Pleiotropy as the Mechanism for Evolving Novelty: Same Signal, Different Result
Abstract
:1. Pleiotropy, the Deus ex Machina (Ghost in the Machine)
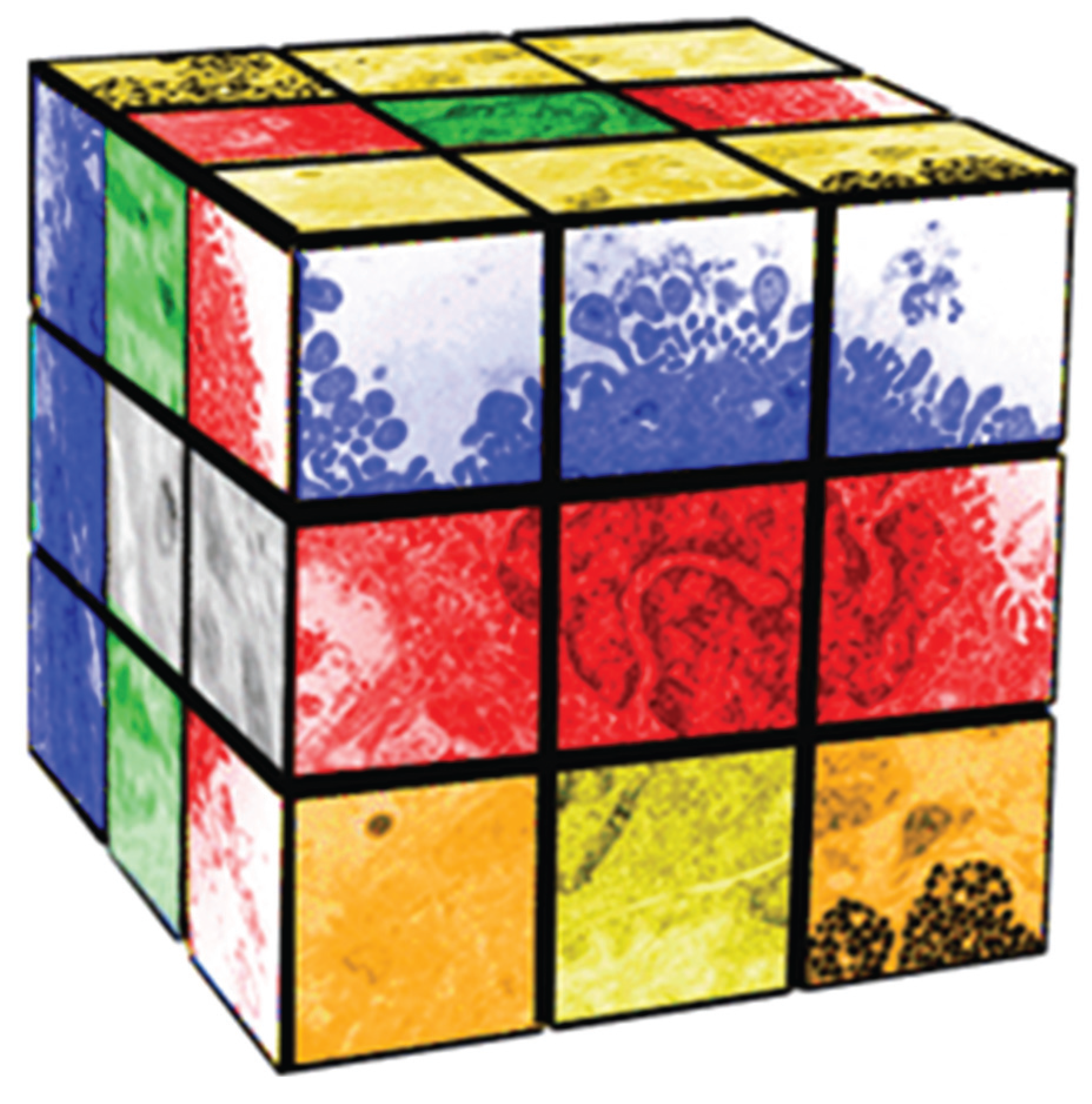
2. Rubik’s Cube as a Metaphor for Pleiotropic Evolution
3. The Lung as the Prototypical Pleiotropic Mechanism
4. The Lung as an Interactive Barrier: Homolog of the Plasma Membrane, Skin and Brain
5. NKX2.1, Thyroid, Pituitary and Lung Pleiotropy
6. The Phylogeny of the Thyroid
7. An Evolutionary Vertical Integration of the Phylogeny and Ontogeny of the Thyroid
8. A Retrospective Understanding of Evolution

9. Denouement
10. Conclusions
Conflicts of Interest
References
- Williams, G.C. Pleiotropy, natural selection, and the evolution of senescence. Evolution 1957, 11, 398–411. [Google Scholar] [CrossRef]
- Torday, J.S. On the evolution of development. Trends Dev. Biol. 2014, 8, 17–37. [Google Scholar] [PubMed]
- Sagert, B. The 1970s (American Popular Culture through History); Greenwood Press: Westport, CT, USA, 2007. [Google Scholar]
- Winklbauer, R.; Damm, E.W. Internalizing the vegetal cell mass before and during amphibian gastrulation: Vegetal rotation and related movements. Wiley Interdiscip. Rev. Dev. Biol. 2012, 1, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Grobstein, C. Mechanisms of organogenetic tissue interaction. Natl. Cancer Inst. Monogr. 1967, 26, 279–299. [Google Scholar] [PubMed]
- Zhang, J.; Wagner, G.P. On the definition and measurement of pleiotropy. Trends Genet. 2013, 29, 383–384. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S.; Wingfield, J.C. The concept of allostasis in biology and biomedicine. Horm. Behav. 2003, 43, 2–15. [Google Scholar] [CrossRef]
- Torday, J.S.; Rehan, V.K. Deconvoluting lung evolution using functional/comparative genomics. Am. J. Respir. Cell Mol. Biol. 2004, 31, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Torday, J.; Rehan, V. Evolutionary Biology, Cell-Cell Communication and Complex Disease; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Torday, J.S. Evolutionary biology redux. Perspect. Biol. Med. 2013, 56, 455–484. [Google Scholar] [CrossRef] [PubMed]
- Torday, J.S. A central theory of biology. Med. Hypotheses 2015, 85, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Odii, B.O.; Coussons, P. Biological functionalities of transglutaminase 2 and the possibility of its compensation by other members of the transglutaminase family. Sci. World J. 2014. [Google Scholar] [CrossRef] [PubMed]
- Aune, T.M.; Collins, P.L.; Collier, S.P.; Henderson, M.A.; Chang, S. Epigenetic activation and silencing of the gene that encodes IFN-γ. Front. Immunol. 2013. [Google Scholar] [CrossRef] [PubMed]
