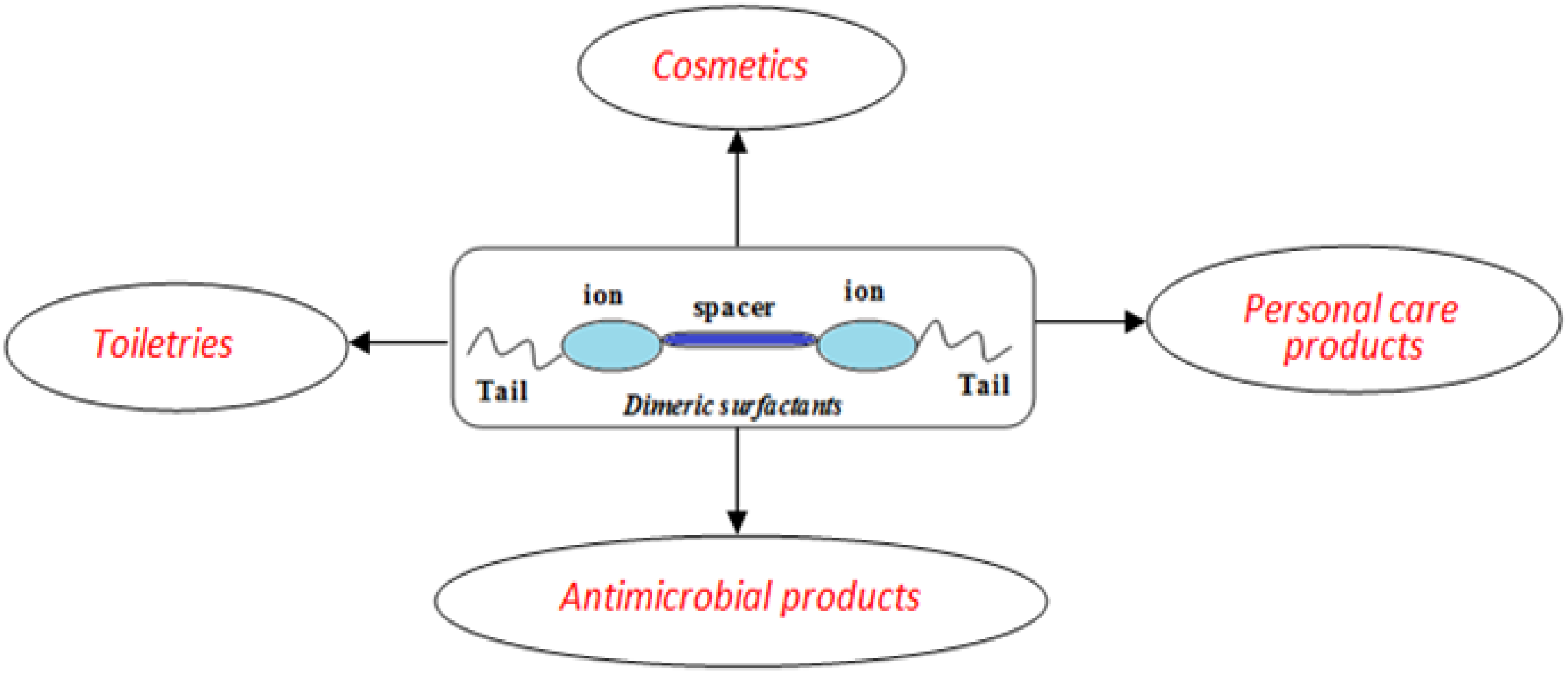
Dimeric Surfactants: Promising Ingredients of Cosmetics and Toiletries
Abstract
:1. Introduction
2. Physico-Chemical Characteristics
2.1. Surface Activity and Micellization
2.2. Solubilization Capacity and Krafft Point
2.3. Foaming and Froth Ability
2.4. Emulsifying Properties
3. Uses of Dimeric Surfactants in Cosmetics and Personal Care Products
3.1. In Cosmetic Formulations
3.2. Skin and Eye Irritation
3.3. Hair Care Products
3.4. Antimicrobial Activity
3.5. Biodegradability
3.6. Economical Aspects
4. Conclusions
Conflicts of Interest
References
- Myers, D. Surfactant Science and Technology, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Choi, T.S.; Shimizu, Y.; Shirai, H.; Hamada, K. Disperse dyeing of polyester fiber using gemini surfactants containing ammonium cations as auxiliaries. Dyes Pigments 2001, 50, 55–65. [Google Scholar] [CrossRef]
- Tracy, D.J.; Li, R.; Dahanayake, M.; Yang, J. Nonionic Gemini Surfactants. U.S. Patent 6204297, 20 March 2001. [Google Scholar]
- Karlsson, L.; Eijk, M.C.; Soderman, O. Compaction of DNA by gemini surfactants: Effects of surfactant architecture. J. Colloid Interface Sci. 2002, 252, 290–296. [Google Scholar] [CrossRef]
- Holmberg, K. Novel Surfactants Preparation, Applications, and Biodegradability; Marcel Dekker: New York, NY, USA, 2003. [Google Scholar]
- Zana, R.; Xia, J. Gemini Surfactants Synthesis, Interfacial and Solution-Phase Behavior, and Applicalions; Marcel Dekker: New York, NY, USA, 2004. [Google Scholar]
- Wattebled, L.; Laschewsky, A. New anionic gemini surfactant based on EDTA accessible by convenient synthesis. Colloid Polym. Sci. 2007, 285, 1387–1393. [Google Scholar] [CrossRef]
- Hait, S.K.; Moulik, S.P. Gemini surfactants: A distinct class of self-assembling molecules. Curr. Sci. 2002, 82, 1101–1111. [Google Scholar]
- Bai, G.; Wang, J.; Yan, H.; Li, Z.; Thomas, K. Thermodynamics of molecular self-assembly of cationic gemini and related double chain surfactants in aqueous solution. J. Phys. Chem. B 2001, 105, 3105–3108. [Google Scholar] [CrossRef]
- Lim, J.C.; Park, J.M.; Park, C.J.; Lee, B.M. Synthesis and surface active properties of a gemini type surfactant linked by a quaternary ammonium group. Colloid Polym. Sci. 2013, 291, 855–866. [Google Scholar] [CrossRef]
