1. Introduction
In dentistry, the specialty dealing with root canals is endodontics. Root canal preparation is the most essential part in root canal treatment (RCT). The preparation aims to clean all inflamed tissues, disinfect the root canal system, and provide a regular-shaped canal that can be sealed from bacteria by using the appropriate filling materials. In fact, cleaning and shaping are not totally separate procedures. To provide sufficient space for obturating materials, the cutting of infected dentine and residual pulp tissue are usually occurring at the same time. Various kinds of hand and rotary endodontic instruments are used routinely in the preparation [
1].
Results have shown that mechanical instrumentation is capable of greatly reducing the amount of microorganisms remaining in a root canal system [
2]. Furthermore, mechanical cleaning has also been shown as a method that could reduce bacteria without any endodontic irrigant [
3,
4]. However, despite a good cutting efficiency, the drawback of metal instruments, such as those made of stainless steel, is obvious and not rare in clinic. Iatrogenesis is a negligent adverse effect or complication, which is commonly resulted from medical treatment or device. In endodontics, as mechanical cleaning could be achieved by instrumentation, several kinds of iatrogenic damages might be caused in a clinical procedure. Some studies have described several kinds of potential iatrogenic damages that could occur during root canal preparation with conventional stainless steel instruments. We suggest, for example, zip, elbow, ledging, perforation, outer widening, damage of the apical foramen, and blockage of the root canal system [
5,
6,
7,
8].
In general, fiber-based materials find many applications in contemporary dentistry [
9]. In particular, nylon, which is a synthetic aliphatic polyamide, is a relatively soft material and it has been used in endodontics as a canal brush [
10]. One study focused on evaluating the removal of a smear layer by using a new kind of CanalBrush, and it was reported that the CanalBrush was more effective in the narrow parts of the root canal, e.g., apical and middle area, where it could have a better contact with the root canal wall [
10].
The objective of this laboratory study was to develop and study a novel way of root canal cleaning, proposed by the authors, which could overcome some safety issues present in traditional endodontic treatment, as mentioned above. We hypothesize that the cleaning efficiency of nylon fibers, using various hand-pieces, would be the same as NiTi files, and would not damage root canals.
2. Experimental Section
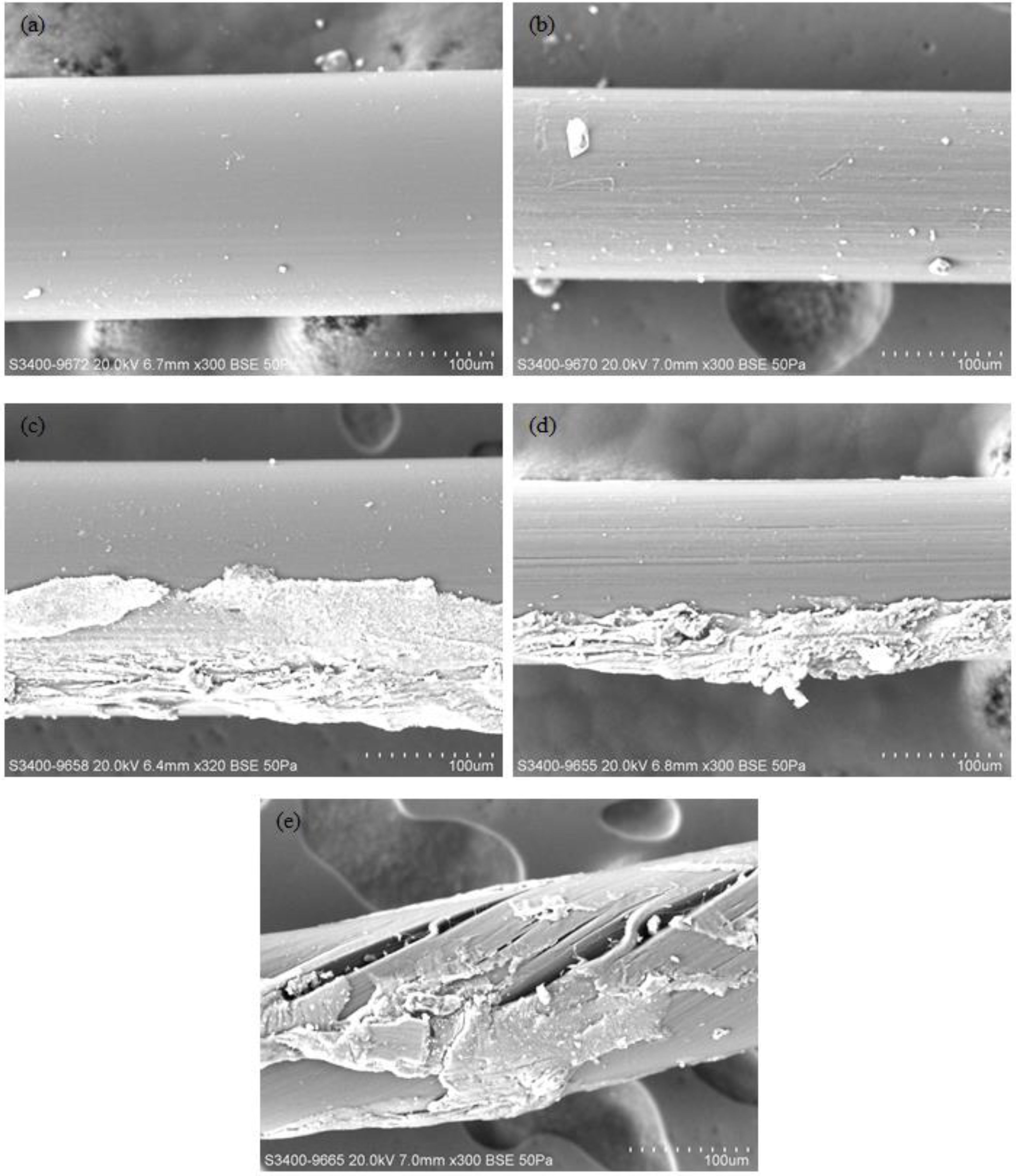
