A Review on Biodiversity, Ecosystem Services, and Perceptions of New Zealand’s Mangroves: Can We Make Informed Decisions about Their Removal?
Abstract
:1. Introduction
- What biodiversity and ecosystem service information exists on New Zealand’s mangroves?
- What are the knowledge gaps in terms of potential species and ecosystem services occupying New Zealand mangroves?
- How can we better integrate data for comprehensive and effective management decisions regarding the removal and preservation of these ecosystems?
1.1. Mangrove Ecosystems
1.2. Mangroves, Biodiversity, and Ecosystem Services
1.3. Temperate Mangroves
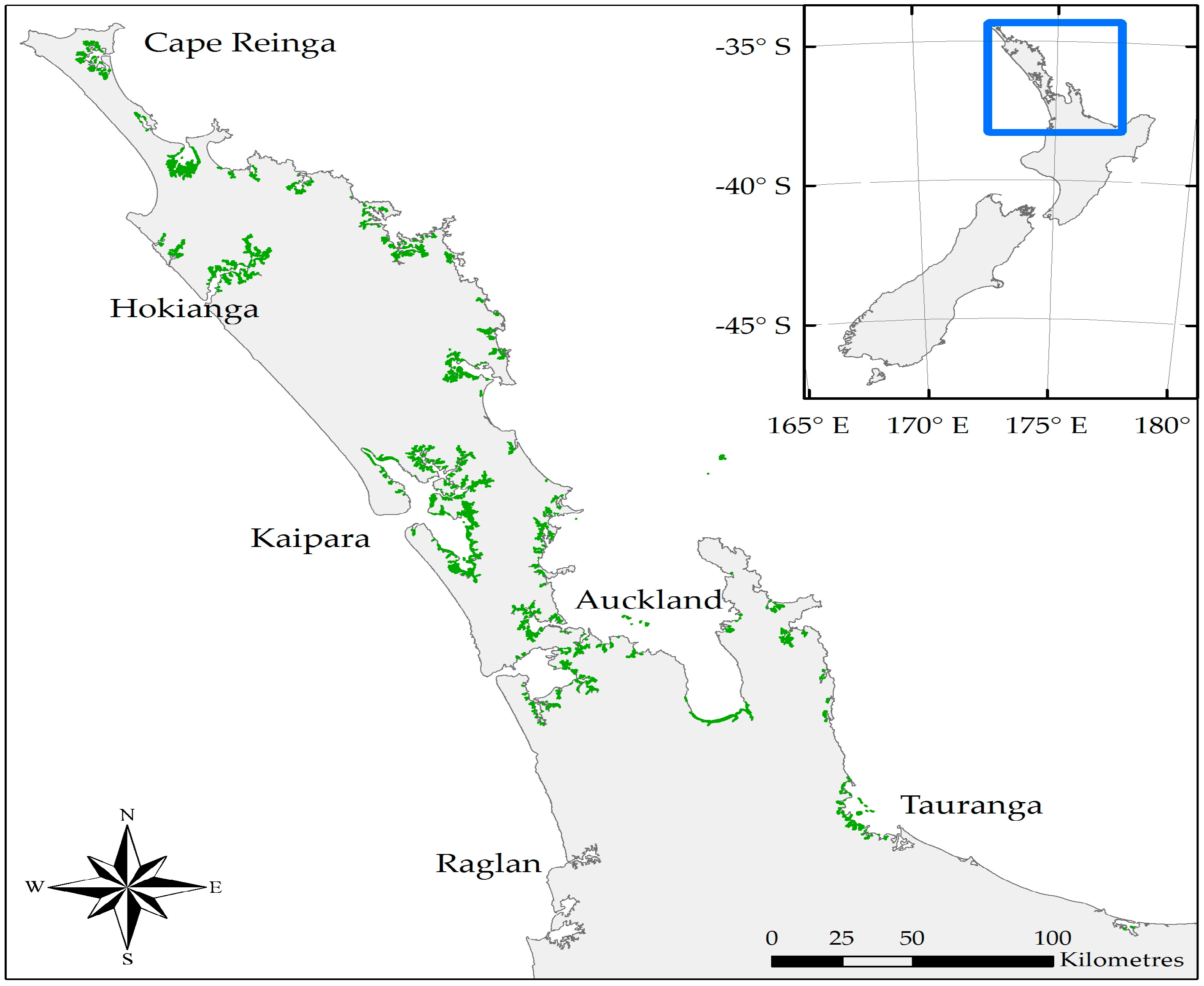
1.3.1. New Zealand’s Mangroves
1.3.2. Conservation Status and Policy: Treaty of Waitangi and the Resource Management Act
sustaining the potential of natural and physical resources (excluding minerals) to meet the reasonably foreseeable needs of future generations; andsafeguarding the life-supporting capacity of air, water, soil, and ecosystems; andavoiding, remedying, or mitigating any adverse effects of activities on the environment.([36], Part 2, Section 5)
1.3.3. Mangrove Expansion and Removal
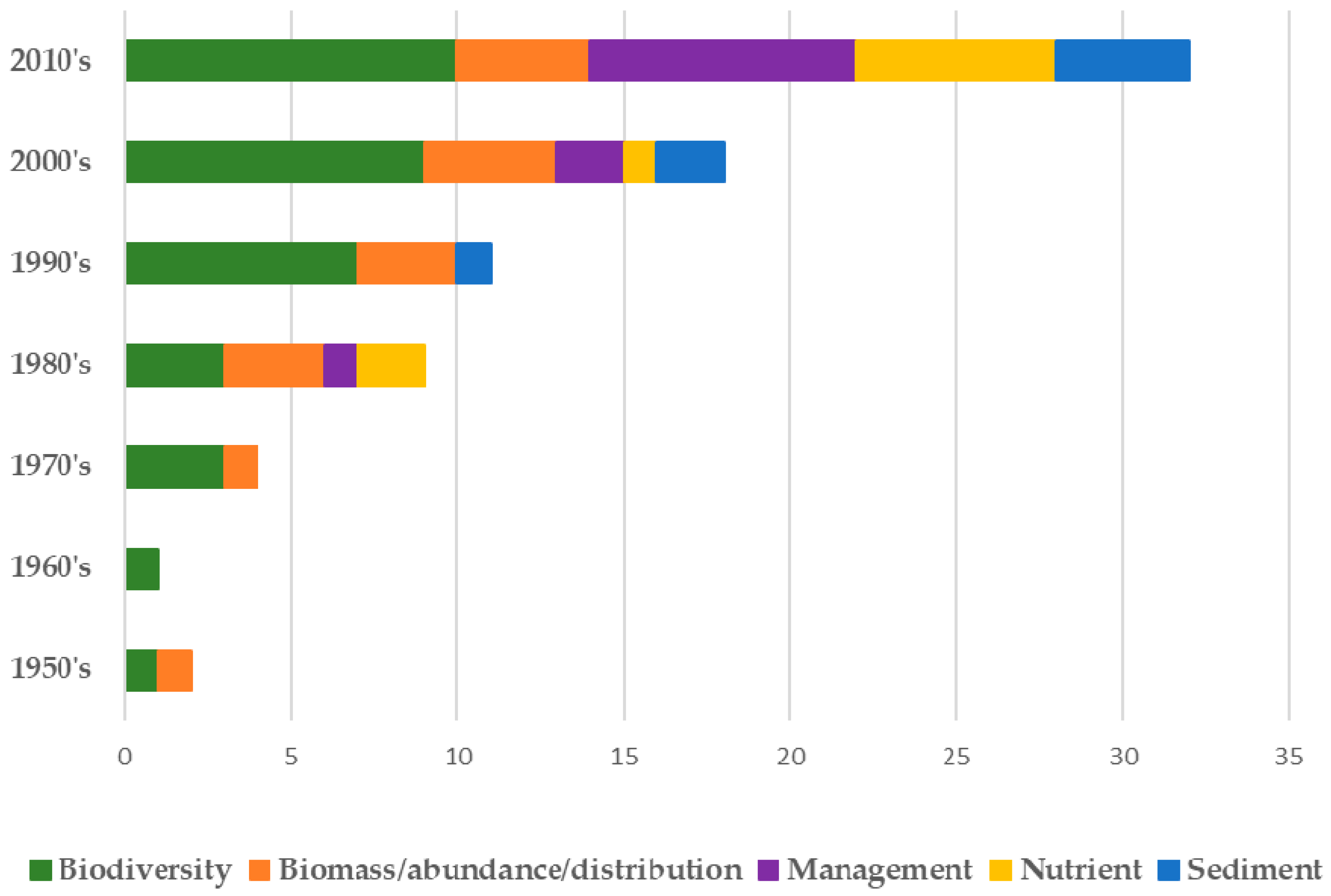
2. Materials and Methods
3. Results
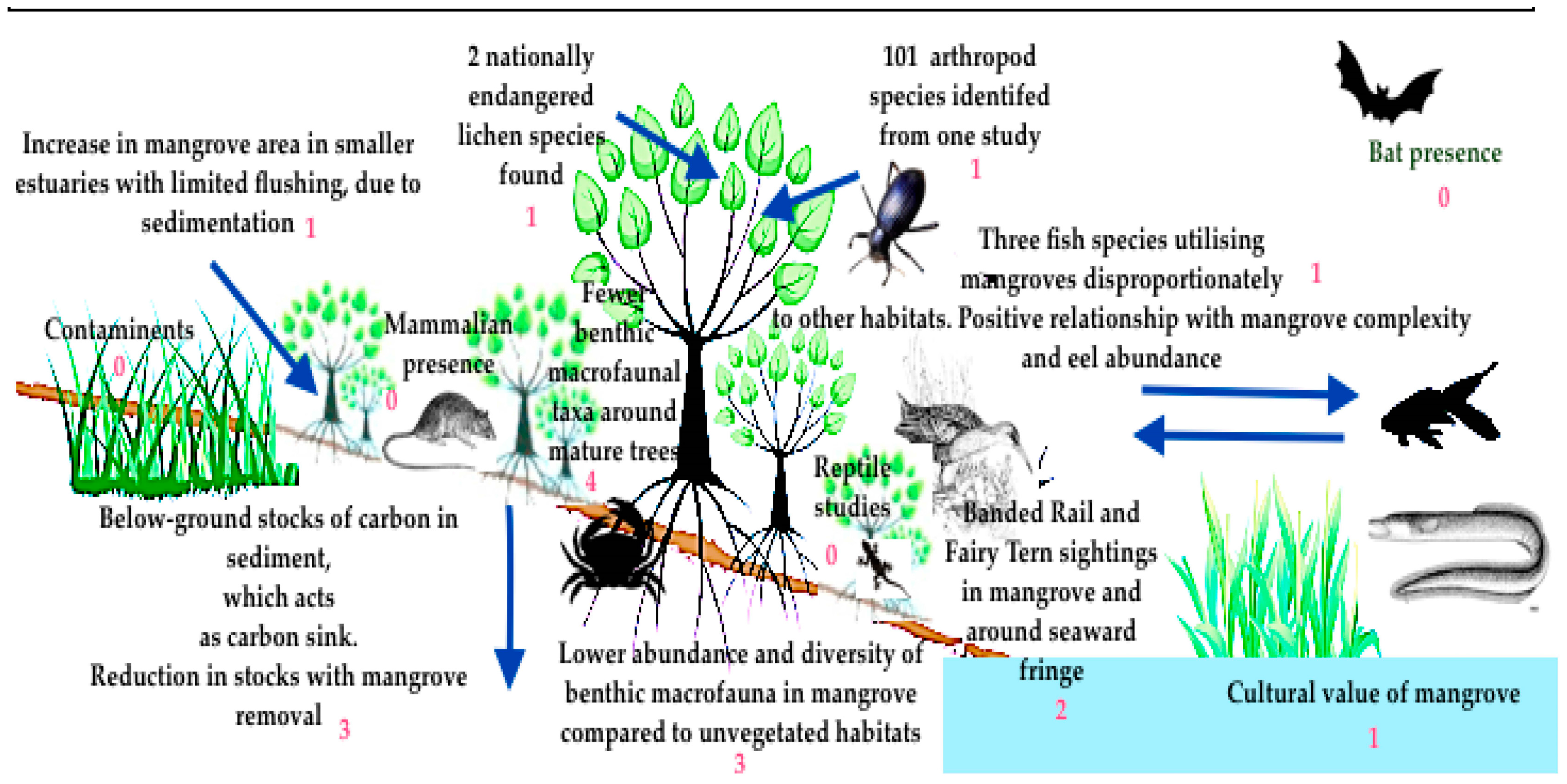
3.1. Biodiversity
3.1.1. Macrobenthic Invertebrate Studies
3.1.2. Birds
3.1.3. Insects and Spiders
3.1.4. Fish
3.1.5. Mammals
3.1.6. Lichens
3.2. Attributes-Regulating and Supporting Service Studies
3.2.1. Nutrients
3.2.2. Sediment Accumulation
3.2.3. Biomass/Abundance/Distribution
3.3. Attributes-Social and Economic Studies
3.3.1. Management
3.3.2. Economic Valuation and Cost of Removal
3.3.3. Cultural Studies
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- McMichael, A.J.; Butler, C.D.; Folke, C. New visions for addressing sustainability. Science 2003, 302, 1919–1920. [Google Scholar] [CrossRef] [PubMed]
- Mooney, H.A.; Duraiappah, A.; Larigauderie, A. Evolution of natural and social science interactions in global change research programs. Proc. Natl. Acad. Sci. USA 2013, 110 (Suppl. 1), 3665–3672. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.; Berry, P.; Simpson, G.; Haslett, J.R.; Blicharska, M.; Bucur, M.; Dunford, R.; Egoh, B.; Garcia-Llorente, M.; Geamǎnǎ, N.; et al. Linkages between biodiversity attributes and ecosystem services: A systematic review. Ecosyst. Serv. 2014, 9, 191–203. [Google Scholar] [CrossRef]
