The Durability and Performance of Short Fibers for a Newly Developed Alkali-Activated Binder
Abstract
:1. Introduction
2. Experimental Section
2.1. Components for Alkali Activated Binders
2.2. Fibers for the Reinforcement of AAB-Matrix
2.3. Specimen Preparation
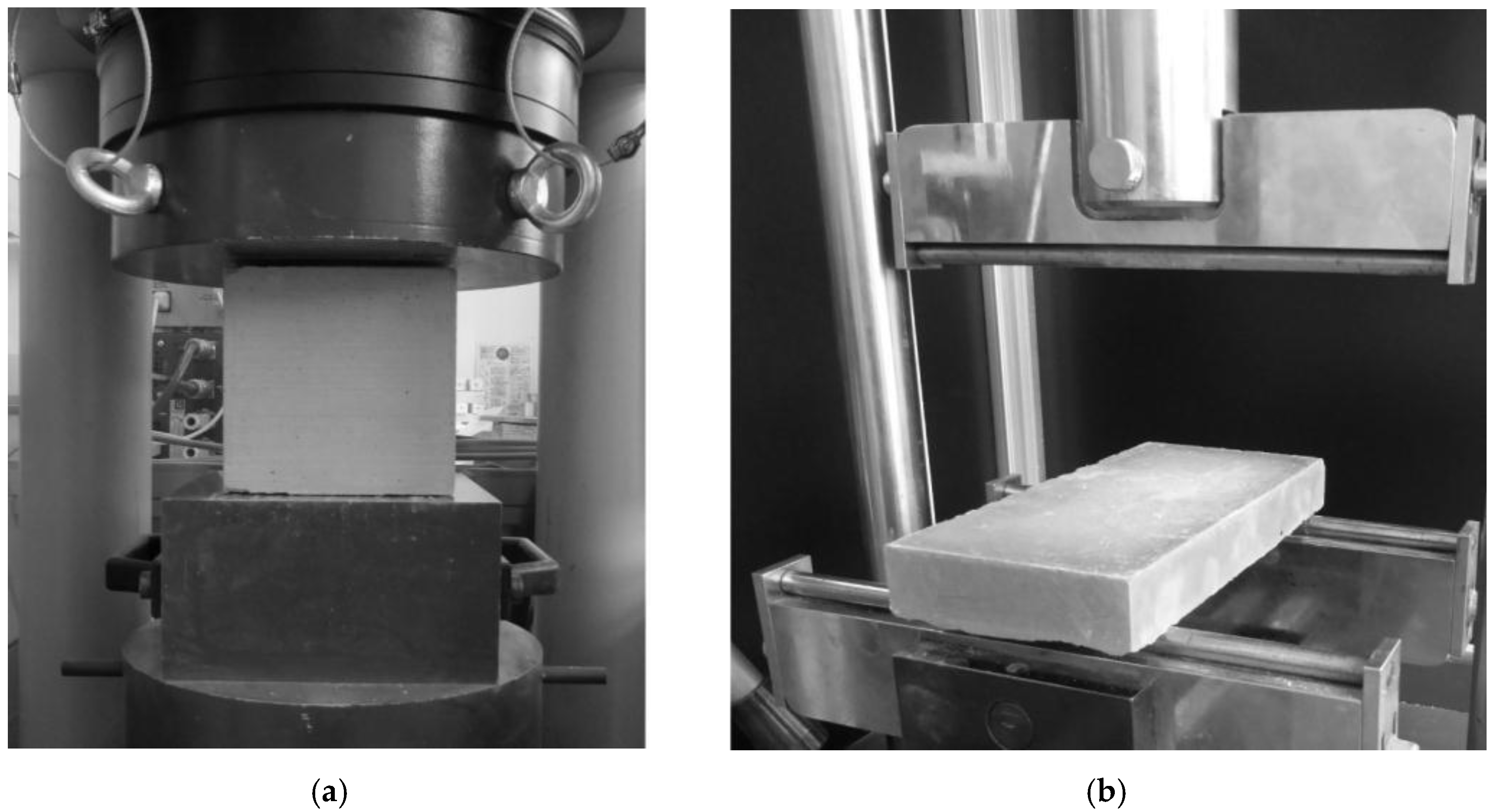
2.4. Test Set-up
3. Results and Discussion
3.1. Basic Properties of Fresh and Hardened AAB
3.2. Thermogravimetry
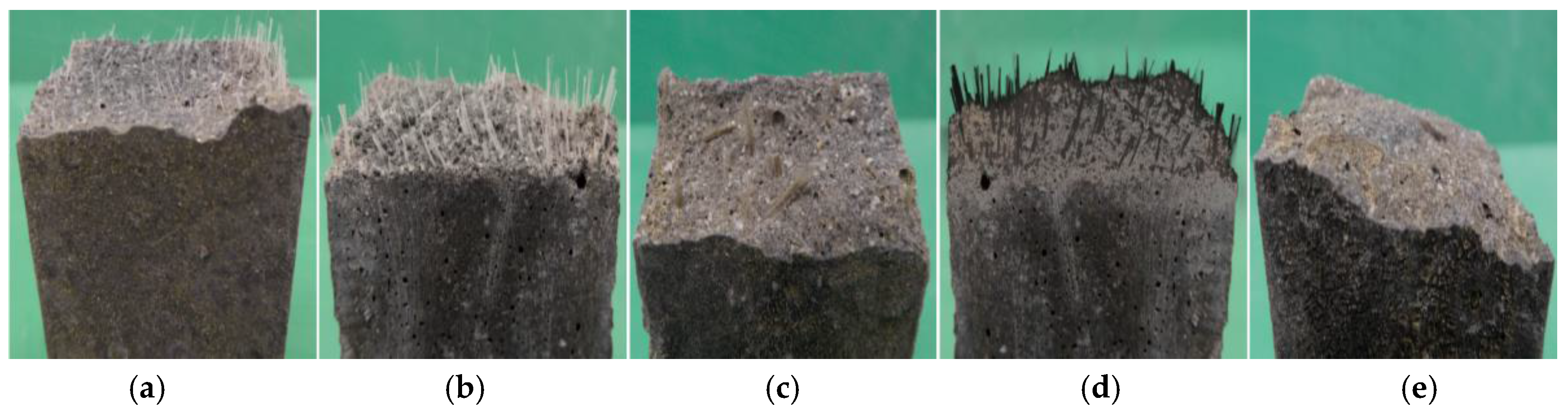
3.3. Durability and Performance of Fibers in the AAB-Matrix
4. Conclusions
- -
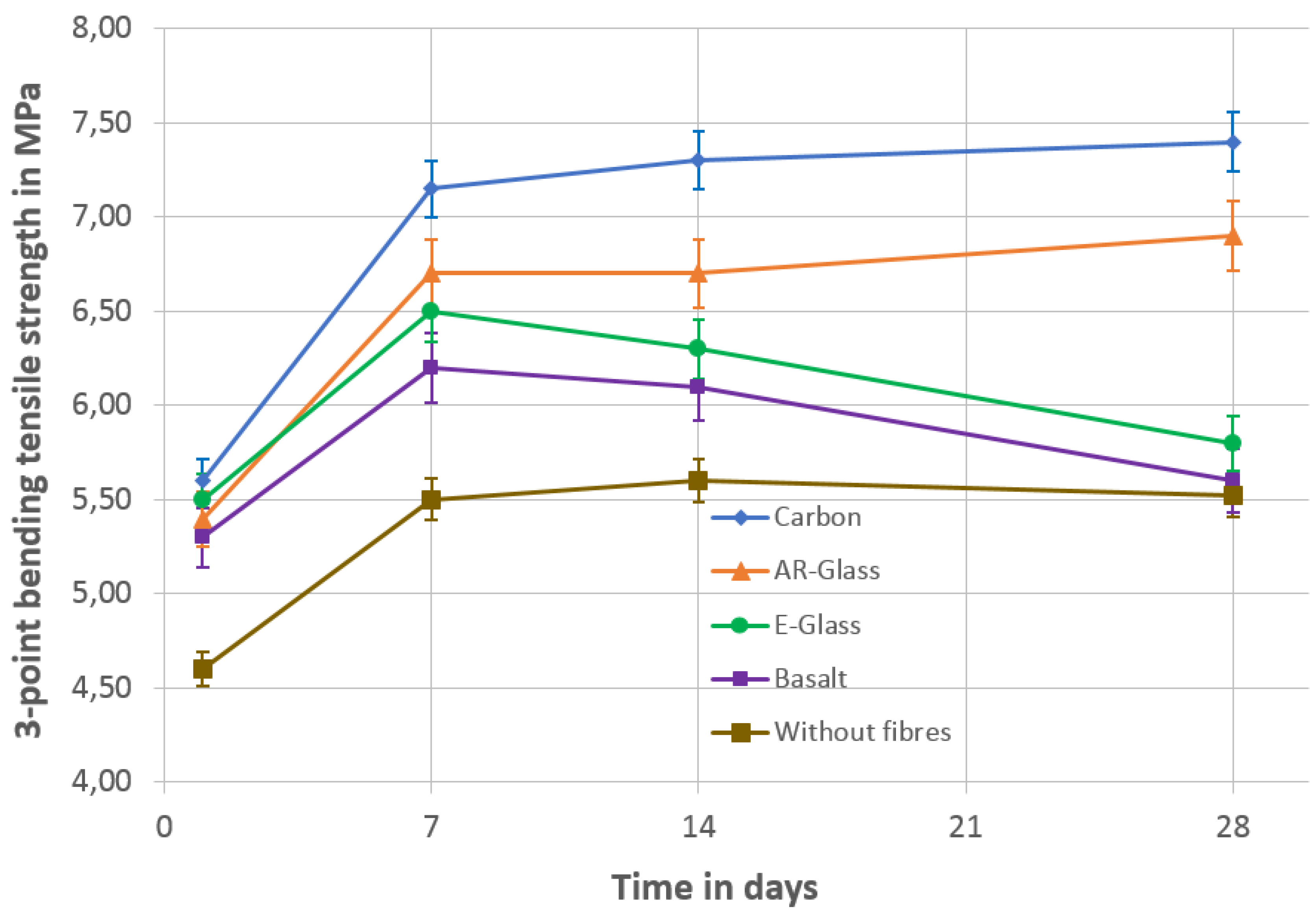
- The adapted molarity for the saturation of the three-point bending tensile strength of the sodium hydroxide solution was 5.5 mol/L. Sodium hydroxide concentrations over 5.5 mol/L did not result in an increase of the three-point bending tensile strength after seven days.
- -
- By the integration of short fibers, the three-point bending tensile strength of the AAB increased from 4.6 MPa (no fibers) up to 5.7 MPa (carbon) after one day.
- -
- After 28 days, the three-point bending tensile strength of the AAB increased to 5.6 MPa (no fibers) and 7.3 MPa (carbon), which means an increase of 30%.
- -
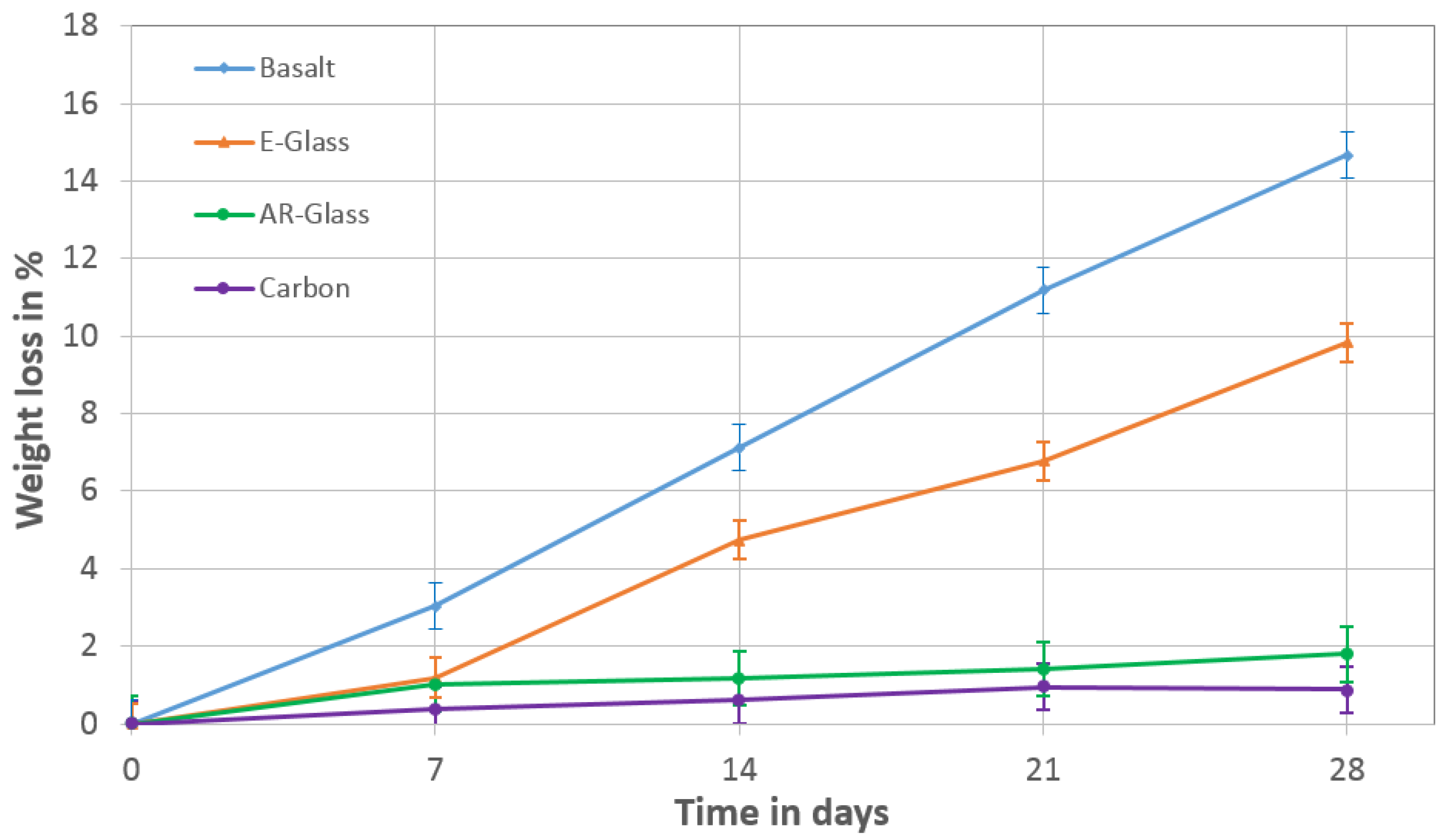
