1. Introduction
The suprachiasmatic nucleus (SCN) in the hypothalamus encompasses the endogenous biological clock, which drives physiological, endocrine, and behavioral rhythms with a periodicity of about 24 h [
1]. The daily environmental light-dark cycle serves as the main synchronizer to entrain these so called circadian rhythms to an exact 24-h cycle in order to remain in phase with the 24-h period of a day on Earth [
2]. Intrinsically photosensitive retinal ganglion cells (ipRGCs) play a key role in this circadian photoentrainment [
3,
4]. ipRGCs express the photopigment melanopsin which enables them to intrinsically decode ambient light levels [
3,
5]. Melanopsin is maximally responsive to short-wavelength (i.e., blue) light with peak sensitivity between 460–480 nm. In addition, ipRGCs receive extrinsic input from rods and cones [
6,
7]. Through direct connections between ipRGCs and the brain, the integrated intrinsic and extrinsic information on environmental light is transferred from the retina to downstream non-image forming brain regions, with the SCN as one of the main targets [
8].
Besides circadian photoentrainment, ipRGCs are also involved in the regulation of mood [
9], alertness [
10], pineal melatonin production [
11], and the pupillary light reflex (PLR) [
6]. The contribution of ipRGCs to the PLR is mediated through direct projections onto the olivary pretectal nucleus (OPN; i.e., the brain region for pupil size control [
12]). The involvement of ipRGCs in the PLR allows for a non-invasive method to measure functionality of the intrinsic melanopsin-based signaling pathway [
13]. More specifically, the characteristic sustained contraction of the pupil after blue light can be almost entirely assigned to the melanopsin-driven delay in ipRGC repolarization after light offset [
14,
15]. This phenomenon, known as the post-illumination pupil response (PIPR) [
8], can thus be used to estimate the functionality of the intrinsic melanopsin-signaling circuitry [
14].
Accordingly, we have previously developed a reliable and robust method to assess individual differences in the maximum PIPR [
16] and showed its relevance for circadian entrainment [
17]. The assessment procedures include pharmacological pupil dilation in the illuminated eye [
18], which is highly uncomfortable for the participants [
19]. The mydriatics that are used for pupil dilation, like tropicamide, phenylephrine, and cyclopentolate, entail several possible side effects including a dry mouth, blurred vision, stinging, and sensitivity of the eyes to light [
20]. In addition, dilation of the pupil may persist up until 24 h after administration causing prolonged reduced sight, which is often experienced as unpleasant and impedes tasks like driving and reading [
21]. A more serious risk is that pharmacological pupil dilation may lead to closed-angle glaucoma [
22]. Ophthalmological expertise is therefore indispensable for the use of mydriatics. This proficiency is usually present in eye research. However, given the myriad of ipRGC-driven downstream photoregulatory functions, PIPR assessments are also highly relevant for non-ophthalmic research on sleep [
17,
23,
24], cognition [
25], alertness [
10,
26], mood and emotion [
27], where ophthalmologic expertise to safely apply pupil dilation may be insufficient. Taken together, to relieve the participant’s burden and to allow for a wide application of safe PIPR measurements, it would be highly desirable to have a protocol that does not rely on the use of mydriatics while preserving the high reliability and robustness of the assessments.
Previous studies have presented reliable PIPR measurements without pharmacological mydriasis [
28,
29]. The PIPR in these previous studies was only transient, however, while we here aimed to elicit a more melanopsin-specific maximum prolonged PIPR. Considering that the PIPR increases with increasing light intensity [
30,
31,
32,
33], the aim of the present study was to examine whether an individual’s maximum PIPR can be validly assessed without mydriatics by instead increasing the intensity of the light stimulus [
14]. We, therefore, examined the within-subject between-day test-retest reliability of an alternative protocol without mydriatics with increased light intensity and compared this to the previously found very high reliability of the protocol with mydriatics [
16]. In a previous different protocol to assess PIPR, outcome measures showed variability across seasons [
34]. We, therefore, evaluated whether our protocol without mydriatics might result in a more stable trait-like biomarker, robust across seasons, by assessing the test-retest reliability across the winter and summer.