- Sakai, K.; Umemoto, N.; Matsuda, W.; Takamatsu, Y.; Matsumoto, M.; Sakai, H.; Abe, M. Oleic acid-based gemini surfactants with carboxylic acid headgroups. J. Oleo Sci. 2011, 60, 411–417. [Google Scholar] [CrossRef]
- Dam, T.; Engbert, J.B.F.N.; Karthauser, J.; Karaborni, S.; van Os, N.M. Synthesis, surface properties and oil solubilisation capacity of cationic gemini surfactants. Colloids Surface A: Physicochem. Eng. Asp. 1996, 118, 41–49. [Google Scholar] [CrossRef]
- De Guertechin, O. Part A: Properties (Surfactant Science). In HandBook of Detergents; Guy, B., Ed.; CRC Press: Boca Raton, FL, USA, 1999; Volume 82, p. 132. [Google Scholar]
- Laschewsky, A.; Lunkenheimer, K.; Rakotoaly, R.H.; Wattebled, L. Spacer effects in dimeric cationic surfactants. Colloid Polym. Sci. 2005, 283, 469–479. [Google Scholar] [CrossRef]
- Laschewsky, A.; Wattebled, L.; Arotcarena, M.; Habib-Jiwan, J.L.; Rakotoaly, R.H. Synthesis and properties of new cationic oligomeric surfactants. Langmuir 2005, 21, 7170–7179. [Google Scholar] [CrossRef]
- Kunieda, H.; Masuda, N.; Tsubone, K. Comparison between phase behavior of anionic dimeric (gemini-type) and monomeric surfactants in water and water-oil. Langmuir 2000, 16, 6438–6444. [Google Scholar] [CrossRef]
- Zhu, Y.P.; Masuyama, A.; Okahara, M. Preparation and surface active properties of amphipathic compounds with two sulfate groups and two lipophilic alkyl chains. J. Am. Oil Chem. Soc. 1990, 67, 459–463. [Google Scholar] [CrossRef]
- Zhu, Y.P.; Masuyama, A.; Okahara, M. Preparation and surface-active properties of new amphipathic compounds with two phosphate groups and two long-chain alkyl groups. J. Am. Oil Chem. Soc. 1991, 68, 268–271. [Google Scholar] [CrossRef]
- Zhu, Y.P.; Masuyama, A.; Nagata, T.; Okahara, M. Preparation and properties of double-chain surfactants bearing two sulfonate groups. J. Jpn. Oil Chem. Soc. (Yukagaku) 1991, 40, 473–477. [Google Scholar] [CrossRef]
- Okano, T.; Egawa, N.; Fujiwara, M.; Fukuda, M. α-Sulfonated fatty acid esters: II. Solution behavior of α-sulfonated fatty acid polyethylene glycol esters. J. Am. Oil Chem. Soc. 1996, 73, 31–37. [Google Scholar] [CrossRef]
- Engles, T.; von Rybinsiki, W.; Schmiedal, P. Structure and dynamics of surfactant-based foams. Progr. Colloid Polym. Sci. 1998, 111, 117–126. [Google Scholar] [CrossRef]