- The E-glass and the basalt fibers had similar deterioration under the alkali immersion and lost weight and strength more significantly than the AR-glass and the carbon fibers. According to this result, the E-Glass and the basalt fibers have no sufficient durability in the alkaline AAB-matrix.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Purnell, P. Material nature versus structural nurture: The embodied carbon of fundamental structural elements. Environ. Sci. Technol. 2011, 46, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Cement Sustainability Initiative. Cement Technology Roadmap 2009. Carbon Emissions Reductions up to 2050. IEA: Paris, France, 2009. [Google Scholar]
- McLellan, B.; Williams, R.; Lay, J.; van Riessen, A.; Corder, G. Costs and carbon emissions for geopolymer pastes in comparison to ordinary Portland cement. J. Clean. Prod. 2011, 19, 1080–1090. [Google Scholar] [CrossRef] [Green Version]
- Davidovits, J. Geopolymer cements to minimize carbon dioxide greenhouse warming. Ceram. Trans. 1993, 37, 165–826. [Google Scholar]
- Komnitas, K.; Zaharaki, D. Geopolymerization: A review and prospects for the minerals industry. Miner. Eng. 2007, 20, 1261–1277. [Google Scholar] [CrossRef]
- Lima, C. Physical properties and mechanical behavior of concrete made with recycled aggregates and fly ash. Cem. Concr. Res. 2011, 41, 750–763. [Google Scholar]
- Shi, C.; Jimenez, A.; Palomo, A. New cements for the 21st century: The pursuit of an alternative to Portland cement. Constr. Build. Mater. 2013, 47, 547–559. [Google Scholar] [CrossRef]
