A Review of the Application of Ultrasound in Bioleaching and Insights from Sonication in (Bio)Chemical Processes
Abstract
:1. Introduction
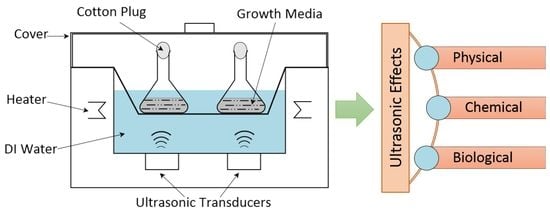
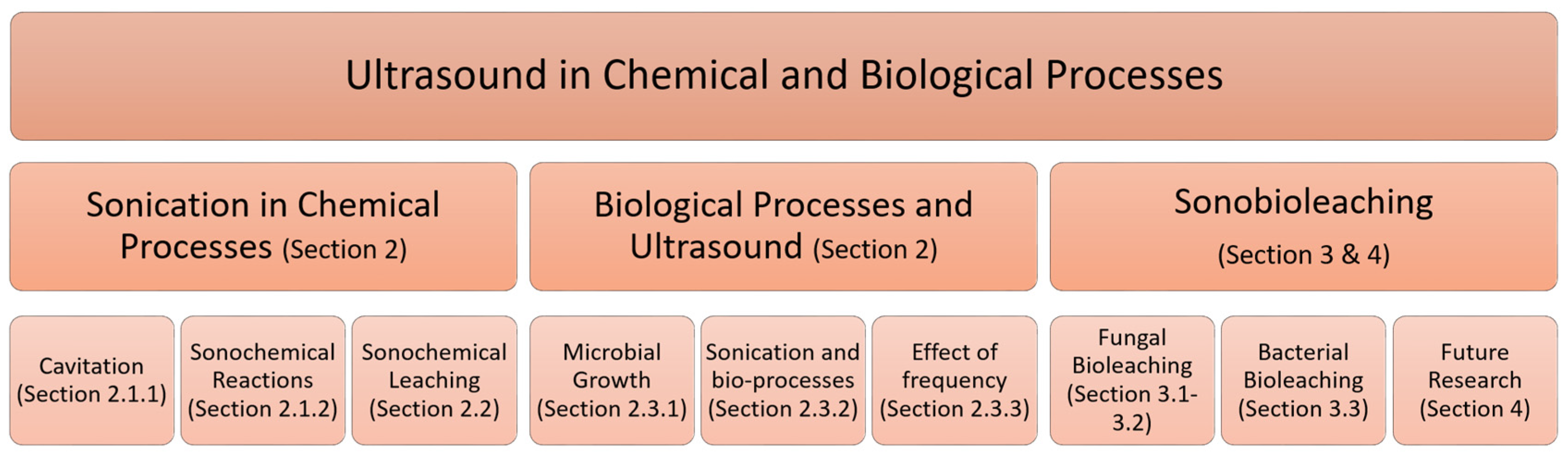
2. Ultrasound in Chemical and Biological Systems
2.1. Sonochemical Reactions and Applications
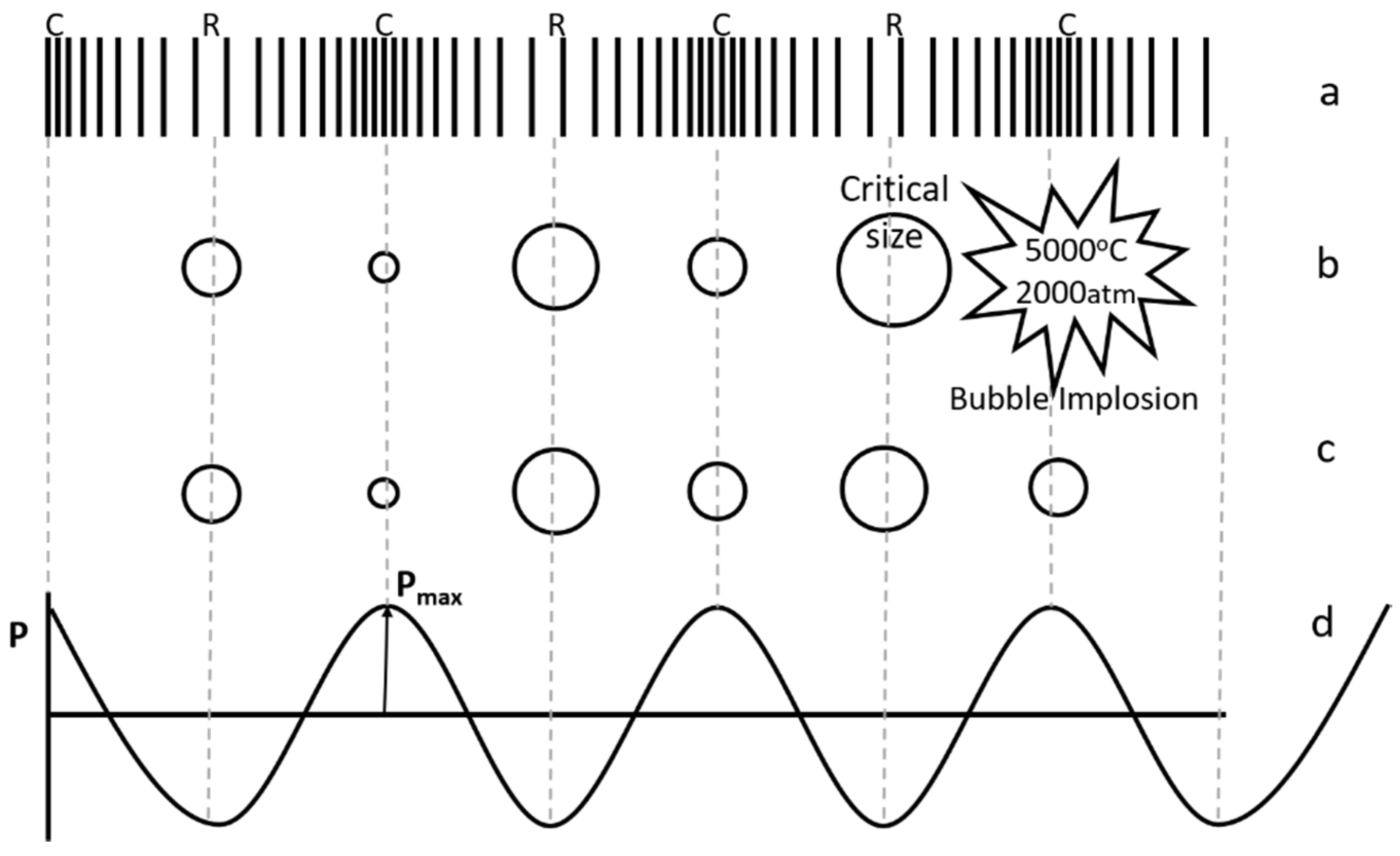
2.1.1. Ultrasound and Cavitation
2.1.2. Impact of Ultrasound on Chemical Reactions
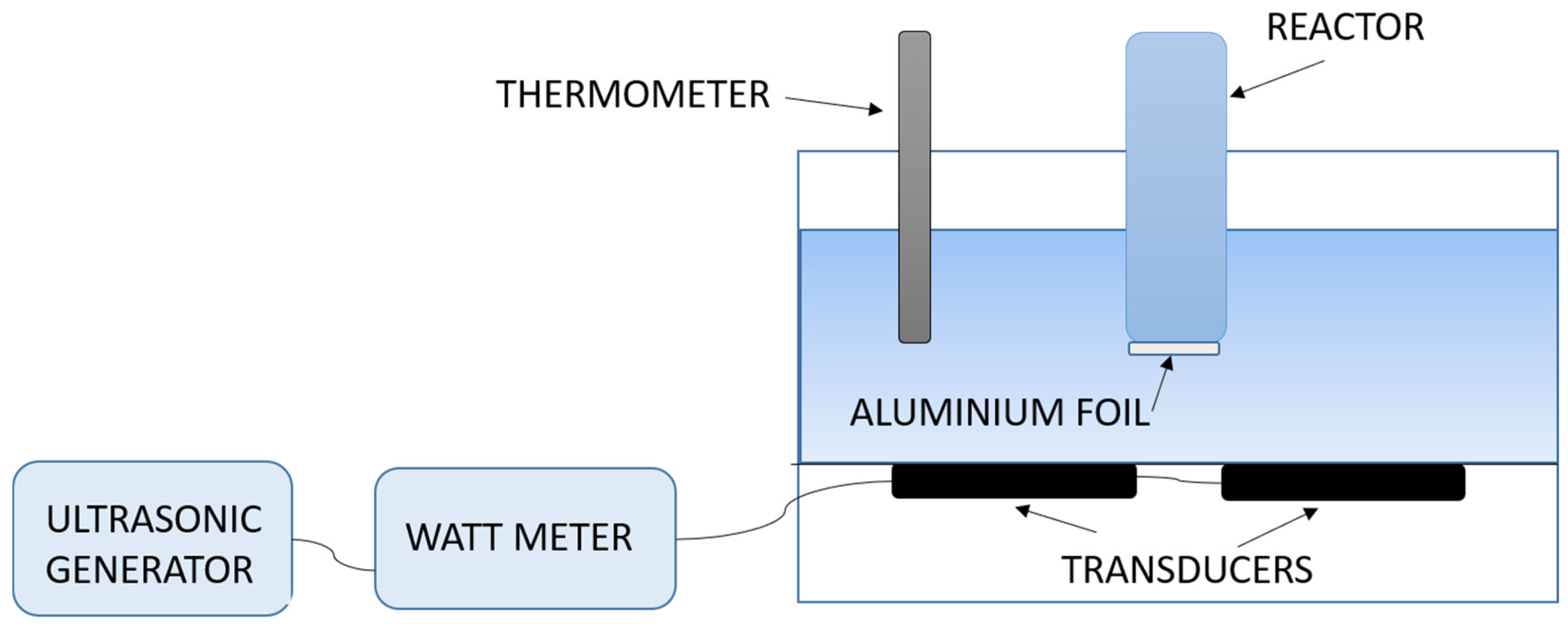
2.2. Sonochemical Leaching
2.2.1. Chemical Leaching and Cavitation
2.2.2. Sonochemical Leaching of Metals
2.2.3. Sonochemical Leaching of Non-Metals
2.3. Ultrasound in Biological Processes
2.3.1. Microbial Growth and Sonication
2.3.2. Impact of Sonication on Biological Processes
2.3.3. Biological Systems and Frequency of Ultrasound
3. Sonobioleaching
3.1. Sonication in One-Step Bioleaching
3.2. Sonication in Two-Step Bioleaching and Spent Medium Leaching
3.3. Sonobioleaching with Bacteria
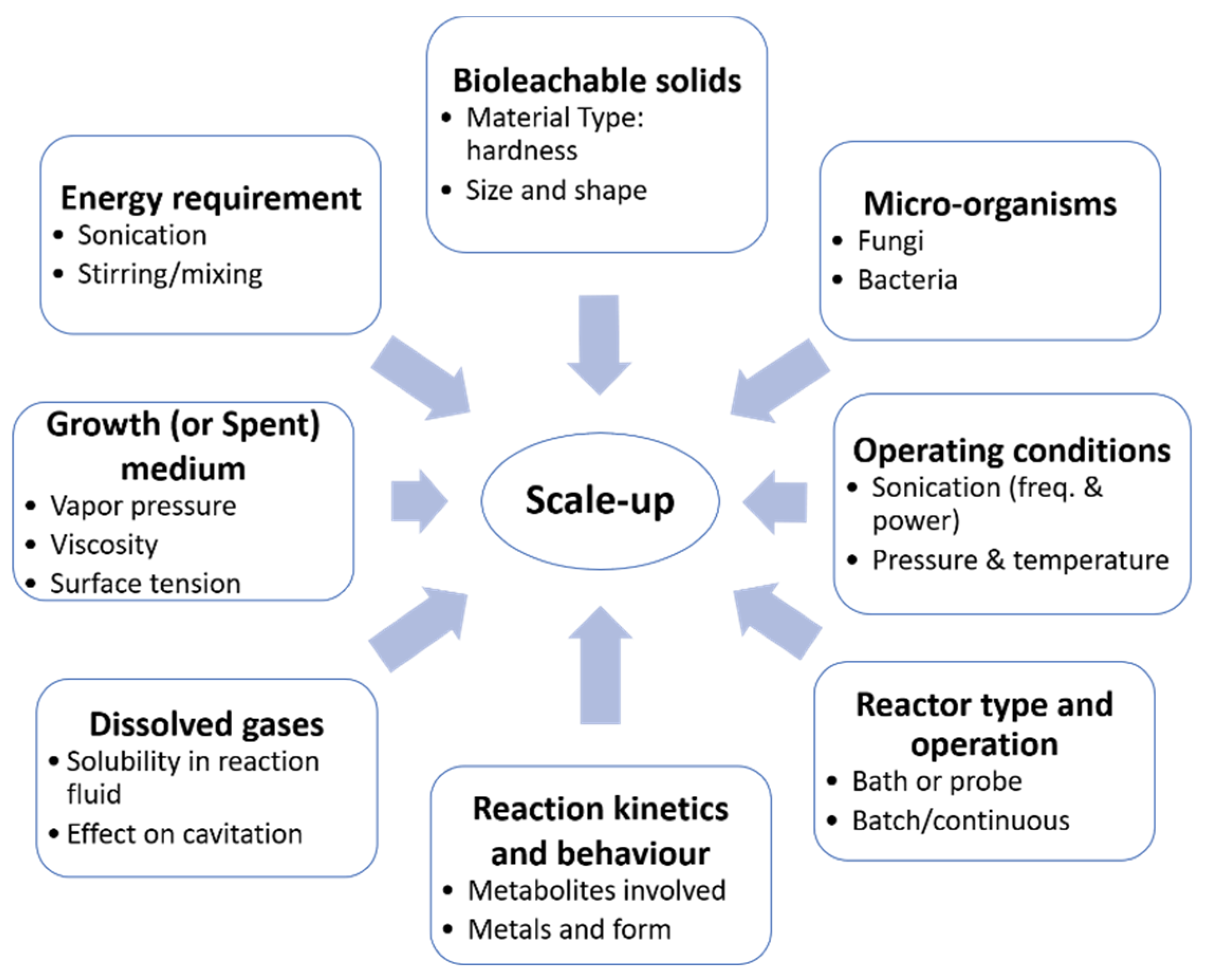
4. Future Directions in Sonobioleaching
Scale-Up Prospects
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Marafi, M.; Stanislaus, A. Spent hydroprocessing catalyst management: A review: Part II. Advances in metal recovery and safe disposal methods. Resour. Conserv. Recycl. 2008, 53, 1–26. [Google Scholar] [CrossRef]
- Akcil, A.; Vegliò, F.; Ferella, F.; Okudan, M.D.; Tuncuk, A. A review of metal recovery from spent petroleum catalysts and ash. Waste Manag. 2015, 45, 420–433. [Google Scholar] [CrossRef] [PubMed]
- Hennebel, T.; Boon, N.; Maes, S.; Lenz, M. Biotechnologies for critical raw material recovery from primary and secondary sources: R&D priorities and future perspectives. New Biotechnol. 2015, 32, 121–127. [Google Scholar] [CrossRef]
- Brierley, J.A. A perspective on developments in biohydrometallurgy. Hydrometallurgy 2008, 94, 2–7. [Google Scholar] [CrossRef]
- Borja, D.; Lee, E.; Silva, R.A.; Kim, H.; Park, J.H.; Kim, H. Column bioleaching of arsenic from mine tailings using a mixed acidophilic culture: A technical feasibility assessment. J. Korean Inst. Resour. Recycl. 2015, 24, 69–77. [Google Scholar] [CrossRef]
- Silva, R.A.; Borja, D.; Hwang, G.; Hong, G.; Gupta, V.; Bradford, S.A.; Zhang, Y.; Kim, H. Analysis of the effects of natural organic matter in zinc beneficiation. J. Clean. Prod. 2017, 168, 814–822. [Google Scholar] [CrossRef]
- Borja, D.; Nguyen, K.A.; Silva, R.A.; Park, J.H.; Gupta, V.; Han, Y.; Lee, Y.; Kim, H. Experiences and future challenges of bioleaching research in South Korea. Minerals 2016, 6, 128. [Google Scholar] [CrossRef]
