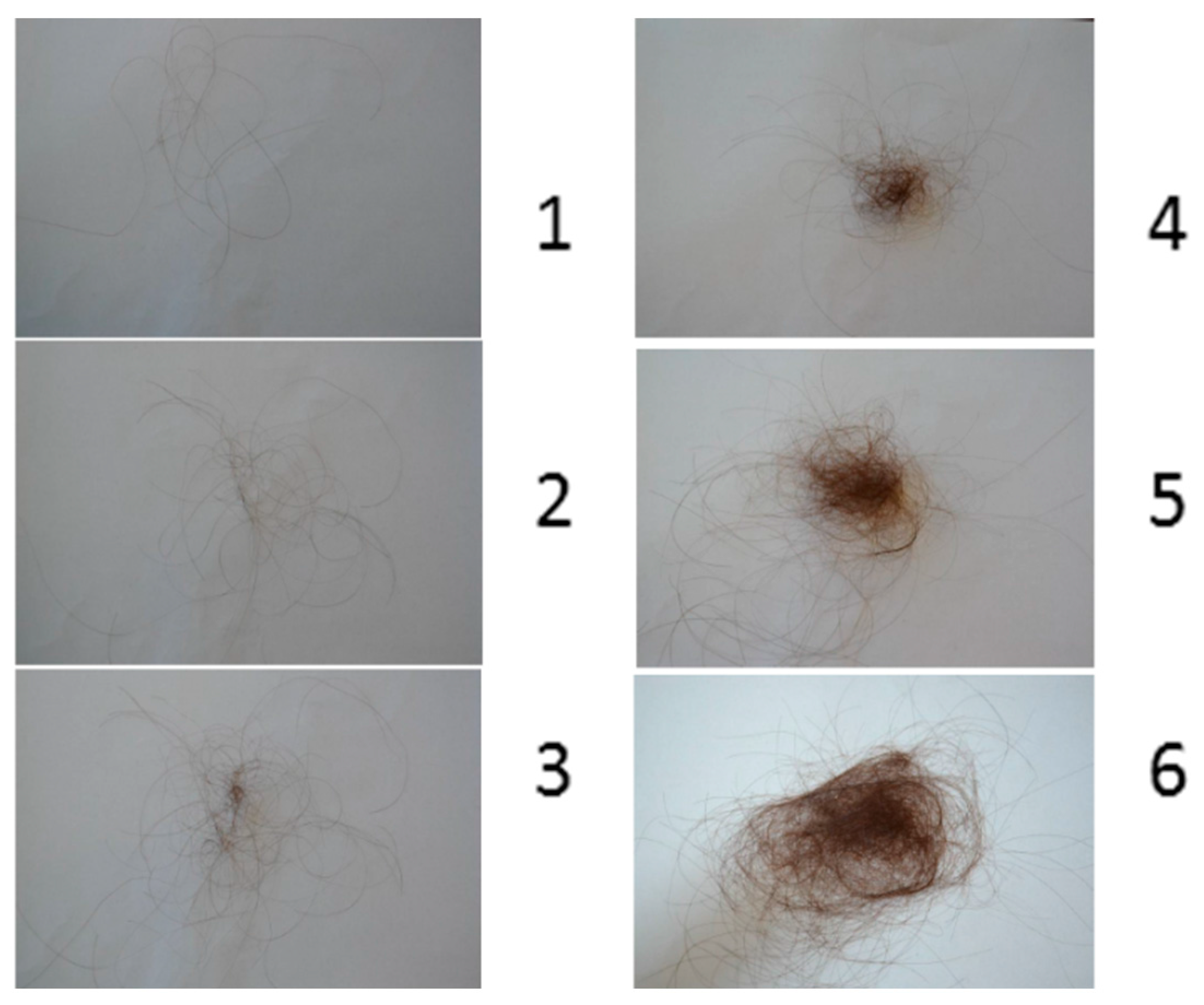
To measure hair shedding in women, a validated visual analogue scale has been developed. Hair shedding is scored on a scale of 1–6 (
Figure 2). Women are asked to look at the scale and point to the photograph that best correlates with the amount of hair shed on an average day. Grades 1–4 are considered normal. Grades 5 and 6 indicate excessive shedding [
3].
The trichogram from a hair pluck sample usually shows more than 25% telogen hairs in acute telogen effluvium. In another test, a 60-second timed hair count will usually be in excess of 100 hairs (the normal value is 10 hairs). This method, which involves combing the hair forward for 60 s over a contrasting cloth before shampooing, can be used to assess the disease progression and resolution [
13].