- Cortázar, D.; Kunz, C.; Selfridge, J.; Lettieri, T.; Saito, Y.; MacDougall, E.; Wirz, A.; Schuermann, D.; Jacobs, A.L.; Siegrist, F.; et al. Embryonic lethal phenotype reveals a function of TDG in maintaining epigenetic stability. Nature 2011, 470, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Glenny, R.W.; Robertson, H.T. Spatial distribution of ventilation and perfusion: Mechanisms and regulation. Compr. Physiol. 2011, 1, 375–395. [Google Scholar] [PubMed]
- Carlström, M.; Wilcox, C.S.; Arendshorst, W.J. Renal autoregulation in health and disease. Physiol. Rev. 2015, 95, 405–511. [Google Scholar] [CrossRef] [PubMed]
- Triposkiadis, F.K.; Skoularigis, J. Prevalence and importance of comorbidities in patients with heart failure. Curr. Heart Fail. Rep. 2012, 9, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Rehan, V.K.; Torday, J.S. Lower parathyroid hormone-related protein content of tracheal aspirates in very low birth weight infants who develop bronchopulmonary dysplasia. Pediatr. Res. 2006, 60, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Bosch, R.J.; Rodríguez-Puyol, D.; Bover, J.; Rodríguez-Puyol, M. Parathyroid hormone-related protein: Roles in the glomerulus. Exp. Nephrol. 1999, 7, 212–216. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, B.A.; Sund, M.; Grant, M.A.; Pfaff, K.L.; Holthaus, K.; Zon, L.I.; Kalluri, R. Zebrafish to humans: Evolution of the α3-chain of type IV collagen and emergence of the autoimmune epitopes associated with Goodpasture syndrome. Blood 2006, 107, 1908–1915. [Google Scholar] [CrossRef] [PubMed]
- Torday, J.S.; Rehan, V.K. Stretch-stimulated surfactant synthesis is coordinated by the paracrine actions of PTHrP and leptin. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 283, L130–L135. [Google Scholar] [CrossRef] [PubMed]
- Schlöndorff, D. Roles of the mesangium in glomerular function. Kidney Int. 1996, 49, 1583–1585. [Google Scholar] [CrossRef] [PubMed]
- Romer, A. The Vertebrate Story; University of Chicago Press: Chicago, IL, USA, 1949. [Google Scholar]
- Whitsett, J.A.; Wert, S.E.; Weaver, T.E. Diseases of pulmonary surfactant homeostasis. Annu. Rev. Pathol. 2015, 10, 371–393. [Google Scholar] [CrossRef] [PubMed]
- Maina, J.N.; West, J.B. Thin and strong! The bioengineering dilemma in the structural and functional design of the blood-gas barrier. Physiol. Rev. 2005, 85, 811–844. [Google Scholar] [CrossRef] [PubMed]
- Daniels, C.B.; Orgeig, S.; Sullivan, L.C.; Ling, N.; Bennett, M.B.; Schürch, S.; Val, A.L.; Brauner, C.J. The origin and evolution of the surfactant system in fish: Insights into the evolution of lungs and swim bladders. Physiol. Biochem. Zool. 2004, 77, 732–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, W.; Wang, Z.; Collins, J.E.; Andrews, R.M.; Stemple, D.; Gong, Z. Comparative transcriptome analyses indicate molecular homology of zebrafish swimbladder and mammalian lung. PLoS ONE 2011, 6, e24019. [Google Scholar] [CrossRef] [PubMed]