- Tomlinson, P.B. The Botany of Mangroves, 2nd ed.; Cambridge University Press: Cambridge, UK, 1986; 413p, ISBN 978-1-107-08067-6. [Google Scholar]
- Quisthoudt, K.; Schmitz, N.; Randin, C.F.; Dahdouh-Guebas, F.; Robert, E.M.; Koedam, N. Temperature variation among mangrove latitudinal range limits worldwide. Trees 2012, 26, 1919–1931. [Google Scholar] [CrossRef]
- Austin, M.P.; Heyligers, P.C. Vegetation survey design for conservation: Gradsect sampling of forests in north-eastern New South Wales. Biol. Conserv. 1989, 50, 13–32. [Google Scholar] [CrossRef]
- Alongi, D.M. Present state and future of the world’s mangrove forests. Environ. Conserv. 2002, 29, 331–349. [Google Scholar] [CrossRef]
- Millennium Ecosystem Assessment. Ecosystems and Human Well-Being: Synthesis; Island Press: Washington, DC, USA, 2005; 155p, ISBN 1-59726-040-1. [Google Scholar]
- Richards, D.R.; Friess, D.A. Rates and drivers of mangrove deforestation in Southeast Asia, 2000–2012. Proc. Natl. Acad. Sci. USA 2016, 113, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations. The World’s Mangroves 1980–2005; FAO Forestry Paper No. 153; Forest Resources Division, FAO: Rome, Italy, 2007; 77p, ISBN 978-92-5-105856-5. [Google Scholar]
- Convention on Biological Diversity. Available online: https://www.cbd.int/ (accessed on 25 March 2017).
- Diaz, S.; Fargione, J.; Chapin, F.S., III; Tilman, D. Biodiversity Loss Threatens Human Well-Being. PLoS Biol. 2006, 4, e277. [Google Scholar] [CrossRef] [PubMed]
- Huxham, M.; Dencer-Brown, A.; Diele, K.; Kathiresan, K.; Nagelkerken, I.; Wanjiru, C. Mangrove Ecosystem Services for Local Communities and their Vulnerability to Climate Change. In Mangrove Ecosystems: A Global Biogeographic Perspective on Structure, Function and Services; Springer: Cham, Switzerland, 2017; pp. 245–274. ISBN 978-3-319-62206-4. [Google Scholar]
- Spaninks, F.; Beukering, P.V. Economic Valuation of Mangrove Ecosystems: Potential and Limitations. CREED Working Paper 14. 1997. Available online: http://pubs.iied.org/pdfs/8133IIED.pdf (accessed on 21 April 2017).
- McLeod, E.; Salm, R.V. Managing Mangroves for Resilience to Climate Change; IUCN: Gland, Switzerland, 2006; 64p, ISBN 2-8317-0953-9. Available online: http://cmsdata.iucn.org/downloads/managing_mangroves_for_resilience_to_climate_change.pdf (accessed on 15 June 2017).