- Gu, W.; Bai, J.; Dong, B.; Zhuang, X.; Zhao, J.; Zhang, C.; Wang, J.; Shih, K. Enhanced bioleaching efficiency of copper from waste printed circuit board driven by nitrogen-doped carbon nanotubes modified electrode. Chem. Eng. J. 2017, 324, 122–129. [Google Scholar] [CrossRef]
- Vyas, S.; Ting, Y.-P. Sequential biological process for molybdenum extraction from hydrodesulphurization spent catalyst. Chemosphere 2016, 160, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, A.; Ting, Y.-P. Bioleaching of spent hydrotreating catalyst by acidophilic thermophile Acidianus brierleyi: Leaching mechanism and effect of decoking. Bioresour. Technol. 2013, 130, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Swamy, K.M.; Narayana, K.L.; Misra, V.N. Bioleaching with ultrasound. Ultrason. Sonochem. 2005, 12, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Olson, G.J.; Brierley, J.A.; Brierley, C.L. Bioleaching review part B: Progress in bioleaching: Applications of microbial processes by the minerals industries. Appl. Microbiol. Biotechnol. 2003, 63, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Silva, R.A.; Park, J.; Lee, E.; Park, J.; Kim, H. Adaptation of a mixed culture of acidophiles for a tank biooxidation of refractory gold concentrates containing a high concentration of arsenic. J. Biosci. Bioeng. 2016, 121, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Avvaru, B.; Roy, S.B.; Chowdhury, S.; Hareendran, K.N.; Pandit, A.B. Enhancement of the leaching rate of uranium in the presence of ultrasound. Ind. Eng. Chem. Res. 2006, 45, 7639–7648. [Google Scholar] [CrossRef]
- Thompson, L.H.; Doraiswamy, L.K. Sonochemistry: Science and engineering. Ind. Eng. Chem. Res. 1999, 38, 1215–1249. [Google Scholar] [CrossRef]
- Margulis, M.A. Fundamental problems of sonochemistry and cavitation. Ultrason. Sonochem. 1994, 1, S87–S90. [Google Scholar] [CrossRef]
- Margulis, M.A.; Margulis, I.M. Contemporary review on nature of sonoluminescence and sonochemical reactions. Ultrason. Sonochem. 2002, 9, 1–10. [Google Scholar] [CrossRef]
- Santos, H.M.; Lodeiro, C.; Capelo-Martínez, J.-L. The power of ultrasound. In Ultrasound in Chemistry; Capelo-Martínez, J.-L., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2009; pp. 1–16. [Google Scholar]
- Ashokkumar, M. The characterization of acoustic cavitation bubbles—An overview. Ultrason. Sonochem. 2011, 18, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Peters, D. Ultrasound in materials chemistry. J. Mater. Chem. 1996, 6, 1605–1618. [Google Scholar] [CrossRef]
- Makino, K.; Mossoba, M.M.; Riesz, P. Chemical effects of ultrasound on aqueous solutions. Formation of hydroxyl radicals and hydrogen atoms. J. Phys. Chem. 1983, 87, 1369–1377. [Google Scholar] [CrossRef]