- Rosen, M.J.; Zhu, Z.H.; Hua, X.Y. Relationship of structure to properties of surfactants. 16. Linear decyldiphenylether sulfonates. J. Am. Oil Chem. Soc. 1992, 69, 30–33. [Google Scholar] [CrossRef]
- Rosen, M.J.; Zhu, Z.H. Enhancement of wetting properties of water-insoluble surfactants viasolubilization. J. Am. Oil Chem. Soc. 1993, 70, 65–68. [Google Scholar] [CrossRef]
- Ono, D.; Tanaka, T.; Masuyana, A.; Nakatsuji, Y.; Okahara, M. Preparation and properties of bis(sodium carboxylate) types of cleavable surfactants derived from diethyl tartrate and fatty carbonyl compounds. J. Japan Oil Chem. Soc. (Yakagaku) 1993, 42, 10–16. [Google Scholar] [CrossRef]
- Fujikura, Y.; Kita, K.; Kitsuki, T.; Maruta, K.; Nakano, A.; Tamura, S.; Tosaka, M.; Uno, M.; Yahagi, K. 2-Hydroxypropanediamine derivative and detergent composition containing the same. U.S. Patent 5714457, 3 February 1998. [Google Scholar]
- Pinazo, A.; Perez, L.; Infante, M.R.; Franses, E.I. Relation of foam stability to solution and surface properties of gemini cationic surfactants derived from arginine. Colloids Surfaces A: Phys. Eng. Asp. 2001, 189, 225–235. [Google Scholar] [CrossRef]
- Janusz, N. Emulsion properties and phase equilibrium and of new asymmetric gemini surfactants consisting of fatty acid esters of polyethoxylated alcohol or phenol. J. Surfact. Deterg. 2010, 13, 195–199. [Google Scholar] [CrossRef]
- Qun, X.; Liyan, W.; Fenglan, X. Synthesis and properties of dissymmetric gemini surfactants. J. Surfact. Deterg. 2011, 14, 85–90. [Google Scholar] [CrossRef]
- Dreja, M.; Tieke, B. Polymerization of styrene in microemulsion using gemini surfactants with hydrophilic and hydrophobic spacer groups. Berichte der Bunsengesellschaft fur physikalische Chemie 1998, 102, 1705–1709. [Google Scholar] [CrossRef]
- Umbach, W. The importance of colloid chemistry in industrial practice. Progr. Colloid Polym. Sci. 1998, 111, 9–16. [Google Scholar] [CrossRef]
- Schueller, R.; Romanowski, P. The science of reactive hair-care products. Cosmet. Toiletries 1998, 113, 39–44. [Google Scholar]
- Kwetkat, K. Gemini surfactants—Applications in real life. In Proceedings of CESIO 5th World Surfactant Congress, Firenze, Italy, 29 May–2 June 2000; Volume 2, pp. 1094–1096.