A biopsy is rarely required in acute cases, although it will provide reassuring prognostic information in an anxious patient. It can also rule out female pattern hair loss, diffuse alopecia areata, secondary syphilis, systemic lupus erythematosus and dermatomyositis, which can all present with increased hair shedding. The histology of acute telogen effluvium shows increased number of telogen hairs without inflammation and there is no significant increase in the vellus hair count to suggest androgenic alopecia [
5].
Trichoscopy, the term coined for dermoscopy imaging of the scalp and hair, shows decreased hair density with the presence of empty follicles. It can be easily differentiated from Androgenetic Alopecia due to absence of hair shaft diameter variation and peripilar halo. In trichoscopy, telogen effluvium is a diagnosis of exclusion [
14].
A full blood count, iron studies, thyroid function test, syphilis serology, serum zinc, and anti-nuclear antibody should be performed to exclude other causes of diffuse telogen hair loss [
5].
When an obvious explanation exists for the recent onset telogen effluvium, expectant management and observation is appropriate. Shedding can be expected to cease within 3–6 months and thereafter recovery should be complete. Empirically, topical minoxidil may hasten resolution by prolonging anagen and stimulating telogen hairs to re-enter anagen [
1].
4.2. Chronic Diffuse Hair Loss
Chronic diffuse hair loss is the diagnosis used to describe telogen hair shedding that persists longer than six months. It can be due to a primary chronic telogen effluvium or secondary to a variety of causes. The diagnosis can be ascertained if the relationship between the trigger and the hair loss is reversible and reproducible.
Accepted causes of chronic diffuse telogen hair loss are thyroid disorders, profound iron deficiency anaemia, acrodermatitis enteropathica, and malnutrition [
15].
Both hyperthyroidism and hypothyroidism cause diffuse hair loss in approximately 50% and 33% of patients, respectively, as can drug induced hypothyroidism. It is believed that hypothyroidism inhibits cell division both in the epidermis and in the skin appendages. In a proportion of patients, this inhibition of mitosis induces catagen and delays re-entry of telogen hairs into anagen. The mechanism of hair loss in hyperthyroidism is unknown. The hair loss may precede the other manifestations by months. There appears to be no relationship between the duration or severity of hypothyroidism and the duration of alopecia. Replacement therapy generally resolves hair loss except in long standing hypothyroidism where hair follicles are atrophied [
16].
Profound iron deficiency anaemia can cause diffuse hair loss. In about 20% of cases, the iron deficiency occurs in the absence of anaemia and manifests solely with a serum ferritin below 20 µg/L. However, the relationship between iron deficiency with no anaemia or only mild anaemia and chronic diffuse hair loss is more complex and controversial. It is thought that iron deficiency reduces proliferation of matrix cells, because iron is an essential cofactor for ribonuclease reductase which involves DNA synthesis. As a result, hair follicles that shed their hair at the end of telogen may temporarily fail to re-enter anagen leading to slow onset diffuse hair loss. Patients should be treated with oral iron supplements until ferritin returns to normal. Some have advocated that treatment should be continued until ferritin rises above 40 µg/L; however, evidence is unconvincing [
5].
Acrodermatitis enteropathica and acquired zinc deficiency can lead to severe telogen effluvium. However subclinical zinc deficiency without other manifestation does not cause diffuse hair shedding. Therefore, correction of sub clinical zinc deficiency does not halt hair shedding and alternate forms of alopecia such as androgenetic alopecia should be excluded [
5,
17].
Crash dieting with severe protein-calorie malnutrition can precipitate hair loss [
18]. Marasmus may result in dry, lustreless, fine, straight hair that is sparse and easily pluckable, and it is often accompanied by hair shaft abnormalities [
19]. Kwashiorkor results in periods of interrupted hair growth where either hair sends into telogen or, if less severe affects the calibre of the hair more than its linear growth, hence producing multiple Pohl Pinkus lines. Another prominent feature is the hair colour change. Dark hair changes to brown or red, while brown hair becomes blonde. This colour change together with the periodic constrictions produces “flag signs” of Kwashiorkor [
19]. Essential fatty acid deficiency also produces marked hair loss with lightening of hair colour [
20].
Metabolic disturbances such as liver disease and chronic renal failure produce sparse scalp hair [
21].
Hair loss in advanced malignant disease may be due to hypoproteinaemia rather than malignancy itself, although, in Hodgkin’s disease, alopecia is an early sign [
22].
Systemic lupus erythematosus and Dermatomyositis can also cause telogen hair loss. Diffuse hair loss may occur in secondary syphilis, but the characteristic moth-eaten appearance is not always present [
23].