- Mason, T.J.; Lorimer, J.P. Sonochemistry in Environmental Protection and Remediation. In Applied Sonochemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2002; pp. 131–156. [Google Scholar]
- Miyaji, A.; Kohno, M.; Inoue, Y.; Baba, T. Hydroxyl radical generation by dissociation of water molecules during 1.65 MHz frequency ultrasound irradiation under aerobic conditions. Biochem. Biophys. Res. Commun. 2017, 483, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Riesz, P.; Kondo, T.; Krishna, C.M. Free radical formation by ultrasound in aqueous solutions. A spin trapping study. Free Radic. Res. Commun. 1990, 10, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Cella, R.; Stefani, H.A. Ultrasonic reactions. In Green Techniques for Organic Synthesis and Medicinal Chemistry; Zhang, W., Cue, B.W., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2012; pp. 343–361. [Google Scholar]
- Ji, J.; Wang, J.; Li, Y.; Yu, Y.; Xu, Z. Preparation of biodiesel with the help of ultrasonic and hydrodynamic cavitation. Ultrasonics 2006, 44, e411–e414. [Google Scholar] [CrossRef] [PubMed]
- Stavarache, C.; Vinatoru, M.; Maeda, Y.; Bandow, H. Ultrasonically driven continuous process for vegetable oil transesterification. Ultrason. Sonochem. 2007, 14, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Avvaru, B.; Roy, S.B.; Ladola, Y.; Chowdhury, S.; Hareendran, K.N.; Pandit, A.B. Sono-chemical leaching of uranium. Chem. Eng. Process. Process Intensif. 2008, 47, 2107–2113. [Google Scholar] [CrossRef]
- Hatanaka, S.; Mitome, H.; Yasui, K.; Hayashi, S. Single-bubble sonochemiluminescence in aqueous luminol solutions. J. Am. Chem. Soc. 2002, 124, 10250–10251. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Bhatia, B.; Nayyar, N.K. Transition metal-promoted free-radical reactions in organic synthesis: The formation of carbon-carbon bonds. Chem. Rev. 1994, 94, 519–564. [Google Scholar] [CrossRef]
- Bhanvase, B.A.; Pinjari, D.V.; Sonawane, S.H.; Gogate, P.R.; B, A. Pandit, Analysis of semibatch emulsion polymerization: Role of ultrasound and initiator. Ultrason. Sonochem. 2012, 19, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Bhanvase, B.A.; Sonawane, S.H. Ultrasound assisted in situ emulsion polymerization for polymer nanocomposite: A review. Chem. Eng. Process. Process Intensif. 2014, 85, 86–107. [Google Scholar] [CrossRef]
- Mason, T.J.; Lorimer, J.P. Polymers. In Applied Sonochemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2002; pp. 157–224. [Google Scholar]
- Henglein, A.; Ulrich, R.; Lilie, J. Luminescence and chemical action by pulsed ultrasound. J. Am. Chem. Soc. 1989, 111, 1974–1979. [Google Scholar] [CrossRef]