- Dumbarton, T.C.; Stoyek, M.; Croll, R.P.; Smith, F.M. Adrenergic control of swimbladder deflation in the zebrafish (Danio rerio). J. Exp. Biol. 2010, 213, 2536–2546. [Google Scholar] [CrossRef] [PubMed]
- Torday, J.S.; Rehan, V.K. Cell-cell signaling drives the evolution of complex traits: Introduction-lung evo-devo. Integr. Comp. Biol. 2009, 49, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Torday, J.; Rehan, V. Neutral lipid trafficking regulates alveolar type II cell surfactant phospholipid and surfactant protein expression. Exp. Lung Res. 2011, 37, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Schultz, C.J.; Torres, E.; Londos, C.; Torday, J.S. Role of adipocyte differentiation-related protein in surfactant phospholipid synthesis by type II cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 283, L288–L296. [Google Scholar] [CrossRef] [PubMed]
- Londos, C.; Sztalryd, C.; Tansey, J.T.; Kimmel, A.R. Role of PAT proteins in lipid metabolism. Biochimie 2005, 87, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Torday, J.S.; Torday, D.P.; Gutnick, J.; Qin, J.; Rehan, V. Biologic role of fetal lung fibroblast triglycerides as antioxidants. Pediatr. Res. 2001, 49, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Bellusci, S.; Warburton, D. Lung development and adult lung diseases. Chest 2007, 132, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Aberg, K.M.; Man, M.Q.; Gallo, R.L.; Ganz, T.; Crumrine, D.; Brown, B.E.; Choi, E.H.; Kim, D.K.; Schröder, J.M.; Feingold, K.R.; et al. Co-regulation and interdependence of the mammalian epidermal permeability and antimicrobial barriers. J. Investig. Dermatol. 2008, 128, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Dietl, P.; Liss, B.; Felder, E.; Miklavc, P.; Wirtz, H. Lamellar body exocytosis by cell stretch or purinergic stimulation: Possible physiological roles, messengers and mechanisms. Cell. Physiol. Biochem. 2010, 25, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Oren, A.; Ganz, T.; Liu, L.; Meerloo, T. In human epidermis, β-defensin 2 is packaged in lamellar bodies. Exp. Mol. Pathol. 2003, 74, 180–182. [Google Scholar] [CrossRef]
- Brown, M.S.; Goldstein, J.L. Scientific side trips: Six excursions from the beaten path. J. Biol. Chem. 2012, 287, 22418–22435. [Google Scholar] [CrossRef] [PubMed]
- Ribelin, W.E.; Kintner, L.D. Lipodystrophy of the central nervous system in a dog; a disease with similarities to Tay-Sachs disease of man. Cornell Vet. 1956, 46, 532–537. [Google Scholar] [PubMed]
- Rohrbach, M.; Clarke, J.T. Treatment of lysosomal storage disorders: Progress with enzyme replacement therapy. Drugs 2007, 67, 2697–2716. [Google Scholar] [CrossRef] [PubMed]