- Kettunen, M.; Vakrou, A.; Wittmer, H. The Economics of Ecosystems and Biodiversity (TEEB) 2010. Available online: http://www.teebweb.org/ (accessed on 24 December 2017).
- Davidson-Hunt, I.J.; Suich, H.; Meijer, S.S.; Olsen, N. (Eds.) People in Nature: Valuing the Diversity of Interrelationships between People and Nature; IUCN: Gland, Switzerland, 2016; 108p, ISBN 978-2-8317-1798-2. Available online: https://portals.iucn.org/library/node/46207 (accessed on 11 April 2017).
- Salem, M.E.; Mercer, D.E. The economic value of mangroves: A meta-analysis. Sustainability 2012, 4, 359–383. [Google Scholar] [CrossRef]
- Brander, L.M.; Wagtendonk, A.J.; Hussain, S.S.; McVittie, A.; Verburg, P.H.; de Groot, R.S.; van der Ploeg, S. Ecosystem service values for mangroves in Southeast Asia: A meta-analysis and value transfer application. Ecosyst. Serv. 2012, 1, 62–69. [Google Scholar] [CrossRef]
- Morrisey, D.J.; Swales, A.; Dittmann, S.; Morrison, M.A.; Lovelock, C.E.; Beard, C.M. The ecology and management of temperate mangroves. In Oceanography and Marine Biology: An Annual Review; Gibson, R.N., Ed.; CRC Press: Boca Raton, FL, USA, 2010; Volume 48, pp. 43–160. [Google Scholar]
- Statistics Times. 2016. Available online: https://statisticstimes.com/ecomony/projected-world-gdp-capita-ranking.php (accessed on 22 April 2017).
- Sutherland, J.I. Miocene petrified wood and associated borings and termite faecal pellets from Hukatere Peninsula, Kaipara Harbour, North Auckland, New Zealand. J. R. Soc. N. Z. 2003, 33, 395–414. [Google Scholar] [CrossRef]
- Pocknall, D.T. Late Eocene to Early Miocene vegetation and climate history of New Zealand. J. R. Soc. N. Z. 1989, 19, 1–18. [Google Scholar] [CrossRef]
- Hume, T.M.; Snelder, T.; Weatherhead, M.; Liefting, R. A controlling factor approach to estuary classification. Ocean Coast. Manag. 2007, 50, 905–929. [Google Scholar] [CrossRef]
- Carbines, M.; Vaughan, M.; Walker, J. Tracking Mangrove Expansion and Infilling in Auckland; Prepared by Research and Evaluations Unit for Auckland Council; Auckland, New Zealand, 2017; Unpublished work. [Google Scholar]
- Morrisey, D.; Beard, C.; Morrison, M.; Craggs, R.; Lowe, M. The New Zealand mangrove: Review of the current state of knowledge. In Auckland Regional Council Technical Publication; No. 325; Auckland Regional Council: Auckland, New Zealand, 2007. Available online: http://www.nzpcn.org.nz/publications/ARC-325%20Mangrove-review.pdf (accessed on 15 March 2017).