- Lorant, R.; Lahousse, F. Cosmetic composition comprising a gemini surfactant and high levels of solid fatty substance. WO 2013057118A2, 25 April 2013. [Google Scholar]
- Villa, C.; Baldassari, S.; Martino, D.F.C.; Spinella, A.; Caponetti, E. Green synthesis, molecular characterization and associative behavior of some gemini surfactants without a spacer group. Materials 2013, 6, 1506–1519. [Google Scholar] [CrossRef] [Green Version]
- Schmitz, A. Novel betaine derivatives and hair washing preparation containing them. U.K. Patent 1149140(A), 10 October 1967. [Google Scholar]
- Dahanayake, M.; Li, J.; Reierson, R.L.; Tracy, D.J. Amphoteric Surfactants having Multiple Hydrophobic and Hydrophilic Groups. U.S. Patent 5656586 A, 12 August 1997. [Google Scholar]
- Puchta, R.; Krings, P.; Sandkuhler, P. A new generation of softeners. Tenside Surfactants Deterg. 1993, 30, 186–191. [Google Scholar]
- Okano, T.; Fukuda, M.; Tanabe, J.; Ono, M.; Akabane, Y.; Takahashi, H.; Egawa, N.; Sakotani, T.; Kanao, H.; Yoneyanna, Y. Detergent Composition Having Low Skin Irritability. U.S. Patent 5681803, 28 October 1997. [Google Scholar]
- Tracy, D.J.; Li, R.; Yang, J. Anionic surfactants having multiple hydrophobic and hydrophilic groups. U.S. Patent 5789371, 4 August 1998. [Google Scholar]
- Raths, H.-C.; Biermann, M.; Maurer, K.H. Deodorizing Formulation. U.S. Patent 6277359 B1, 21 August 2001. [Google Scholar]
- Rosen, M.J.; Tracy, D.J. Gemini surfactants. J. Surfact. Deterg. 1998, 1, 547–554. [Google Scholar] [CrossRef]
- Li, R.; Tracy, D.J. Anionic Gemini Surfactants and Methods for Their Preparation. U.S. Patent 5952290A, 14 September 1999. [Google Scholar]
- Diz, M.; Manresa, A.; Pinazo, A.; Erra, P.; Infante, M.R. Synthesis, surface active properties and 302 antimicrobial activity of new bis quaternary ammonium compounds. J. Chem. Soc. Perkin Trans. 1994, 2, 1871–1879. [Google Scholar]
- Kim, T.S.; Kida, T.; Nakatsuji, Y.; Hirao, T.; Ikeda, I. Surface active properties of novel cationic surfactants with two alkyl chains and two ammonium groups. J. Am. Oil Chem. Soc. 1996, 73, 907–912. [Google Scholar] [CrossRef]
- Pavlikova, M.; Lacko, I.; Devinsky, F.; Mlynarcik, D. Quantitative relationships between structure, aggregation properties and antimicrobial activity of quaternary ammonium bolaamphiphiles. Collect. Czech. Chem. Commun. 1995, 60, 1213–1228. [Google Scholar] [CrossRef]
- Pinazo, A.; Diz, M.; Solans, C.; Erra, P.; Infante, M.R. Synthesis and properties of cationic surfactants containing a disulphide bond. J. Am. Oil Chem. Soc. 1993, 70, 37–42. [Google Scholar] [CrossRef]
- Piera, E.; Infante, M.R.; Clapes, P. Chemoenzymatic synthesis of arginine based gemini surfactants. Biotechnol. Bioeng. 2000, 70, 323–331. [Google Scholar] [CrossRef]