- Cochran, G.; Hardy, J.; Harpending, H. Natural history of Ashkenazi intelligence. J. Biosoc. Sci. 2006, 38, 659–693. [Google Scholar] [CrossRef] [PubMed]
- Rubin, L.P.; Kifor, O.; Hua, J.; Brown, E.M.; Torday, J.S. Parathyroid hormone (PTH) and PTH-related protein stimulate surfactant phospholipid synthesis in rat fetal lung, apparently by a mesenchymal-epithelial mechanism. Biochim. Biophys. Acta 1994, 1223, 91–100. [Google Scholar] [CrossRef]
- Bosch, R.J.; Rojo-Linares, P.; Torrecillas-Casamayor, G.; Iglesias-Cruz, M.C.; Rodríguez-Puyol, D.; Rodríguez-Puyol, M. Effects of parathyroid hormone-related protein on human mesangial cells in culture. Am. J. Physiol. 1999, 277, E990–E995. [Google Scholar] [PubMed]
- Philbrick, W.M.; Wysolmerski, J.J.; Galbraith, S.; Holt, E.; Orloff, J.J.; Yang, K.H.; Vasavada, R.C.; Weir, E.C.; Broadus, A.E.; Stewart, A.F. Defining the roles of parathyroid hormone-related protein in normal physiology. Physiol. Rev. 1996, 76, 127–173. [Google Scholar] [PubMed]
- Kovacs, C.S. Bone development in the fetus and neonate: Role of the calciotropic hormones. Curr. Osteoporos. Rep. 2011, 9, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Raj, J.U. Parathyroid hormone-related protein-mediated responses in pulmonary arteries and veins of newborn lambs. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 289, L60–L66. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.L.; Demiralp, B.; Schneider, A.; Koh, A.J.; Silve, C.; Wang, C.Y.; McCauley, L.K. Parathyroid hormone and parathyroid hormone-related protein exert both pro- and anti-apoptotic effects in mesenchymal cells. J. Biol. Chem. 2002, 277, 19374–19381. [Google Scholar] [CrossRef] [PubMed]
- Isowa, S.; Shimo, T.; Ibaragi, S.; Kurio, N.; Okui, T.; Matsubara, K.; Hassan, N.M.; Kishimoto, K.; Sasaki, A. PTHrP regulates angiogenesis and bone resorption via VEGF expression. Anticancer Res. 2010, 30, 2755–2767. [Google Scholar] [PubMed]
- Smith, H. From Fish to Philosopher; Little, Brown & Co.: Boston, MA, USA, 1953. [Google Scholar]
- Bingle, C.D. Thyroid transcription factor-1. Int. J. Biochem. Cell Biol. 1997, 29, 1471–1473. [Google Scholar] [CrossRef]
- Jacobs, D.K.; Hughes, N.C.; Fitz-Gibbon, S.T.; Winchell, C.J. Terminal addition, the Cambrian radiation and the Phanerozoic evolution of bilaterian form. Evol. Dev. 2005, 7, 498–514. [Google Scholar] [CrossRef] [PubMed]
- Arendt, D.; Technau, U.; Wittbrodt, J. Evolution of the bilaterian larval foregut. Nature 2001, 409, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Jankovic, J. NKX2-1-Related Disorders. In GeneReviews; Pagon, R., Adam, M., Ardinger, H., Wallace, S., Amemiya, A., Bean, L., Bird, T., Dolan, C., Fong, C., Smith, R., et al., Eds.; University of Washington: Seattle, WA, USA; pp. 1993–2015.