- Vectors New Zealand. Available online: https://vectors.co.nz/wpcontent/uploads/Blank_Map_Of_New_Zealand.png (accessed on 4 May 2017).
- Land Information New Zealand (LINZ). Available online: https://data.linz.govt.nz/layer/88131-northland-04m-rural-aerial-photos-2014-16/ (accessed on 15 April 2017).
- Küchler, A.W. The Mangrove in New Zealand. N. Z. Geogr. 1972, 28, 113–129. [Google Scholar] [CrossRef]
- Dingwall, P.R. Overcoming problems in the management of New Zealand mangrove forests. In Physiology and Management of Mangroves; Teas, H.G., Ed.; Dr. W. Junk Publishers: The Hague, The Netherlands, 1984; pp. 97–106. [Google Scholar]
- Crisp, P.; Daniel, L.; Tortell, P. Mangroves in New Zealand: Trees in the Tide; GP Books: Wellington, New Zealand, 1990; 69p, ISBN 0-477-01396-1. [Google Scholar]
- Schwarz, A.-M. Spreading mangroves: A New Zealand phenomenon or global trend? Water Atmos. 2003, 11, 8–10. [Google Scholar]
- Park, S.; (Waikato Regional Council, Tauranga, New Zealand). Personal communication, 2017.
- Waitangi Tribunal. Available online: http://archives.govt.nz/exhibitions/treaty (accessed on 1 November 2017).
- Wai 262. Waitangi Tribunal Report. Available online: https://forms.justice.govt.nz/search/Documents/WT/wt_DOC_68356054/KoAotearoaTeneiTT1W.pdf (accessed on 11 April 2017).
- Resource Management Act. Available online: http://www.legislation.govt.nz/act/public/1991/0069/latest/DLM231905.html (accessed on 3 April 2017).
- Auckland Council Unitary Plan. Available online: http://www.aucklandcouncil.govt.nz/EN/planspoliciesprojects/plansstrategies/unitaryplan/Documents/Section32report/Appendices/Appendix%203.32.7.pdf (accessed on 24 June 2017).
- Harmsworth, G.R.; Awatere, S. Indigenous Māori knowledge and perspectives of ecosystems. In Ecosystem Services in New Zealand: Conditions and Trends; Landcare Research, Palmerston North; Manaaki Whenua Press: Lincoln, New Zealand, 2013; pp. 274–286. Available online: https://www.landcareresearch.co.nz/__data/assets/pdf_file/0007/77047/2_1_Harmsworth.pdf (accessed on 12 May 2017).
- Taiepa, T. Maori participation in environmental planning: Institutional reform and collaborative management. He Pukenga Korero 1999, 5, 34–39. [Google Scholar]
- Selby, R.; Moore, P.; Mulholland, M. Māori and the Environment: Kaitiaki; Huia Publishers: Wellington, New Zealand, 2010; 372p, ISBN 9781869694029. [Google Scholar]
- Green, M.; Ellis, J.; Schwarz, A.; Green, N.; Lind, D.; Bluck, B. For and against mangrove control. NIWA Inf. Ser. 2003, 31–39. [Google Scholar]
- De Luca, S. Mangroves in NZ—Misunderstandings and Management. Available online: http://www.boffamiskell.co.nz/downloads/publications/mangroves-in-nz-misunderstandings-and-management.pdf (accessed on 14 May 2017).
- Alfaro, A.C. Benthic macro-invertebrate community composition within a mangrove/seagrass estuary in northern New Zealand. Estuar. Coast. Shelf Sci. 2006, 66, 97–110. [Google Scholar] [CrossRef]
- Harty, C. Mangrove planning and management in New Zealand and South East Australia–A reflection on approaches. Ocean Coast. Manag. 2009, 52, 278–286. [Google Scholar] [CrossRef]
- Stokes, D.J.; Healy, T.R.; Cooke, P.J. Surface elevation changes and sediment characteristics of intertidal surfaces undergoing mangrove expansion and mangrove removal, Waikaraka Estuary, Tauranga Harbour, New Zealand. Int. J. Ecol. Dev. 2009, 12, 88–106. [Google Scholar]
- Lundquist, C.J.; Hailes, S.F.; Carter, K.R.; Burgess, T.C. Ecological Status of Mangrove Removal Sites in the Auckland Region. Prepared by the National Institute of Water and Atmospheric Research for Auckland Council. Auckland Council Technical Report, 2014a, TR2014/033. Available online: http://temp.aucklandcouncil.govt.nz/SiteCollectionDocuments/aboutcouncil/planspoliciespublications/technicalpublications/tr2014033ecologicalstatusofmangroveremovalsitesintheaklregion.pdf (accessed on 5 May 2017).
- Kaly, U.L.; Eugelink, G.; Robertson, A.I. Soil conditions in damaged North Queensland Mangroves. Estuaries 1997, 20, 291–300. [Google Scholar] [CrossRef]
- Gladstone, W.; Schreider, M.J. Effects of pruning a temperate mangrove forest on the associated assemblages of macroinvertebrates. Mar. Freshw. Res. 2003, 54, 683–690. [Google Scholar] [CrossRef]
- New Zealand Landcare Trust. 2016. Available online: http://www.landcare.org.nz/Regional-Focus/Manawatu-Whanganui-Office/Citizen-Science-Meets-Environmental-Restoration (accessed on 8 February 2018).
- Peters, M. An Inventory of Citizen Science Initiatives, Resources and Learning Opportunities in New Zealand; A Report Prepared for the NZ Landcare Trust; NZ Landcare Trust: Hamilton, New Zealand, 2016; p. 55. [Google Scholar]
- Bay of Plenty Regional Council 2016. Available online: http://www.boprc.govt.nz/mangroves (accessed on 10 February 2018).
- Schwarz, A.; Burns, B.; Alfaro, A. Guidelines for Community-Focused Ecological Monitoring of Mangrove Habitats in Estuaries. Environment Waikato Technical Report; 2005. Available online: http://www.waikatoregion.govt.nz/PageFiles/3331/tr05-12.pdf (accessed on 10 February 2018).