- Perez, L.; Torres, J.L.; Manresa, A.; Solans, C.; Infante, M.R. Synthesis, aggregation, and biological properties of a new class of gemini cationic amphiphilic compounds from arginine, bis(Args). Langmuir 1996, 12, 5296–5301. [Google Scholar] [CrossRef]
- Holmberg, K. Natural surfactants. Curr. Opin. Colloid Interface Sci. 2001, 6, 148–159. [Google Scholar] [CrossRef]
- Infante, M.R.; Pinazo, A.; Seguer, J. Non-conventional surfactants from amino acids and glycolipids: Structure, preparation and properties. Colloids Surf. A Physicochem. Eng. Asp. 1997, 49–70. [Google Scholar] [CrossRef]
- Yoshimura, T.; Sakato, A.; Esumi, K. Solution properties and emulsification properties of amino acid-based gemini surfactants derived from cysteine. J. Oleo Sci. 2013, 62, 579–586. [Google Scholar] [CrossRef]
- Masuyama, A.; Endo, C.; Takeda, S.; Nojima, M. Ozone-cleavable gemini surfactants, a new candidate for an environmentally friendly surfactant. Chem. Commun. 1998, 18, 2023–2024. [Google Scholar] [CrossRef]
- Masuyama, A.; Endo, C.; Takeda, S.Y.; Nojima, M.; Ono, D.; Takeda, T. Ozone-cleavable gemini surfactants. Their surface-active properties, ozonolysis, and biodegradability. Langmuir 2000, 16, 368–373. [Google Scholar] [CrossRef]
- Tatsumi, T.; Zhang, W.; Kida, T.; Nakatsuji, Y.; Ono, D.; Takeda, T.; Ikeda, I. Novel hydrolyzable and biodegradable cationic gemini surfactants: 1,3-bis[(acyloxyalkyl)-dimethylammonio]-2-hydroxypropane dichloride. J. Surfact. Deterg. 2000, 3, 167–172. [Google Scholar] [CrossRef]
- Perez, L.; García, T.; Ribosa, I.; Vinardell, P.; Manresa, A.; Infante, M.R. Biological properties of arginine-based gemini cationic surfactants. Environ. Toxicol. Chem. 2002, 21, 1279–1285. [Google Scholar] [CrossRef]
- Li, J.; Dahanayake, M.; Reierson, R.L.; Tracy, D.J. Amphoteric surfactants having multiple hydrophobic and hydrophilic groups. U.S. Patent 5914310, 22 June 1999. [Google Scholar]
- O’Lenick, A.J.; O’Lenick, K.A. Surfactants Based upon Alkyl Polyglycosides. U.S. Patent 6627612, 30 September 2003. [Google Scholar]
- Zoller, U.; Sosis, P. Handbook of Detergents, Part F: Production (Surfactant Science); CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kumar, N.; Tyagi, R. Dimeric Surfactants: Promising Ingredients of Cosmetics and Toiletries. Cosmetics 2014, 1, 3-13. https://doi.org/10.3390/cosmetics1010003
Kumar N, Tyagi R. Dimeric Surfactants: Promising Ingredients of Cosmetics and Toiletries. Cosmetics. 2014; 1(1):3-13. https://doi.org/10.3390/cosmetics1010003
Chicago/Turabian StyleKumar, Naveen, and Rashmi Tyagi. 2014. "Dimeric Surfactants: Promising Ingredients of Cosmetics and Toiletries" Cosmetics 1, no. 1: 3-13. https://doi.org/10.3390/cosmetics1010003