- Cañestro, C.; Bassham, S.; Postlethwait, J.H. Evolution of the thyroid: Anterior-posterior regionalization of the Oikopleura endostyle revealed by Otx, Pax2/5/8, and Hox1 expression. Dev. Dyn. 2008, 237, 1490–1499. [Google Scholar] [CrossRef] [PubMed]
- Troedsson, C.; Ganot, P.; Bouquet, J.M.; Aksnes, D.L.; Thompson, E.M. Endostyle cell recruitment as a frame of reference for development and growth in the Urochordate Oikopleura dioica. Biol. Bull. 2007, 213, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Satoh, N.; Tagawa, K.; Lowe, C.J.; Yu, J.K.; Kawashima, T.; Takahashi, H.; Ogasawara, M.; Kirschner, M.; Hisata, K.; Su, Y.H.; et al. On a possible evolutionary link of the stomochord of hemichordates to pharyngeal organs of chordates. Genesis 2014, 52, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Kluge, B.; Renault, N.; Rohr, K.B. Anatomical and molecular reinvestigation of lamprey endostyle development provides new insight into thyroid gland evolution. Dev. Genes Evol. 2005, 215, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Ota, K.G.; Kuratani, S. Cyclostome embryology and early evolutionary history of vertebrates. Integr. Comp. Biol. 2007, 47, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Wang, X. PKC and PKA, but not PKG mediate LPS-induced CGRP release and [Ca2+](i) elevation in DRG neurons of neonatal rats. J. Neurosci. Res. 2001, 66, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.J.; Woo, N.R.; Shin, I.C.; Kim, S.G. H89, an inhibitor of PKA and MSK, inhibits cyclic-AMP response element binding protein-mediated MAPK phosphatase-1 induction by lipopolysaccharide. Inflamm. Res. 2009, 58, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Antunes, T.T.; Gagnon, A.; Bell, A.; Sorisky, A. Thyroid-stimulating hormone stimulates interleukin-6 release from 3T3-L1 adipocytes through a cAMP-protein kinase A pathway. Obes. Res. 2005, 13, 2066–2071. [Google Scholar] [CrossRef] [PubMed]
- Maquet, E.; Costagliola, S.; Parma, J.; Christophe-Hobertus, C.; Oligny, L.L.; Fournet, J.C.; Robitaille, Y.; Vuissoz, J.M.; Payot, A.; Laberge, S.; et al. Lethal respiratory failure and mild primary hypothyroidism in a term girl with a de novo heterozygous mutation in the TITF1/NKX2.1 gene. J. Clin. Endocrinol. Metab. 2009, 94, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Carré, A.; Szinnai, G.; Castanet, M.; Sura-Trueba, S.; Tron, E.; Broutin-L’Hermite, I.; Barat, P.; Goizet, C.; Lacombe, D.; Moutard, M.L.; et al. Five new TTF1/NKX2.1 mutations in brain-lung-thyroid syndrome: Rescue by PAX8 synergism in one case. Hum. Mol. Genet. 2009, 18, 2266–2276. [Google Scholar] [CrossRef] [PubMed]
- De Felice, M.; di Lauro, R. Murine models for the study of thyroid gland development. Endocr. Dev. 2007, 10, 1–14. [Google Scholar] [PubMed]
- Melmed, S.; Polonsky, K.; Larsen, P.; Kronenberg, H. Willliams Textbook of Endocrinology; Elsevier: Philadelphia, PA, USA, 2011. [Google Scholar]
- Csete, M.; Walikonis, J.; Slawny, N.; Wei, Y.; Korsnes, S.; Doyle, J.C.; Wold, B. Oxygen-mediated regulation of skeletal muscle satellite cell proliferation and adipogenesis in culture. J. Cell. Physiol. 2001, 89, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Torday, J.S.; Sun, H.; Wang, L.; Torres, E.; Sunday, M.E.; Rubin, L.P. Leptin mediates the parathyroid hormone-related protein paracrine stimulation of fetal lung maturation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 282, L405–L410. [Google Scholar] [PubMed]