- Tandiono; Ohl, S.-W.; Ow, D.S.; Klaseboer, E.; Wong, V.V.; Dumke, R.; Ohl, C.-D. Sonochemistry and sonoluminescence in microfluidics. Proc. Natl. Acad. Sci. USA 2011, 108, 5996–5998. [Google Scholar] [CrossRef] [PubMed]
- Terasaki, N.; Yamada, H.; Xu, C.-N. Ultrasonic wave induced mechanoluminescence and its application for photocatalysis as ubiquitous light source. Catal. Today 2013, 201, 203–208. [Google Scholar] [CrossRef]
- Suslick, K.S.; Price, G.J. Applications of ultrasound to materials chemistry. Annu. Rev. Mater. Sci. 1999, 29, 295–326. [Google Scholar] [CrossRef]
- Pradhan, D.; Mishra, D.; Kim, D.J.; Ahn, J.G.; Chaudhury, G.R.; Lee, S.W. Bioleaching kinetics and multivariate analysis of spent petroleum catalyst dissolution using two acidophiles. J. Hazard. Mater. 2010, 175, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Beolchini, F.; Fonti, V.; Ferella, F.; Vegliò, F. Metal recovery from spent refinery catalysts by means of biotechnological strategies. J. Hazard. Mater. 2010, 178, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Marafi, M.; Stanislaus, A. Waste catalyst utilization: extraction of valuable metals from spent hydroprocessing catalysts by ultrasonic-assisted leaching with acids. Ind. Eng. Chem. Res. 2011, 50, 9495–9501. [Google Scholar] [CrossRef]
- Narayana, K.L.; Swamy, K.M.; Rao, K.S.; Murty, J.S. Leaching of metals from ores with ultrasound. Miner. Process. Extr. Metall. Rev. 1997, 16, 239–259. [Google Scholar] [CrossRef]
- Swamy, K.M.; Narayana, K.L. Intensification of leaching process by dual-frequency ultrasound. Ultrason. Sonochem. 2001, 8, 341–346. [Google Scholar] [CrossRef]
- Makino, K.; Mossoba, M.M.; Riesz, P. Chemical effects of ultrasound on aqueous solutions. Evidence for hydroxyl and hydrogen free radicals (.cntdot.OH and.cntdot.H) by spin trapping. J. Am. Chem. Soc. 1982, 104, 3537–3539. [Google Scholar] [CrossRef]
- Oncel, M. Leaching of silver from solid waste using ultrasound assisted thiourea method. Ultrason. Sonochem. 2005, 12, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Pinto, I.S.S.; Soares, H.M.V.M. Selective leaching of molybdenum from spent hydrodesulphurisation catalysts using ultrasound and microwave methods. Hydrometallurgy 2012, 129–130, 19–25. [Google Scholar] [CrossRef]
- Tangy, A.; Pulidindi, I.N.; Perkas, N.; Gedanken, A. Continuous flow through a microwave oven for the large-scale production of biodiesel from waste cooking oil. Bioresour. Technol. 2017, 224, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Lu, X.; Du, Y.; Mao, W.; Wang, Y.; Li, J. Ultrasonic-assisted oxidation leaching of nickel sulfide concentrate. Chin. J. Chem. Eng. 2010, 18, 948–953. [Google Scholar] [CrossRef]