- Bernal, S.A. MgO content of slag controls phase evolution and structural changes induced by accelerated carbonation in alkali-activated binders. Cem. Concr. Res. 2014, 57, 33–43. [Google Scholar] [CrossRef]
- Funke, H.; Gelbrich, S. Alkalisch aktivierte Bindemittel zur Generierung von faserverstärkten Vergussmassen. In 19. Internationale Baustofftagung „Ibausil", Weimar, Germany, 16–18 September 2015; pp. 859–866. (In German)
- Damtoft, J. Sustainable development and climate change initiatives. Cem. Concr. Res. 2008, 38, 115–127. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers: Inorganic polymeric new materials. J. Therm. Anal. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Cheng, T.W. Development and application of geopolymer technology: A review. Min. Metall. 2010, 54, 141–157. [Google Scholar]
- Schneider, M. Sustainable cement production—Present and future. Ceme. Concr. Res. 2011, 41, 642–650. [Google Scholar] [CrossRef]
- Duxson, P. The role of inorganic polymer technology in the development of green concrete. Ceme. Concr. Res. 2007, 37, 1590–1597. [Google Scholar] [CrossRef]
- Duxson, P.; Lukey, G.; van Deventer, J. The thermal evolution of metakaolin geopolymers: Part 2—Phase stability and structural development. J. Non Cryst. Solids 2007, 353, 2186–2200. [Google Scholar] [CrossRef]
- Li, W.; Xu, J. Mechanical properties of basalt fiber-reinforced geopolymeric concrete under impact loading. Mater. Sci. Eng. 2009, 505, 178–186. [Google Scholar] [CrossRef]