- Ingold, T. The Perception of the Environment: Essays in Livelihood, Dwelling and Skill; Routledge: London, UK; New York, NY, USA, 2000; 465p, ISBN 9780415617475. [Google Scholar]
- Ellis, J.; Nicholls, P.; Craggs, R.; Hofstra, D.; Hewitt, J. Effects of terrigenous sedimentation on mangrove physiology and associated macrobenthic communities. Mar. Ecol. Prog. Ser. 2004, 207, 71–82. [Google Scholar] [CrossRef]
- Alfaro, A.C. Effects of mangrove removal on benthic communities and sediment characteristics at Mangawhai Harbour, northern New Zealand. ICES J. Mar. Sci. J. Cons. 2010, 67, 1087–1104. [Google Scholar] [CrossRef]
- Morrisey, D.J.; Skilleter, G.A.; Ellis, J.I.; Burns, B.R.; Kemp, C.E.; Burt, K. Differences in benthic fauna and sediment among mangrove. (Avicennia marina var. australasica) stands of different ages in New Zealand. Estuar. Coast. Shelf Sci. 2003, 56, 581–592. [Google Scholar] [CrossRef]
- Bulmer, R.H.; Schwendenmann, L.; Lohrer, A.M.; Lundquist, C.J. Sediment carbon and nutrient fluxes from cleared and intact temperate mangrove ecosystems and adjacent sandflats. Sci. Total Environ. 2017, 599–600, 1874–1884. [Google Scholar] [CrossRef] [PubMed]
- Stokes, D.J.; Healy, T.R.; Cooke, P.J. Expansion dynamics of monospecific, temperate mangroves and sedimentation in two embayments of a barrier-enclosed lagoon, Tauranga Harbour, New Zealand. J. Coast. Res. 2010, 113–122. [Google Scholar] [CrossRef]
- Cox, G.J. Utilization of New Zealand Mangrove Swamps by Birds. Master’s Thesis, University of Auckland, Auckland, New Zealand, 1977. [Google Scholar]
- Baird, K.; Ismar, S.M.; Wilson, D.; Plowman, S.; Zimmerman, R.; Bellingham, M. Sightings of New Zealand Fairy Tern (Sternula nereis davisae) in the Kaipara Harbour following nest failure. Notornis 2013, 60, 183–185. [Google Scholar]
- Ismar, S.M.H.; Trnski, T.; Beauchamp, T.; Bury, S.; Wilson, D.; Kannemeyer, R.; Bellingham, M.; Baird, K. Foraging ecology and choice of feeding habitat in the New Zealand Fairy Tern Sternula nereis davisae. Bird Conserv. Int. 2014, 24, 72–87. [Google Scholar] [CrossRef]
- Dugdale, J.S. Reassessment of Ctenopseustis Meyrick and Planotortrix Dugdale with descriptions of two new genera (Lepidoptera: Tortricidae). N. Z. J. Zool. 1990, 17, 437–465. [Google Scholar] [CrossRef]
- Lamb, K.P. New plant galls: 1—Mite and insect galls. Trans. R. Soc. N. Z. 1952, 79, 349–362. [Google Scholar]
- Clapperton, B.K.; Tilley, J.A.V.; Pierce, R.J. Distribution and abundance of the Asian paper wasp Polistes chinensis antennalis Perez and the Australian paper wasp P. humilis (Fab.) (Hymenoptera: Vespidae) in New Zealand. N. Z. J. Zool. 1996, 23, 19–25. [Google Scholar] [CrossRef]
- Brejaart, R.; Brownell, B. Mangroves: The cornerstone of a dynamic coastal environment. In Muddy Feet: Firth of Thames Ramsar Site Update 2004; Brownell, B., Ed.; Ecoquest Education Foundation: Pokeno, New Zealand; pp. 234–252. ISBN 978-1-877416-87-3.
- Ward, D.F.; Harris, R.J. Invasibility of Native Habitats by Argentine Ants, Linepithema Humile, in New Zealand. N. Z. J. Ecol. 2005, 215–219. Available online: http://newzealandecology.org/nzje/2273 (accessed on 28 February 2017).
- Stephens, A.E.; Suckling, D.M.; Burnip, G.M.; Richmond, J.; Flynn, A. Field records of painted apple moth (Teia anartoides Walker: Lepidoptera: Lymantriidae) on plants and inanimate objects in Auckland, New Zealand. Aust. J. Entomol. 2007, 46, 152–159. [Google Scholar] [CrossRef]
- Doyle, E.J. Assessing the Use of Molecular Techniques to Determine Terrestrial Invertebrate Diversity in New Zealand Mangroves and Detection of Blue Ducks in New Zealand Streams. Master’s Thesis, University of Waikato, Hamilton, New Zealand, 2015. [Google Scholar]
- Ritchie, L.D. Fish and fisheries. “Why are mangroves important?”. In Proceedings of the Nature Conservation Council/Friends of the Earth Symposium, 1976. [Google Scholar]
- Lowe, M.L. Factors Affecting the Habitat Usage of Estuarine Juvenile Fish in Northern New Zealand (Doctoral Dissertation, ResearchSpace@ Auckland). 2013. Available online: https://researchspace.auckland.ac.nz/bitstream/handle/2292/20691/whole.pdf?sequence=6 (accessed on 12 April 2017).