- Pandey, A.D.; Mallick, K.K.; Pandey, P.C. Intensification of the reaction between Udaipur rock phosphate and sulphuric acid by means of ultrasound. Ultrasonics 1980, 18, 115–119. [Google Scholar] [CrossRef]
- Tekin, T. Use of ultrasound in the dissolution kinetics of phosphate rock in HCl. Hydrometallurgy 2002, 64, 187–192. [Google Scholar] [CrossRef]
- Tekin, T.; Tekin, D.; Bayramoğlu, M. Effect of ultrasound on the dissolution kinetics of phosphate rock in HNO3. Ultrason. Sonochem. 2001, 8, 373–377. [Google Scholar] [CrossRef]
- Balakrishnan, S.; Reddy, V.M.; Nagarajan, R. Ultrasonic coal washing to leach alkali elements from coals. Ultrason. Sonochem. 2015, 27, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Abramov, O.; Abramov, V.; Myasnikov, S.; Mullakaev, M. Extraction of bitumen, crude oil and its products from tar sand and contaminated sandy soil under effect of ultrasound. Ultrason. Sonochem. 2009, 16, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Barba, F.J.; Koubaa, M.; do Prado-Silva, L.; Orlien, V.; de Souza Sant’Ana, A. Mild processing applied to the inactivation of the main foodborne bacterial pathogens: A review. Trends Food Sci. Technol. 2017, 66, 20–35. [Google Scholar] [CrossRef]
- Piyasena, P.; Mohareb, E.; McKellar, R. Inactivation of microbes using ultrasound: A review. Int. J. Food Microbiol. 2003, 87, 207–216. [Google Scholar] [CrossRef]
- Hwang, G.; Han, Y.; Choi, S.Q.; Cho, S.; Kim, H. Bacterial inactivation by ultrasonic waves: Role of ionic strength, humic acid, and temperature. Water Air Soil Pollut. 2015, 226, 304. [Google Scholar] [CrossRef]
- Thacker, J. An approach to the mechanism of killing of cells in suspension by ultrasound. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1973, 304, 240–248. [Google Scholar] [CrossRef]
- Cameron, M.; McMaster, L.D.; Britz, T.J. Electron microscopic analysis of dairy microbes inactivated by ultrasound. Ultrason. Sonochem. 2008, 15, 960–964. [Google Scholar] [CrossRef] [PubMed]
- Drakopoulou, S.; Terzakis, S.; Fountoulakis, M.S.; Mantzavinos, D.; Manios, T. Ultrasound-induced inactivation of gram-negative and gram-positive bacteria in secondary treated municipal wastewater. Ultrason. Sonochem. 2009, 16, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Cameron, M. Impact of Low-Frequency High-Power Ultrasound on Spoilage and Potentially Pathogenic Dairy Microbes. University of Stellenbosch, 2007. Available online: https://scholar.sun.ac.za/bitstream/handle/10019.1/1163/cameron_impact_2007.pdf?sequence=3 (accessed on 22 February 2016).