3. Results
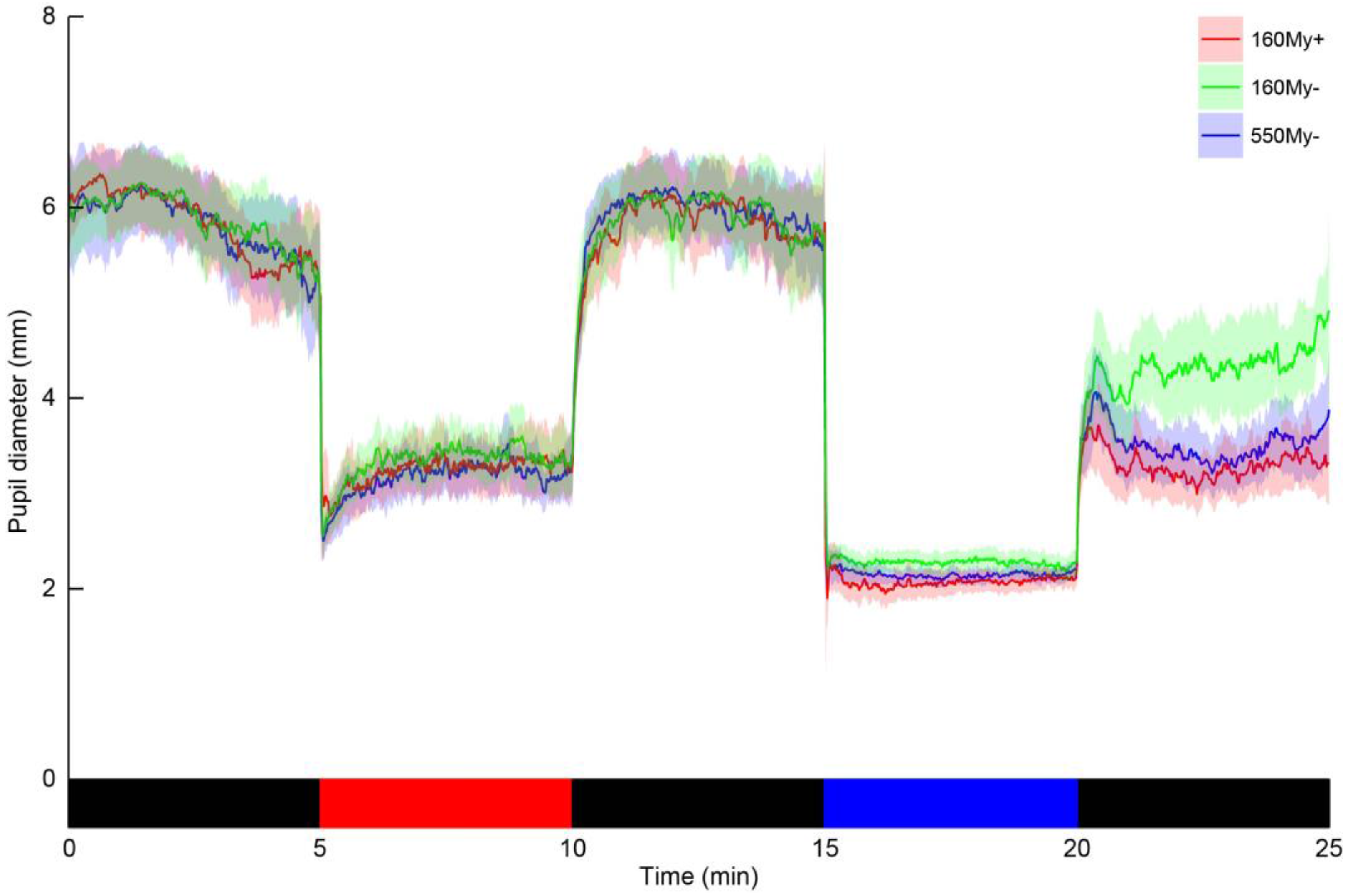
The values for baseline and post-blue pupil diameter, PIPR-mm and PIPR-%, are shown in
Table 2. Retinal illuminance during blue light was estimated based on the mean pupil diameter during blue light to be 18850 Td in 160My+, 1545 Td in 160My−, and 4897 Td in 550My−. Bland-Altman bias indicated low agreement between 160My+ and 160My− for the PIPR-mm and PIPR-%. In addition, there was low reliability between the PIPR outcome parameters in these conditions, as indicated by the ICC (PIPR-mm: −0.12; PIPR-%: −0.27) (
Table 2). Negative ICC estimates indicate within-subject variability exceeding between-subject variability, and thus a very poor ICC [
44]. There was also a low agreement between 160My+ and 160My− for the post-blue pupil diameter (ICC = 0.03).