- Blom, C.M. Anthropogenic Impacts on the Benthic Fauna and Forest Structure of the New Zealand Mangrove Avicennia Marina Var. Resinifera. Master’s Thesis, University of Auckland, Auckland, New Zealand, 1992. [Google Scholar]
- Reynolds, C.L.; Er, O.A.H.; Winder, L.; Blanchon, D.J. Distribution and community composition of lichens on mature mangroves (Avicennia marina subsp. australasica (Walp.) J. Everett) in New Zealand. PLoS ONE 2017, 12, e0180525. [Google Scholar] [CrossRef] [PubMed]
- Gritcan, I.; Duxbury, M.; Leuzinger, S.; Alfaro, A. Leaf Stable Isotope and Nutrient Status of Temperate Mangroves as Ecological Indicators to Assess Anthropogenic Activity and Recovery from Eutrophication. Front. Plant Sci. 2017, 1977. [Google Scholar] [CrossRef] [PubMed]
- Bulmer, R.H.; Schwendenmann, L.; Lundquist, C.J. Allometric models for estimating aboveground biomass, carbon and nitrogen stocks in temperate Avicennia marina forests. Wetlands 2016, 36, 841–848. [Google Scholar] [CrossRef]
- Tran, P.; Gritcan, I.; Cusens, J.; Alfaro, A.C.; Leuzinger, S. Biomass and nutrient composition of temperate mangrove Avicennia marina var. australasica, New Zealand. N. Z. J. Mar. Freshw. Res. 2016, 51, 427–442. [Google Scholar] [CrossRef]
- Bulmer, R.H.; Schwendenmann, L.; Lundquist, C.J. Carbon and nitrogen stocks and below-ground allometry in temperate mangroves. Front. Mar. Sci. 2016, 3, 150. [Google Scholar] [CrossRef]
- Pérez, A.; Machado, W.; Gutierrez, D.; Stokes, D.; Sanders, L.; Smoak, J.M.; Sanders, C.J. Changes in organic carbon accumulation driven by mangrove expansion and deforestation in a New Zealand estuary. Estuar. Coast. Shelf Sci. 2017, 192, 108–116. [Google Scholar] [CrossRef]
- Bulmer, R.H.; Lundquist, C.J.; Schwendenmann, L. Sediment properties and CO2 efflux from intact and cleared temperate mangrove forests. Biogeosciences 2015, 12, 6169–6180. [Google Scholar] [CrossRef]
- Swales, A.; Bell, R.G.; Gorman, R.; Oldman, J.W.; Altenberger, A.; Hart, C.; Claydon, L.; Wadhwa, S.; Ovenden, R. Potential future changes in mangrove-habitat in Auckland’s east-coast estuaries. In Auckland Regional Council Technical Publication Number TR 2009/079; NIWA: Auckland, New Zealand, 2009. [Google Scholar]
- Swales, A.; Denys, P.; Pickett, V.I.; Lovelock, C. Evaluating deep subsidence in a rapidly-accreting mangrove forest using GPS monitoring of surface-elevation benchmarks and sedimentary records. Mar. Geol. 2016, 380, 205–218. [Google Scholar] [CrossRef]
- McBride, G.; Reeve, G.; Pritchard, M.; Lundquist, C.; Daigneault, A.; Bell, R.; Blackett, P.; Swales, A.; Wadhwa, S.; Tait, A.; et al. The Firth of Thames and Lower Waihou River. Synthesis Report RA2, Coastal Case Study. Climate Changes, Impacts and Implications (CCII) for New Zealand to 2100. MBIE Contract C01X1225. 2016. Available online: http://ccii.org.nz/wp-content/uploads/2017/02/RA2-Coastal-Case-Study-Synthesis-report.pdf (accessed on 27 June 2016).
- Chapman, V.J.; Ronaldson, J.W. The Mangrove and Salt-marsh Flats of the Auckland Isthmus; Department of Scientific and Industrial Research: Wellington, New Zealand, 1958; 79p.
- Yang, J.; Gao, J.; Cheung, A.; Liu, B.; Schwendenmann, L.; Costello, M.J. Vegetation and sediment characteristics in an expanding mangrove forest in New Zealand. Estuar. Coast. Shelf Sci. 2013, 134, 11–18. [Google Scholar] [CrossRef]
- Gladstone-Gallagher, R.V.; Lundquist, C.J.; Pilditch, C.A. Mangrove (Avicennia marina subsp. australasica) litter production and decomposition in a temperate estuary. N. Z. J. Mar. Freshw. Res. 2014, 48, 24–37. [Google Scholar] [CrossRef]
- Lundquist, C.J.; Morrisey, D.J.; Gladstone-Gallagher, R.V.; Swales, A. Managing mangrove habitat expansion in New Zealand. In Mangrove Ecosystems of Asia; Springer: New York, NY, USA, 2014; pp. 415–438. ISBN 978-1-4614-8582-7. [Google Scholar]
- Lundquist, C.; Carter, K.; Hailes, S.; Bulmer, R. Guidelines for Managing Mangroves (Mānawa) Expansion in New Zealand; NIWA Information Series No. 85; National Institute of Water & Atmospheric Research Ltd.: Newmarket, Auckland, New Zealand, 2017. [Google Scholar]
- Murray, C. An Assessment of Cost-Benefit Analysis Approaches to Mangrove Management; Auckland Council Technical Report, 2013/006; Auckland Council: Auckland, New Zealand, 2013; ISBN 978-1-927216-31-6. Available online: http://temp.aucklandcouncil.govt.nz/EN/planspoliciesprojects/reports/technicalpublications/Documents/TR2013006anassessmentofcostbenefitanalysisapproachestomangrovemanagement.pdf (accessed on 5 May 2017).
- Patterson, M.G.; Cole, A.O. Total economic value of New Zealand’s land-based ecosystems and their services. In Ecosystem Services in New Zealand–Conditions and Trends; Manaaki Whenua Press: Lincoln, New Zealand, 2013; pp. 496–510. [Google Scholar]
- Ngāti Te Ata Waiohua. Proposal for Mangrove Removal on Behalf of the Mangere-Ōtāhuhu Local Board, Mangere Inlet, Manukau Harbour; Cultural Impact Assessment: Auckland, New Zealand, 2015. [Google Scholar]
- Morton, J.; Miller, M. The New Zealand Sea Shore; Collins: London, UK, 1968; 653p, ISBN 9780002115551. [Google Scholar]
- Henriques, P.R. Faunal community structure of eight soft shore, intertidal habitats in the Manukau Harbour. N. Z. J. Ecol. 1980, 97–103. [Google Scholar]
- Taylor, F.J. The New Zealand mangrove association. In Biology and Ecology of Mangroves; Teas, H.J., Ed.; 1980; pp. 77–79. ISBN 978-90-481-8526-9. [Google Scholar]
- Alfaro, A.C.; Thomas, F.C.M.; Sergent, L.; Duxbury, M. Identification of trophic interactions within an estuarine food web (northern New Zealand) using fatty acid biomarkers and stable isotopes. Estuar. Coast. Shelf Sci. 2006, 70, 271–286. [Google Scholar] [CrossRef]