- Pitt, W.G.; Ross, S.A. Ultrasound increases the rate of bacterial cell growth. Biotechnol. Progress 2003, 19, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Lewis, G.D.; Ashokkumar, M.; Hemar, Y. Inactivation of microorganisms by low-frequency high-power ultrasound: 1. Effect of growth phase and capsule properties of the bacteria. Ultrason. Sonochem. 2014, 21, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Koda, S.; Miyamoto, M.; Toma, M.; Matsuoka, T.; Maebayashi, M. Inactivation of Escherichia coli and Streptococcus mutans by ultrasound at 500 kHz. Ultrason. Sonochem. 2009, 16, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Ter Haar, G. Therapeutic applications of ultrasound. Progress Biophys. Mol. Biol. 2007, 93, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, K.; Feril, L.B., Jr.; Ikeda-Dantsuji, Y. Sonodynamic therapy. Ultrasonics 2008, 48, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Child, S.Z.; Hartman, C.L.; Schery, L.A.; Carstensen, E.L. Lung damage from exposure to pulsed ultrasound. Ultrasound Med. Biol. 1990, 16, 817–825. [Google Scholar] [CrossRef]
- Holland, C.K.; Deng, C.X.; Apfel, R.E.; Alderman, J.L.; Fernandez, L.A.; Taylor, K.J.W. Direct evidence of cavitation in vivo from diagnostic ultrasound. Ultrasound Med. Biol. 1996, 22, 917–925. [Google Scholar] [CrossRef]
- Baker, K.G.; Robertson, V.J.; Duck, F.A. A review of therapeutic ultrasound: biophysical effects. Phys. Ther. 2001, 81, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, S.; Mitragotri, S. Therapeutic opportunities in biological responses of ultrasound. Ultrasonics 2008, 48, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Feril, L.B., Jr.; Tachibana, K. Use of ultrasound in drug delivery systems: Emphasis on experimental methodology and mechanisms. Int. J. Hyperth. 2012, 28, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Husseini, G.A.; Pitt, W.G. The use of ultrasound and micelles in cancer treatment. J. Nanosci. Nanotechnol. 2008, 8, 2205–2215. [Google Scholar] [CrossRef] [PubMed]
- Mitragotri, S. Healing sound: The use of ultrasound in drug delivery and other therapeutic applications. Nat. Rev. Drug Discov. 2005, 4, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.-L.; Zeng, W.-C.; Lu, X.-L. Influence of ultrasound to the activity of tyrosinase. Ultrason. Sonochem. 2013, 20, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Purcell, D.; Parsons, S.A.; Jefferson, B. The influence of ultrasound frequency and power, on the algal species Microcystis aeruginosa, Aphanizomenon flos-aquae, Scenedesmus subspicatus and Melosira sp. Environ. Technol. 2013, 34, 2477–2490. [Google Scholar] [CrossRef] [PubMed]
- Vargas, L.H.M.; Pião, A.C.S.; Domingos, R.N.; Carmona, E.C. Ultrasound effects on invertase from Aspergillus niger. World J. Microbiol. Biotechnol. 2004, 20, 137–142. [Google Scholar] [CrossRef]
- Liu, H.; Yan, Y.; Wang, W.; Yu, Y. Low intensity ultrasound stimulates biological activity of aerobic activated sludge. Front. Environ. Sci. Eng. China 2007, 1, 67–72. [Google Scholar] [CrossRef]
- Pérez-Elvira, S.; Fdz-Polanco, M.; Plaza, F.I.; Garralón, G.; Fdz-Polanco, F. Ultrasound pre-treatment for anaerobic digestion improvement. Water Sci. Technol. 2009, 60, 1525–1532. [Google Scholar] [CrossRef] [PubMed]
- Herrán, N.S.; López, J.L.C.; Pérez, J.A.S.; Chisti, Y. Effects of ultrasound on culture of Aspergillus terreus. J. Chem. Technol. Biotechnol. 2008, 83, 593–600. [Google Scholar] [CrossRef]
- Gogate, P.R.; Kabadi, A.M. A review of applications of cavitation in biochemical engineering/biotechnology. Biochem. Eng. J. 2009, 44, 60–72. [Google Scholar] [CrossRef]