- Funke, H.; Gelbrich, S.; Ehrlich, A.; Kroll, L. Rheological and mechanical development of a fiber-reinforced concrete for an application in civil engineering. SOJ Mater. Sci. Eng. 2014, 2, 1–4. [Google Scholar] [CrossRef]
- Ohno, M.; Li, V. A feasibility study of strain hardening fiber-reinforced fly ash based geopolymer composites. Constr. Build. Mater. 2014, 57, 163–168. [Google Scholar]
- Puertas, F.; Amat, T.; Fernández-Jiménez, A.; Vázquez, T. Mechanical and durable behavior of alkaline cement mortars reinforced with polypropylene fibers. Ceme. Concr. Res. 2003, 33, 2031–2036. [Google Scholar] [CrossRef]
- Buchwald, A. Der Einfluss des Kalziums auf die Kondensation von (Alumo-)Silikaten in alkali-aktivierten Binder. Habilitation Thesis, Fakultät Bauingenieurwesen, Bauhaus-Universität Weimar, Weimar, Germany, 2012. [Google Scholar]
- Provis, J.; Lukey, G.; Deventer, J. Do Geopolymers Actually Contain Nanocrystalline Zeolites? A Reexamination of Existing Results. Chem. Mater. 2005, 17, 3075–3085. [Google Scholar] [CrossRef]
- Bernal, S. Mechanical and thermal characterization of geopolymers based on silicate activated metakaolin/slag blends. J. Mater. Sci. 2011, 46, 5477–5486. [Google Scholar] [CrossRef]


| Component | AAB | OPCC |
|---|---|---|
| Granulated slag | 500 | - |
| Coal fly ash | 100 | - |
| CEM I 52.5 R | - | 500 |
| Quartz sand 0–2 mm | 1070 | 1780 |
| NaOH-solution | 340 | - |