Drug-induced diffuse hair telogen hair loss usually starts 6–12 weeks after ingestion of treatment and is progressive while the drug is continued [
24,
25]. It is most commonly due to immediate anagen release. The diagnosis is made by demonstrating compatible chronology of drug exposure and the onset of hair loss. If a particular drug is suspected, testing involves suspending its use for at least three months. The conclusion can be made if regrowth occurs following discontinuation and recurrence on re-exposure. A dose-related diffuse telogen hair loss is common with acitretin but less common with isotretinoin [
26]. The retinoids appear to cause a telogen anchorage defect and reduce duration of anagen. Minoxidil has been reported to cause a short-lived telogen shedding by immediate release [
27]. Individual susceptibility exists in drug-induced telogen effluvium. Drugs known to cause telogen effluvium are Heparin Retinoid, Propranalol, Captopril, Allopurinol, Boric acid, Phenytoin, Glibenclamide, Amphetamines, Levodopa, Bromocriptine, Methysergide, Albendazole/Mebendazole, Cimetidine, Colchicine (low dose), Sulfasalazine, Penicillamine and Gold.
4.3. Primary Chronic Telogen Effluvium
Chronic telogen effluvium is an idiopathic, self-limiting condition with increased telogen shedding at least six months in duration, but not associated with the widening of the central part and miniaturization of hair follicles upon scalp biopsy [
1]. It is common in females between 30 and 50 years of age [
28,
29]. Some cases of telogen effluvium follow an acute telogen effluvium, with a known trigger; in most cases, a trigger cannot be identified. Any of the functional types of telogen effluvium could account for chronic telogen effluvium, but it is believed to be related to shortening of the anagen phase of the hair cycle [
30]. Diagnosis is made by exclusion of other causes of hair loss (
Figure 3).
Affected women commonly present with persistent, severe shedding that has fluctuating causes over several years. They may give a history of the ability to grow their hair very long in childhood, suggestive of a long anagen phase duration, and report a high hair density prior to the onset of hair loss. They usually have negative family history of androgenetic alopecia and examination reveals marked temporal recession without widening of the central part. However, these criteria are not absolute and androgenic alopecia can mimic this presentation [
31].
The natural history, prognosis and treatment for chronic telogen effluvium and androgenetic alopecia are different. The diagnosis of chronic telogen effluvium can usually be suspected from the history and examination, but a scalp biopsy is required to differentiate the two conditions. The optimal scalp biopsy is a 4 mm punch biopsy taken from the vertex of the scalp for horizontal embedding [
32]. The vertex is the chosen site as androgenetic alopecia is a patterned disease that preferentially affects the vertex of the scalp, so diagnostic yield is greatest in this area. Histology of the scalp biopsy shows an anagen:telogen ratio of 8:1 compared to the ratio of 14:1 in normal scalp biopsies. The total number of hairs in chronic telogen effluvium is the same as that found in normal scalps and the terminal:vellus-like hair ratio is similar in both averaging eight terminal hairs per vellus-like hair. In androgenetic alopecia, the terminal:vellus ratio is 1.9:1 [
1].
The condition usually resolves spontaneously after 3–4 years. Occasionally, the condition may persist, lasting 10 or more years.
5. New Treatments
Novel cosmetic treatments for thinning hair in telogen effluvium have been reported. It is comprised of a leave-on technology combination (caffeine, niacin amide, panthenol, dimethazone, and an acrylate polymer (CNPDA) that significantly increases hair diameter by 2–3 µm, yielding an increase in the cross sectional area of approximately 10%. In addition, CNPDA thickened fibres have the ability to withstand force without breaking. However, efficacy in telogen effluvium remains to be established [
33].
Stemoxydine, a molecule that mimics hypoxic signalling, is a novel approach to sustain hair growth and cycling. Stemoxydine is a potent P4H inhibitor (prolyl-4-hydroxylase). It has an ability to induce hypoxia-like signalling. Based on
in vitro studies, it is hypothesized that induction of hypoxia signalling may be important in maintaining hair follicle stem cell functionality.
In vivo clinical studies showed that following daily topical treatment with 5% solution for three months resulted in increased follicular density compared to volunteers receiving placebo [
34].
Nioxin is a cleanser scalp therapy and scalp serum. Nioxin is based upon bio-nutrient actives and protectives. It enhances hair with moisture and vitamin nourishment. These include vitamins and minerals such as copper, iron, biotin and caffeine, and herbal remedies such as ginseng, ginkgo and saw palmetto [
35].
6. Conclusions
Telogen effluvium is the common cause of diffuse hair loss. Since acute telogen effluvium is a self-limiting event, a wait and see approach is needed until spontaneous resolution. Chronic telogen effluvium should only be diagnosed after other causes of diffuse telogen hair loss have been excluded, including androgenetic alopecia.