- Bergen, H.T.; Cherlet, T.C.; Manuel, P.; Scott, J.E. Identification of leptin receptors in lung and isolated fetal type II cells. Am. J. Respir. Cell Mol. Biol. 2002, 27, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Warburton, D.; Parton, L.; Buckley, S.; Cosico, L.; Saluna, T. Effects of beta-2 agonist on tracheal fluid flow, surfactant and pulmonary mechanics in the fetal lamb. J. Pharmacol. Exp. Ther. 1987, 242, 394–398. [Google Scholar] [PubMed]
- Solc, D. The heart and heart conducting system in the kingdom of animals: A comparative approach to its evolution. Exp. Clin. Cardiol. 2007, 12, 113–118. [Google Scholar] [PubMed]
- Jensen, B.; Wang, T.; Christoffels, V.M.; Moorman, A.F. Evolution and development of the building plan of the vertebrate heart. Biochim. Biophys. Acta 2013, 1833, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Zheng, M.; Goldfarb, M.; Zaret, K.S. Initiation of mammalian liver development from endoderm by fibroblast growth factors. Science 1999, 284, 1998–2003. [Google Scholar] [CrossRef] [PubMed]
- Brekke, E.; Morken, T.S.; Sonnewald, U. Glucose metabolism and astrocyte-neuron interactions in the neonatal brain. Neurochem. Int. 2015, 82, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Shestov, A.A.; Emir, U.E.; Kumar, A.; Henry, P.G.; Seaquist, E.R.; Öz, G. Simultaneous measurement of glucose transport and utilization in the human brain. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E1040–E1049. [Google Scholar] [CrossRef] [PubMed]
- Venturi, S.; Donati, F.M.; Venturi, A.; Venturi, M.; Grossi, L.; Guidi, A. Role of iodine in evolution and carcinogenesis of thyroid, breast and stomach. Adv. Clin. Pathol. 2000, 4, 11–17. [Google Scholar]
- Falkowski, P.G.; Katz, M.E.; Milligan, A.J.; Fennel, K.; Cramer, B.S.; Aubry, M.P.; Berner, R.A.; Novacek, M.J.; Zapol, W.M. The rise of oxygen over the past 205 million years and the evolution of large placental mammals. Science 2005, 309, 2202–2204. [Google Scholar] [CrossRef] [PubMed]
- Hume, R.; Richard, K.; Kaptein, E.; Stanley, E.L.; Visser, T.J.; Coughtrie, M.W. Thyroid hormone metabolism and the developing human lung. Biol. Neonate 2001, 80, 8–21. [Google Scholar] [CrossRef]
- Klieverik, L.P.; Coomans, C.P.; Endert, E.; Sauerwein, H.P.; Havekes, L.M.; Voshol, P.J.; Rensen, P.C.; Romijn, J.A.; Kalsbeek, A.; Fliers, E. Thyroid hormone effects on whole-body energy homeostasis and tissue-specific fatty acid uptake in vivo. Endocrinology 2009, 150, 5639–5648. [Google Scholar] [CrossRef] [PubMed]
- Mogulkoc, R.; Baltaci, A.K.; Oztekin, E.; Sivrikaya, A.; Aydin, L. Effects of hyperthyroidism induced by L-thyroxin administration on lipid peroxidation in various rat tissues. Acta Biol. Hung. 2006, 57, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Sáenz, J.P.; Sezgin, E.; Schwille, P.; Simons, K. Functional convergence of hopanoids and sterols in membrane ordering. Proc. Natl. Acad. Sci. USA 2012, 109, 14236–14240. [Google Scholar] [CrossRef] [PubMed]