- Bulmer, R.; Lewis, M.; O’Donnell, E.; Lundquist, C.J. Assessing mangrove clearance methods to minimise adverse impacts and maximise the potential to achieve restoration objectives. N. Z. J. Mar. Freshw. Res. 2017, 51. [Google Scholar] [CrossRef]
- Laird, M. New Zealand’s northern mosquito survey, 1988–89. J. Am. Mosq. Control Assoc. 1990, 6, 287–289. [Google Scholar] [PubMed]
- Gill, B.J. Breeding of the grey warbler Gerygone igata at Kaikoura, New Zealand. Ibis 1982, 124, 123–147. [Google Scholar] [CrossRef]
- Miller, P.; Miller, K. Bitterns using mangroves. Notornis 1991, 38, 79. [Google Scholar]
- Beauchamp, A.J.; Parrish, G.R. Bird use of the sediment settling ponds and roost areas at Port Whangarei. Notornis 1999, 46, 470–483. [Google Scholar]
- Robertson, H.; Heather, B. Hand Guide to the Birds of New Zealand; Oxford University Press: Oxford, UK, 2001; 168p, ISBN 9780198508311. [Google Scholar]
- Parrish, R.; Williams, M. Decline of brown teal (Anas chlorotis) in Northland, New Zealand, 1988–99. Notornis 2001, 48, 131–136. [Google Scholar]
- Balke, T.; Swales, A.; Lovelock, C.E.; Herman, P.M.; Bouma, T.J. Limits to seaward expansion of mangroves: Translating physical disturbance mechanisms into seedling survival gradients. J. Exp. Mar. Biol. Ecol. 2015, 467, 16–25. [Google Scholar] [CrossRef]
- Santini, N.S.; Schmitz, N.; Lovelock, C.E. Variation in wood density and anatomy in a widespread mangrove species. Trees 2012, 26, 1555–1563. [Google Scholar] [CrossRef]
- Mildenhall, D.C. Middle Holocene mangroves in Hawke’s Bay, New Zealand. N. Z. J. Bot. 2001, 39, 517–521. [Google Scholar] [CrossRef]
- Nichol, S.L.; Augustinus, P.C.; Gregory, M.R.; Creese, R.; Horrocks, M. Geomorphic and sedimentary evidence of human impact on the New Zealand. Phys. Geogr. 2000, 21, 109–132. [Google Scholar]
- Gao, J. A comparative study on spatial and spectral resolutions of satellite data in mapping mangrove forests. Int. J. Remote Sens. 1999, 20, 2823–2833. [Google Scholar] [CrossRef]
- May, J.D. Spatial variation in litter production by the mangrove Avicennia marina var. australasica in Rangaunu Harbour, Northland, New Zealand. N. Z. J. Mar. Freshw. Res. 1999, 33, 163–172. [Google Scholar] [CrossRef]
- Osunkoya, O.; Creese, R.G. Population structure, spatial pattern and seedling establishment of the grey mangrove, Avicennia marina var. australasica, in New Zealand. Aust. J. Bot. 1997, 45, 707–725. [Google Scholar]
- Burns, B.R.; Ogden, J. The demography of the temperate mangrove [Avicennia marina (Forsk.) Vierh.] at its southern limit in New Zealand. Aust. Ecol. 1985, 10, 125–133. [Google Scholar] [CrossRef]
- Woodroffe, C.D. Studies of a mangrove basin, Tuff Crater, New Zealand: I. Mangrove biomass and production of detritus. Estuar. Coast. Shelf Sci. 1985, 20, 265–280. [Google Scholar] [CrossRef]
- Woodroffe, C.D. Litter production and decomposition in the New Zealand mangrove, Avicennia marina var. resinifera. N. Z. J. Mar. Freshw. Res. 1982, 16, 179–188. [Google Scholar] [CrossRef]
- Bay of Plenty Regional Council. Available online: https://www.boprc.govt.nz/media/520778/tauranga-harbour-mangrove-management-literature-review.pdf (accessed on 26 April 2017).
- Lovelock, C.E.; Feller, I.C.; Ellis, J.; Schwarz, A.M.; Hancock, N.; Nichols, P.; Sorrell, B. Mangrove growth in New Zealand estuaries: The role of nutrient enrichment at sites with contrasting rates of sedimentation. Oecologia 2007, 153, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Dromgoole, F.I. Carbon dioxide fixation in aerial roots of the New Zealand mangrove Avicennia marina var. resinifera. N. Z. J. Mar. Freshw. Res. 1988, 22, 617–619. [Google Scholar] [CrossRef]
- Hicks, B.J.; Silvester, W.B. Nitrogen fixation associated with the New Zealand mangrove (Avicennia marina (Forsk.) Vierh. var. resinifera (Forst. f.) Bakh.). Appl. Environ. Microb. 1985, 49, 955–959. [Google Scholar]
- Swales, A.; Bentley, S.J.; Lovelock, C.E. Mangrove-forest evolution in a sediment-rich estuarine system: Opportunists or agents of geomorphic change? Earth Surf. Process. Landf. 2015, 40, 1672–1687. [Google Scholar] [CrossRef]
- Stokes, D.J.; Harris, R.J. Sediment properties and surface erodibility following a large-scale mangrove (Avicennia marina) removal. Cont. Shelf Res. 2015, 107, 1–10. [Google Scholar] [CrossRef]
- Swales, A.; Williamson, R.B.; Van Dam, L.F.; Stroud, M.J.; McGlone, M.S. Reconstruction of urban stormwater contamination of an estuary using catchment history and sediment profile dating. Estuaries 2002, 25, 43–56. [Google Scholar] [CrossRef]
- Young, B.M.; Harvey, E.L. A spatial analysis of the relationship between mangrove (Avicennia marinavar. australasica) physiognomy and sediment accretion in the Hauraki Plains, New Zealand. Estuar. Coast. Shelf Sci. 1996, 42, 231–246. [Google Scholar] [CrossRef]
- Swales, A.; Bentley, S.J.; Lovelock, C.; Bell, R.G. Sediment processes and mangrove-habitat expansion on a rapidly-prograding muddy coast, New Zealand. In Proceedings of the Coastal Sediments, Reston, VA, USA, 13–17 May 2017; pp. 1441–1454. [Google Scholar]
- Meades, L.; Rodgerson, L.; York, A.; French, K. Assessment of the diversity and abundance of terrestrial mangrove arthropods in southern New South Wales, Australia. Aust. Ecol. 2002, 27, 451–458. [Google Scholar] [CrossRef]
- Rog, S.M.; Clarke, R.H.; Cook, C.N. More than marine: Revealing the critical importance of mangrove ecosystems for terrestrial vertebrates. Divers. Distrib. 2017, 23, 221–230. [Google Scholar] [CrossRef]
- Paris, B.; (Auckland Council Biodiversity Officer, Auckland, New Zealand). Personal Communication, 2017.