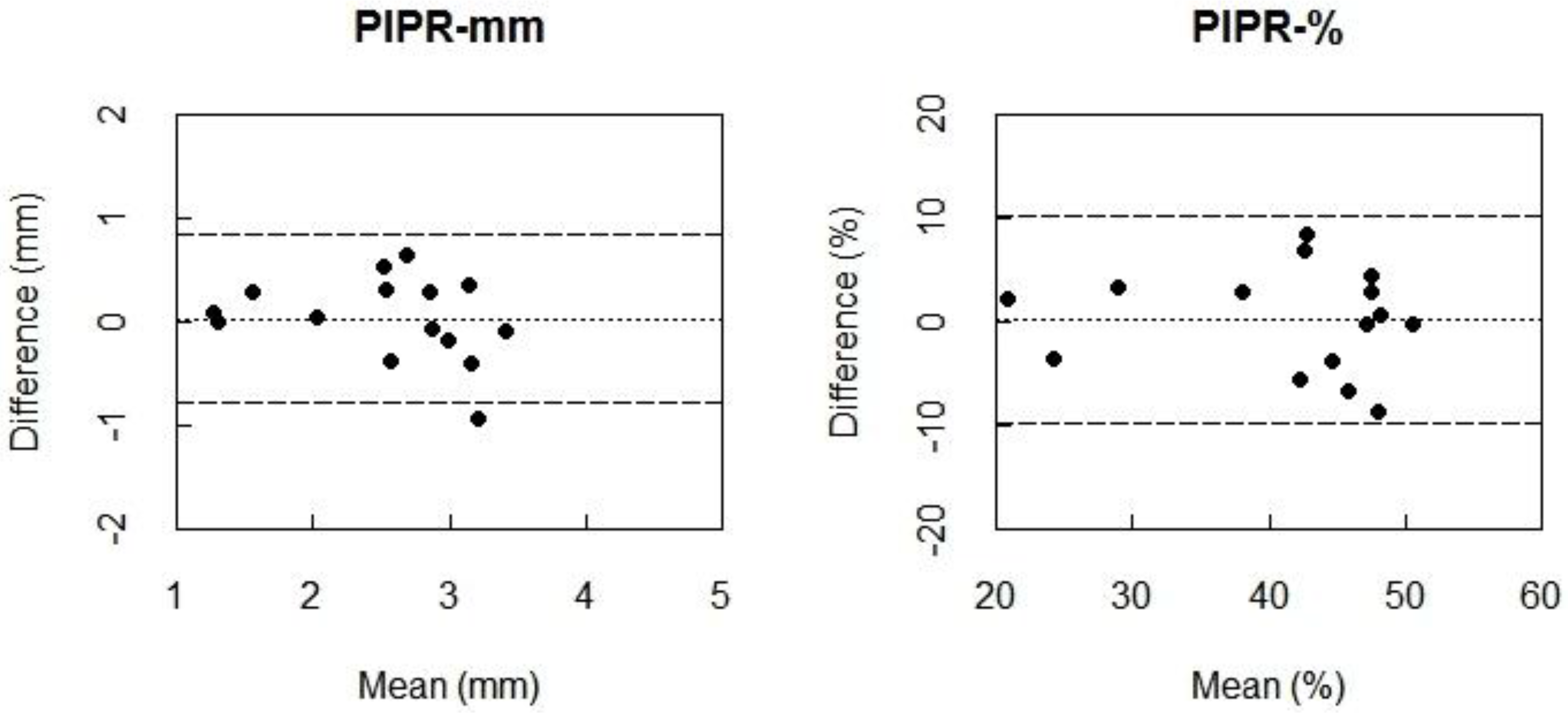
The Bland-Altman plots indicated almost zero bias between 160My+ and 550My− for PIPR-mm and PIPR-% (
Figure 2) indicating high agreement. When comparing the condition 160My+ to the condition 550My−, the ICC values indicate a moderate to strong agreement for the PIPR outcome parameters (PIPR-mm: ICC = 0.67; PIPR-%: ICC = 0.58). The ICC of 0.77 indicated a strong agreement between these conditions for the post-blue pupil diameter, which was confirmed by an almost-zero Bland-Altman bias.
During baseline dark there was an almost perfect agreement between 160My+ and 160My− (ICC = 0.88) and also between 160My + and 550My− (ICC = 0.92). Bland-Altman biases also indicated this high similarity (
Table 3).
Fifteen (5 males, mean age ± SD (range): 36.4 ± 14.3 (23–70) years) out of the 18 participants returned to the lab for two consecutive summer assessments, one day apart. Bland-Altman plots indicated almost zero bias between the outcome measures of the 550My− protocol for PIPR-mm and PIPR-% (
Figure 3), indicating high agreement. The ICC values for the PIPR outcome parameters moreover indicated a very high day-to-day test-retest reliability (PIPR-mm: ICC = 0.85; PIPR-%: ICC = 0.87) (
Table 4).
The Bland-Altman plots also indicated almost zero bias between 550My− winter and 550My− summer measurements for PIPR-mm and PIPR-% (
Figure 4), indicating high agreement. Again, the ICC values indicated a very high reliability for the PIPR outcome parameters (PIPR-mm: ICC = 0.83; PIPR-%: ICC = 0.80) (
Table 5).