- Darwin, C.R. On the Origin of Species by Means of Natural Selection, or the Preservation of Favoured Races in the Struggle for life, 1st ed.; John Murray: London, UK, 1859. [Google Scholar]
- Eldredge, N.; Gould, S. Punctuated equilibria: An alternative to phyletic gradualism. In Models in Paleobiology; Schopf, T., Ed.; Freeman, Cooper and Company: San Francisco, CA, USA, 1972; pp. 82–115. [Google Scholar]
- Graham, J.B.; Dudley, R.; Aguilar, N.; Gans, C. Implications of the late Palaeozoic oxygen pulse for physiology and evolution. Nature 1995, 375, 117–120. [Google Scholar] [CrossRef]
- Michiels, C. Physiological and pathological responses to hypoxia. Am. J. Pathol. 2004, 164, 1875–1882. [Google Scholar] [CrossRef]
- Pinheiro, P.L.; Cardoso, J.C.; Power, D.M.; Canário, A.V. Functional characterization and evolution of PTH/PTHrP receptors: Insights from the chicken. BMC Evol. Biol. 2012, 12, 110–125. [Google Scholar] [CrossRef] [PubMed]
- Aris-Brosou, S.; Chen, X.; Perry, S.F.; Moon, T.W. Timing of the functional diversification of α- and β-adrenoceptors in fish and other vertebrates. Ann. NY Acad. Sci. 2009, 1163, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Bridgham, J.T.; Carroll, S.M.; Thornton, J.W. Evolution of hormone-receptor complexity by molecular exploitation. Science 2006, 312, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Pallottini, V.; Guantario, B.; Martini, C.; Totta, P.; Filippi, I.; Carraro, F.; Trentalance, A. Regulation of HMG-CoA reductase expression by hypoxia. J. Cell. Biochem. 2008, 104, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Bloch, K. The biological synthesis of cholesterol. Vitam. Horm. 1957, 15, 119–150. [Google Scholar] [CrossRef] [PubMed]
- Torday, J.S.; Rehan, V.K. The evolutionary continuum from lung development to homeostasis and repair. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 292, L608–L611. [Google Scholar] [CrossRef] [PubMed]
- Torday, J.S.; Rehan, V.K. Lung evolution as a cipher for physiology. Physiol. Genomics 2009, 38, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Torday, J.S.; Rehan, V.K. Mechanotransduction determines the structure and function of lung and bone: A theoretical model for the pathophysiology of chronic disease. Cell Biochem. Biophys. 2003, 37, 235–246. [Google Scholar] [CrossRef]
- Torday, J.S. Parathyroid hormone-related protein is a gravisensor in lung and bone cell biology. Adv. Space Res. 2003, 32, 1569–1576. [Google Scholar] [CrossRef]
- Taylor, L.; Polgar, P.; McAteer, J.A.; Douglas, W.H. Prostaglandin production by type II alveolar epithelial cells. Biochim. Biophys. Acta 1979, 572, 502–509. [Google Scholar] [CrossRef]
- Torday, J.S.; Sun, H.; Qin, J. Prostaglandin E2 integrates the effects of fluid distension and glucocorticoid on lung maturation. Am. J. Physiol. 1998, 274, L106–L111. [Google Scholar] [PubMed]
- Hastings, R.H.; Asirvatham, A.; Quintana, R.; Sandoval, R.; Dutta, R.; Burton, D.W.; Deftos, L.J. Parathyroid hormone-related protein-(38–64) regulates lung cell proliferation after silica injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 283, L12–L21. [Google Scholar] [CrossRef] [PubMed]