- Carter, G.G.; Riskin, D.K. Mystacina tuberculata. Mamm. Species 2006, 790, 1–8. [Google Scholar] [CrossRef]
- Mcleod, E.; Chmura, G.L.; Bouillon, S.; Salm, R.; Björk, M.; Duarte, C.M.; Silliman, B.R. A blueprint for blue carbon: Toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front. Ecol. Environ. 2011, 9, 552–560. [Google Scholar] [CrossRef] [Green Version]
- Pendleton, L.; Donato, D.C.; Murray, B.C.; Crooks, S.; Jenkins, W.A.; Sifleet, S.; Megonigal, P. Estimating global “blue carbon” emissions from conversion and degradation of vegetated coastal ecosystems. PLoS ONE 2012, 7, e43542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alongi, D.M. Carbon cycling and storage in mangrove forests. Annu. Rev. Mar. Sci. 2014, 6, 195–219. [Google Scholar] [CrossRef] [PubMed]
- Marchand, C.; Fernandez, J.M.; Moreton, B.; Landi, L.; Lallier-Verges, E.; Baltzer, F. The partitioning of transitional metals (Fe, Mn, Ni, Cr) in mangrove sediments downstream of a ferralitized ultramafic watershed (New Caledonia). Chem. Geol. 2012, 300, 70–80. [Google Scholar] [CrossRef] [Green Version]
- Anonymous; (Auckland, New Zealand). Personal communication, 2017.
- Environmental Protection Agency. Available online: https://www.epa.gov/wetlands/mangrove-swamps (accessed on 13 February 2017).
- Viveiros de Castro, E. Perspectival Anthropology and the Method of Controlled Equivocation. J. Soc. Anthropol. Lowl. S. Am. 1994, 2, 1. [Google Scholar]
- Davies, K.; Fisher, K.; Dickson, M.; Thrush, S.; Le Heron, R. Improving ecosystem service frameworks to address wicked problems. Ecol. Soc. 2015, 20. [Google Scholar] [CrossRef]
- Weihong, J.I.; (Massey University, Auckland, New Zealand). Personal communication, 2017.
- Morrison, M.; (Auckland Council, Auckland, New Zealand). Personal communication, 2017.
- Longman Dictionary of Contemporary English. Available online: https://www.ldoceonline.com/dictionary/beetle (accessed on 22 February 18).
- FishBase (Undated). Available online: http://www.fishbase.se/identification/SpeciesList.php?class=Actinopterygii&order=&famcode=49&subfamily=&genus=&areacode=&c_code=&spines=&fins= (accessed on 22 February 2018).
- Species at Risk Public Registry 2011. Available online: https://www.registrelep-sararegistry.gc.ca/default.asp?lang=En&n=68A63438-1 (accessed on 22 February 2018).
- Pixabay. Available online: https://pixabay.com/en/photos/crab/ (accessed on 22 February 2018).
- Huxham, M.; Whitlock, D.; Njoroge, M.; Dencer-Brown, A. Carbon in the coastal seascape: How interactions between mangrove forests, seagrass meadows and tidal marshes influence carbon storage. Curr. For. Rep. 2018. in review. [Google Scholar]
- Sibr.com. Available online: http://sibr.com/mammals/M140.html (accessed on 8 March 2018).
- Moziru.com. Available online: http://moziru.com/explore/Leopard%20Gecko%20clipart%20silhouette/#go_post_3294_leopard-gecko-clipart-silhouette-14.jpg (accessed on 8 March 2018).
- Clipart Library 2016. Available online: http://clipart-library.com/bat.html (accessed on 8 March 2018).


| Ecosystem Service | Ecological Function | Economic Goods and Service | Value Type |
|---|---|---|---|
| Provisioning | Nursery and habitat for animal and plant species | Commercial & recreational fishing and hunting. Harvesting of natural materials, energy resources | Direct use |
| Cultural | - | Recreation, ecotourism | Direct use |
| Existence, bequest and option values | Non-use | ||
| Regulating | Carbon sequestration | Reduced global warming | Indirect use |
| - | Flood and water flow control, storm buffering, sediment retention, water quality maintenance/ nutrient retention | Flood and storm protection, improved water quality and waste disposal | Indirect use |
| Type | Topic | Location | Notes | Ref. |
|---|---|---|---|---|
| E | Costs of removal | Auckland and Tauranga | Variation of costs ranging from $10,000–$33,000 NZD/Ha for removal | [111] |
| E | Total economic value of land-based ecosystems | New Zealand-wide | Gross value of $144,000,000 NZD for mangroves | [88] |
| E | Cost-benefit analysis of managing mangroves | Auckland | Projected expenditure 2011 local board plans including mangrove removal | [87] |
| C | Cultural impacts of mangrove removal | Auckland | Restoration of mauri of harbour is of most importance | [89] |
| M | Management and Planning review | Tauranga | Harbour-wide management of mangroves difficult to achieve. Need site-specific assessments. | [111] |
| M | Managing mangrove expansion | New Zealand-wide | Likelihood of successful restoration rarely considered, minimal information on long-term trends in ecosystem health of removed areas | [85] |
| M | Management guidelines | General New Zealand | Land-use to reduce sediment loads needs to be better managed, pre-removal baseline data required | [86] |
| Region | Activity | Method | Year | Costs (NZD) | Ref. |
|---|---|---|---|---|---|
| Papakura Auckland | Mangrove and seedling removal over 3 years (27 Ha) | Tractor and helicopter to remove AGB, roots left in situ | 2010–2012 | $1,500,000 | [87] |
| Waimahia | Mangrove and seedling removal | Handsaw, loppers | 2015 | $888,000 (works costs) plus 28 k works management plan and 5 k bird survey (projected) | [111] |
| Mangere and Waitemata | Consenting process and removal of mangroves in Auckland’s two harbours | Hand removal | 2011 Local Board Plans | $780,000 | [87] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dencer-Brown, A.M.; Alfaro, A.C.; Milne, S.; Perrott, J. A Review on Biodiversity, Ecosystem Services, and Perceptions of New Zealand’s Mangroves: Can We Make Informed Decisions about Their Removal? Resources 2018, 7, 23. https://doi.org/10.3390/resources7010023
Dencer-Brown AM, Alfaro AC, Milne S, Perrott J. A Review on Biodiversity, Ecosystem Services, and Perceptions of New Zealand’s Mangroves: Can We Make Informed Decisions about Their Removal? Resources. 2018; 7(1):23. https://doi.org/10.3390/resources7010023
Chicago/Turabian StyleDencer-Brown, Amrit Melissa, Andrea C. Alfaro, Simon Milne, and John Perrott. 2018. "A Review on Biodiversity, Ecosystem Services, and Perceptions of New Zealand’s Mangroves: Can We Make Informed Decisions about Their Removal?" Resources 7, no. 1: 23. https://doi.org/10.3390/resources7010023