| Sodium silicate | 100 | - |
| plugging agent | 4 | - |
| Black pigment | 10 | - |
| superplasticizer | - | 15 |
| w/b | 0.41 | |
| Oxide | Coal Fly Ash | Granulated Slag |
|---|---|---|
| SiO2 | 45 | 52 |
| Al2O3 | 10 | 30 |
| Fe2O3 | - | 10 |
| CaO | 35 | 5 |
| MgO | 10 | 3 |
| Property | AR-glass | E-glass | Basalt | Carbon |
|---|---|---|---|---|
| Fineness in g/1000 m | 45 | 52 | 60 | 50 |
| Length in mm | 12 | |||
| Content of sizing agent in wt% | 1.1 | 0.9 | 1.5 | 1.2 |
| Property | Fresh AAB | Hardened AAB |
|---|---|---|
| pH value | 14 | 10 |
| Geometric bulk density | 2.39 g/cm3 | 2.35 g/cm3 |
| Total shrinkage | 0.45 mm/m | |
| Compressive strength | - | 53.4 MPa |
| three-point bending tensile strength | - | 5.5 MPa |
| Elastic modulus (dynamic) | - | 36 GPa |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Funke, H.; Gelbrich, S.; Kroll, L. The Durability and Performance of Short Fibers for a Newly Developed Alkali-Activated Binder. Fibers 2016, 4, 11. https://doi.org/10.3390/fib4010011
Funke H, Gelbrich S, Kroll L. The Durability and Performance of Short Fibers for a Newly Developed Alkali-Activated Binder. Fibers. 2016; 4(1):11. https://doi.org/10.3390/fib4010011
Chicago/Turabian StyleFunke, Henrik, Sandra Gelbrich, and Lothar Kroll. 2016. "The Durability and Performance of Short Fibers for a Newly Developed Alkali-Activated Binder" Fibers 4, no. 1: 11. https://doi.org/10.3390/fib4010011