4. Discussion
In order to allow for a wide application of assessing the functionality of the melanopsin-signaling circuitry, we here examined whether an individual’s maximum PIPR can be validly assessed without mydriatics by instead increasing the intensity of the light exposure. In our previously established paradigm, with mydriatics, the 5-min duration of the blue light stimulus was expected to saturate the ipRGCs [
16,
37,
40], leading to a maximum PIPR. Shorter blue light stimuli durations of milliseconds to a few seconds, as used in previous studies, may propose less discomfort for the participant but only elicit a transient PIPR (i.e., the post-exposure pupil diameter returns to baseline already during the assessment) [
28,
29,
30,
32]. On the contrary, our use of high intensity light stimuli of longer duration, tailored to the slow photoresponse and low sensitivity of ipRGCs, allows for more specific targeting of melanopsin-signaling pathway. Furthermore, in a previous shorter protocol to assess the PIPR, outcome measures showed variability across seasons [
34]. Our longer protocol may result in a more trait-like biomarker that is stable across the winter and summer.
The previously established blue light stimulus with an irradiance of 160 µW/cm2, evoked a reduced PIPR when the pupil was kept in its natural dynamic state compared to the condition with the use of mydriatics. This indicates that dilation of the pupil or intensifying the light is required to evoke a maximum PIPR.
The retinal illuminance during blue light was over four times higher for the previously established protocol with mydriatics compared to the condition with intensified blue light without mydriatics. In spite of this fourfold difference, the PIPR did not systematically differ between these conditions. This suggests that during both conditions the ipRGCs response was saturated and a maximum PIPR was generated, since there was no further increase in PIPR even though the blue-light retinal illuminance was higher in the previously established protocol.
The moderate to strong agreement between the previously established 160 µW/cm2 blue light with mydriatics protocol and the alternative protocol with intensified blue light with a natural pupil indicates that both approaches are valid for eliciting the maximum PIPR. However, there can be individual variation between the results obtained with these two approaches, indicating that they should not be used interchangeably.
The within-subject test-retest of the protocol with intensified blue light with a natural pupil showed a very high agreement for both PIPR-mm and PIPR-%, approaching the reliability of our formerly established protocol with 160 µW/cm2 blue light and mydriatics (PIPR-mm: ICC = 0.90; PIPR-%: ICC = 0.87). This indicates that although the pupil is dynamic and the amount of light reaching the retina has a larger variation in the protocol with intensified blue light without mydriatics, this does not seem to affect the reliability of this protocol, suggesting saturation of the response at a certain intensity.
Previous research showed that the post-illumination contraction amplitude following blue light was larger in summer compared to winter [
34]. However, there was only a difference between summer and winter for photopically adapted eyes. Our protocol with intensified blue light and a natural pupil showed no effect of season on the PIPR outcome parameters. We showed a very high agreement between the winter and summer measurements. Our protocol did not include an adaption period and provided the light stimulus for a much longer duration (i.e., 5 min instead of 1 s). The high reliability of our protocol with intensified blue light and a natural pupil, between days and even between seasons, provides it as a sensitive and robust possible biomarker to study trait-like individual differences in ipRGCs functioning.
In patients with retinal diseases pharmacological pupil dilation may not be without risk since dilation can lead to a rise in intraocular pressure [
22]. With ipRGCs affected in diverse optic nerve and retinal diseases such as glaucoma [
38,
48], age-related macular degeneration [
49], retinitis pigmentosa [
50], and diabetes [
51], PIPR measurements without dilation may provide a useful diagnostic tool. Excluding pharmacological dilation of the pupil from PIPR assessments is not only beneficial for ophthalmic patients, but also for individuals without eye-related complaints. Since pharmacological pupil dilation may be uncomfortable for participants and can even be dangerous when driving [
52]. With the current reliable PIPR assessment protocol a maximum PIPR can be evoked without pupil dilation making the protocol accessible for non-ophthalmic research fields, such as sleep [
17,
23,
24], cognition [
25], alertness [
10,
26], mood and emotion research [
27]. In addition, considering the important role ipRGCs play in circadian rhythms through their direct projections to the SCN, our current protocol can be easily applied in chronobiological studies.
A limitation of the study is the unequal gender distribution, there were more females included than males, which may have confounded our PIPR results. Accordingly, some parameters of the PLR were shown to be mildly affected by gender [
53]. Future studies should, therefore, use a larger sample with a more even gender distribution to examine the effects of gender. Furthermore, future studies should examine whether the proposed protocol without pupil dilation is applicable in patients suffering from eye diseases and whether it may be used for diagnosing abnormalities in the melanopsin-signaling circuitry.