- Bar, R. Ultrasound enhanced bioprocesses: Cholesterol oxidation by Rhodococcus erythropolis. Biotechnol. Bioeng. 1988, 32, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Erriu, M.; Blus, C.; Szmukler-Moncler, S.; Buogo, S.; Levi, R.; Barbato, G.; Madonnaripa, D.; Denotti, G.; Piras, V.; Orrù, G. Microbial biofilm modulation by ultrasound: Current concepts and controversies. Ultrason. Sonochem. 2014, 21, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Schlicher, R.K.; Radhakrishna, H.; Tolentino, T.P.; Apkarian, R.P.; Zarnitsyn, V.; Prausnitz, M.R. Mechanism of intracellular delivery by acoustic cavitation. Ultrasound Med. Biol. 2006, 32, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Nyborg, W.L. Ultrasonic microstreaming and related phenomena. Br. J. Cancer 1982, 5, 156–160. [Google Scholar]
- Schläfer, O.; Onyeche, T.; Bormann, H.; Schröder, C.; Sievers, M. Ultrasound stimulation of micro-organisms for enhanced biodegradation. Ultrasonics 2002, 40, 25–29. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, P.; Gao, J.; Chen, Y. Using acoustic cavitation to improve the bio-activity of activated sludge. Bioresour. Technol. 2008, 99, 1497–1502. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Bochu, W.; Chuanren, D.; Sakanishi, A. Low ultrasonic stimulates fermentation of riboflavin producing strain Ecemothecium ashbyii. Colloids Surf. B Biointerfaces 2003, 30, 37–41. [Google Scholar] [CrossRef]
- Edmonds, P.D.; Sancier, K.M. Evidence for free radical production by ultrasonic cavitation in biological media. Ultrasound Med. Biol. 1983, 9, 635–639. [Google Scholar] [CrossRef]
- Riesz, P.; Kondo, T. Free radical formation induced by ultrasound and its biological implications. Free Radic. Biol. Med. 1992, 13, 247–270. [Google Scholar] [CrossRef]
- Davies, K.J. Protein damage and degradation by oxygen radicals. I. general aspects. J. Biol. Chem. 1987, 262, 9895–9901. [Google Scholar] [PubMed]
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem.-Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Kakinouchi, K.; Adachi, H.; Matsumura, H.; Inoue, T.; Murakami, S.; Mori, Y.; Koga, Y.; Takano, K.; Kanaya, S. Effect of ultrasonic irradiation on protein crystallization. J. Cryst. Growth 2006, 292, 437–440. [Google Scholar] [CrossRef]
- Swamy, K.M.; Sukla, L.B.; Narayana, K.L.; Kar, R.N.; Panchanadikar, V.V. Use of ultrasound in microbial leaching of nickel from laterites. Ultrason. Sonochem. 1995, 2, S5–S9. [Google Scholar] [CrossRef]
- Sukla, L.B.; Swamy, K.M.; Narayana, K.L.; Kar, R.N.; Panchanadikar, V.V. Bioleaching of Sukinda laterite using ultrasonics. Hydrometallurgy 1995, 37, 387–391. [Google Scholar] [CrossRef]
- Tole, N.M.; Ostensen, H. Basic Physics of Ultrasonographic Imaging; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- Onda Corporation. Tables of Acoustic Properties of Materials, Acoustic Tables of Reference. 2013. Available online: http://www.ondacorp.com/tecref_acoustictable.shtml (accessed on 1 February 2016).
- Kar, R.N.; Sukla, L.B.; Swamy, K.M.; Panchanadikar, V.V.; Narayana, K.L. Bioleaching of lateritic nickel ore by ultrasound. Metall. Mater. Trans. B 1996, 27, 351–354. [Google Scholar] [CrossRef]
- Anjum, F.; Bhatti, H.N.; Ghauri, M.A. Enhanced bioleaching of metals from black shale using ultrasonics. Hydrometallurgy 2010, 100, 122–128. [Google Scholar] [CrossRef]
- Anjum, F.; Shahid, M.; Bukhari, S.; Potgieter, J.H. Combined ultrasonic and bioleaching treatment of hospital waste incinerator bottom ash with simultaneous extraction of selected metals. Environ. Technol. 2014, 35, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Sabra, N.; Dubourguier, H.-C.; Duval, M.-N.; Hamieh, T. Study of canal sediments contaminated with heavy metals: Fungal versus bacterial bioleaching techniques. Environ. Technol. 2011, 32, 1307–1324. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.; Santos, R.; van Audenaerde, A.; Monballiu, A.; van Gerven, T.; Meesschaert, B. Chemoorganotrophic bioleaching of olivine for nickel recovery. Minerals 2014, 4, 553–564. [Google Scholar] [CrossRef]
- Cleaning Technologies Group. Magnetostrictive Versus Piezoelectric Transducers for Power Ultrasonic Applications. 2016. Available online: http://www.ctgclean.com/blog/technology-library/articles/magnetostrictive-versus-piezoelectric-transducers-for-power-ultrasonic-applications/ (accessed on 30 March 2016).