- Mamillapalli, R.; Wysolmerski, J. The calcium-sensing receptor couples to Gαs and regulates PTHrP and ACTH secretion in pituitary cells. J. Endocrinol. 2010, 204, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Rubin, L.P.; Kovacs, C.S.; de Paepe, M.E.; Tsai, S.W.; Torday, J.S.; Kronenberg, H.M. Arrested pulmonary alveolar cytodifferentiation and defective surfactant synthesis in mice missing the gene for parathyroid hormone-related protein. Dev. Dyn. 2004, 230, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, P.; Swenson, E.R.; Bussotti, M.; Revera, M.; Meriggi, P.; Faini, A.; Lombardi, C.; Bilo, G.; Giuliano, A.; Bonacina, D.; et al. High-altitude exposure of three weeks duration increases lung diffusing capacity in humans. J. Appl. Physiol. 2011, 110, 1564–1571. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.S.; Jessen, N.; Jørgensen, J.O.; Møller, N.; Lund, S. Dissecting adipose tissue lipolysis: Molecular regulation and implications for metabolic disease. J. Mol. Endocrinol. 2014, 52, R199–R222. [Google Scholar] [CrossRef] [PubMed]
- Foegh, M.L.; Thomas, G.; Ramwell, P.W. Free radicals, arachidonic acid metabolites, and nutrition. J. Parenteral Enter. Nutr. 1990, 14, 218S–222S. [Google Scholar] [CrossRef]
- Suri, L.N.; McCaig, L.; Picardi, M.V.; Ospina, O.L.; Veldhuizen, R.A.; Staples, J.F.; Possmayer, F.; Yao, L.J.; Perez-Gil, J.; Orgeig, S. Adaptation to low body temperature influences pulmonary surfactant composition thereby increasing fluidity while maintaining appropriately ordered membrane structure and surface activity. Biochim. Biophys. Acta 2012, 1818, 1581–1589. [Google Scholar] [CrossRef] [PubMed]
- Hellmark, T.; Segelmark, M. Diagnosis and classification of Goodpasture’s disease (anti-GBM). J. Autoimmun. 2014, 48–49, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Roux, E. The concept of function in modern physiology. J. Physiol. 2014, 592, 2245–2249. [Google Scholar] [CrossRef] [PubMed]
- Mayr, E. Cause and effect in biology. Science 1961, 134, 1501–1506. [Google Scholar] [CrossRef] [PubMed]
- Keyte, A.L.; Smith, K.K. Heterochrony and developmental timing mechanisms: Changing ontogenies in evolution. Semin. Cell Dev. Biol. 2014, 34, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Horder, T. Heterochrony: Encyclopedia of Life Sciences; John Wiley & Sons: Chichester, UK, 2006. [Google Scholar]
- Kuhn, T. The Structure of Scientific Revolutions; The University of Chicago Press: Chicago, IL, USA, 1962. [Google Scholar]
- Shapiro, J. Evolution: A View from the 21st Century; FT Press Science: Upper Saddle River, NJ, USA, 2011. [Google Scholar]
- Bohr, N. The Quantum Postulate and the Recent Development of Atomic Theory. Nature 1928, 121, 580–590. [Google Scholar] [CrossRef]
- Bohm, D. Wholeness and the Implicate Order; Routlege & Kegan Paul: London, UK, 1980. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torday, J.S. Pleiotropy as the Mechanism for Evolving Novelty: Same Signal, Different Result. Biology 2015, 4, 443-459. https://doi.org/10.3390/biology4020443
Torday JS. Pleiotropy as the Mechanism for Evolving Novelty: Same Signal, Different Result. Biology. 2015; 4(2):443-459. https://doi.org/10.3390/biology4020443
Chicago/Turabian StyleTorday, John S. 2015. "Pleiotropy as the Mechanism for Evolving Novelty: Same Signal, Different Result" Biology 4, no. 2: 443-459. https://doi.org/10.3390/biology4020443