- Aung, K.M.M.; Ting, Y.-P. Bioleaching of spent fluid catalytic cracking catalyst using Aspergillus niger. J. Biotechnol. 2005, 116, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.-J.; Ting, Y.-P. Optimisation on bioleaching of incinerator fly ash by Aspergillus niger—Use of central composite design. Enzyme Microb. Technol. 2004, 35, 444–454. [Google Scholar] [CrossRef]
- Lee, E.; Han, Y.; Park, J.; Hong, J.; Silva, R.A.; Kim, S.; Kim, H. Bioleaching of arsenic from highly contaminated mine tailings using Acidithiobacillus thiooxidans. J. Environ. Manag. 2015, 147, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Ngoma, E.; Shaik, K.; Borja, D.; Smart, M.; Park, J.H.; Kim, H.J.; Petersen, J.; Harrison, S.T.L. Investigating the bioleaching of an arsenic mine tailing using a mixed mesophilic culture. Solid State Phenom. 2017, 262, 668–672. [Google Scholar] [CrossRef]
- Park, J.; Han, Y.; Lee, E.; Choi, U.; Yoo, K.; Song, Y.; Kim, H. Bioleaching of highly concentrated arsenic mine tailings by Acidithiobacillus ferrooxidans. Sep. Purif. Technol. 2014, 133, 291–296. [Google Scholar] [CrossRef]
- Deveci, H. Effect of particle size and shape of solids on the viability of acidophilic bacteria during mixing in stirred tank reactors. Hydrometallurgy 2004, 71, 385–396. [Google Scholar] [CrossRef]
- Casadonte, D.J.; Flores, M.; Petrier, C. The use of pulsed ultrasound technology to improve environmental remediation: a comparative study. Environ. Technol. 2005, 26, 1411–1418. [Google Scholar] [CrossRef] [PubMed]
- Patist, A.; Bates, D. Industrial applications of high power ultrasonics. In Ultrasound Technologies for Food and Bioprocessing; Feng, H., Barbosa-Canovas, G., Weiss, J., Eds.; Springer: New York, NY, USA, 2011; pp. 599–616. [Google Scholar]
- Mason, T.; Peters, D. 4-Large scale sonochemistry. In Practical Sonochemistry, 2nd ed.; Woodhead Publishing: Cambridge, UK, 2002; pp. 82–112. Available online: http://www.sciencedirect.com/science/article/pii/B9781898563839500096 (accessed on 24 March 2016).
- Leonelli, C.; Mason, T.J. Microwave and ultrasonic processing: Now a realistic option for industry. Chem. Eng. Process. Process Intensif. 2010, 49, 885–900. [Google Scholar] [CrossRef]
- Wang, X.; Yang, D.; Srinivasakannan, C.; Peng, J.; Duan, X.; Ju, S. A comparison of the conventional and ultrasound-augmented leaching of zinc residue using sulphuric acid. Arab. J. Sci. Eng. 2013, 39, 163–173. [Google Scholar] [CrossRef]
- Kinoshita, M.; Hynynen, K. Key factors that affect sonoporation efficiency in in vitro settings. In Proceedings of the IEEE Ultrasonics Symposium, Vancouver, BC, Canada, 3–6 October 2006; pp. 852–855. [Google Scholar]

| Ore/Waste | Bioleaching Process | Sonication Parameters | Metal Extraction with US (without US) | References |
|---|---|---|---|---|
| Lateritic nickel ore | One-step | 30 min sonication of broth + spores + ore, 20 kHz, 1.5 W/cm2 | Ni: 95% (24.9%) Fe: 0.2% (5%) all 14 days | [91] |
| Lateritic nickel ore | One-step | 30 min sonication of broth + spores + ore, 43 kHz, 1.5 W/cm2 | Ni: 95%, 14 days (92%, 20 days) Fe: ≈0%, 14 days (12.5%, 20 days) | [92] |
| Lateritic nickel ore | One-step | 30 min sonication of broth + spores + ore, 43 kHz, 1.5 W/cm2 | Ni: 95%, 14 days (92%, 20 days) Fe: ≈0%, 14 days (12.5%, 20 days) | [95] |
| Black shale | Two-step | 7 min sonication, 40 kHz, during 15 days of growth and 36 days of bioleaching | Cu: 92% (78.2%), Zn: 87% (84.4%) Co: 71% (68.2%) over 24 days Al: 92%, 24 days (76.7%, 36 days), Fe: 83%, 21 days (77.0%, 33 days), | [96] |
| Hospital waste incinerator bottom ash | Spent medium leaching | 10 min sonication of spent medium + waste, 40 kHz, 18 days of bioleaching | Increase of around 20 mg/kg Al, 15 mg/kg Fe and 5 mg/kg Zn on sonication | [97] |
| Olivine ore | Two-step | 15 min sonication, 37 kHz, 0.2 W/cm2, during 7 days of growth and 17 days of bioleaching | Ni: 15.7%, 17 days (9.9%, 17 days) marginal increase in Mg, Al, Si & Cr | [99] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vyas, S.; Ting, Y.-P. A Review of the Application of Ultrasound in Bioleaching and Insights from Sonication in (Bio)Chemical Processes. Resources 2018, 7, 3. https://doi.org/10.3390/resources7010003
Vyas S, Ting Y-P. A Review of the Application of Ultrasound in Bioleaching and Insights from Sonication in (Bio)Chemical Processes. Resources. 2018; 7(1):3. https://doi.org/10.3390/resources7010003
Chicago/Turabian StyleVyas, Shruti, and Yen-Peng Ting. 2018. "A Review of the Application of Ultrasound in Bioleaching and Insights from Sonication in (Bio)Chemical Processes" Resources 7, no. 1: 3. https://doi.org/10.3390/resources7010003