Two nylon fibers, “fiber W” and “fiber B”, were purchased and used in this study. Fiber W and fiber B had had average, pretested diameters of 206.90 ± 1.94 μm and 156.40 ± 1.08 μm, respectively. Nine fiber samples from each of the fiber types were carefully examined by using a scanning electron microscope (SEM) (Hitachi S-3400N VP-SEM, Hitachi High Technologies Europe GmbH, Krefeld, Germany) before their use in the experiments. The images were kept on record for further contrast and comparison. These pretested fibers were preserved and used to make fiber files. All the fibers used in this experiment were further investigated using the same SEM, to visually check if any damage or deformation occurred in the fibers after the cleansing.
Another six of each fiber W and fiber B specimens were randomly selected and tested under tension mode. One end of the fiber was fixed and the other end clamped in a U-style jig, and tested using a universal testing machine (ElectroPuls E3000, Instron, UK). A load was applied to the U-style jig, with a constant cross-head speed of 1 mm/min. Then, the dimensional change of the fibers and the load applied were recorded and output was calculated using the Instron software.
Two identical transparent poly(methyl methacrylate) simulated root canal blocks were used. Each block contained a single straight root canal with a length of 20 mm. Prior to dye application, the root canals in the blocks were shaped by dental K files, from 10# to 30#, to confirm that the original shape of the root canal was relatively regular. Then, distilled water was used to wash, and rinse out the debris. After that, both simulated root canal blocks were placed into a centrifuge (Heraeus Primo/Primo R Centrifuge, Hanau, Germany) to discharge the residual water in order to keep the specimens dry. Next, each root canal was colored and filled with red nail polish (No. 470 ANNA SUI, New York, NY, USA) using a plastic syringe (Insulin syringe ½ cc, Terumo, Japan). Because the nail polish liquid was too viscous to flow easily, in order to ensure the completeness of the coloring, the same centrifuge was used to fully fill the whole root canal with the nail polish. The procedure was, in brief, as follows: first, the nail polish liquid was filled into a root canal in the acrylic block using a needle. Then, the simulated root canal was fixed in a 50 mL centrifuge tube (Tube 1). In order to keep the centrifuge balanced, Tube 1 was weighted using a laboratory balance, and another tube (Tube 2) was filled with deionized water to provide an equal mass to Tube 1. Next, these two tubes were then inserted into the centrifuge, which was then operated at a speed of 1000 rpm for 1 min. The aforementioned procedures were repeated three times to ensure that the nail polish could fully fill the root canal evenly and completely. Finally, the simulated root canal block was removed from the centrifuge and allowed to polymerize at room temperature for 24 h.
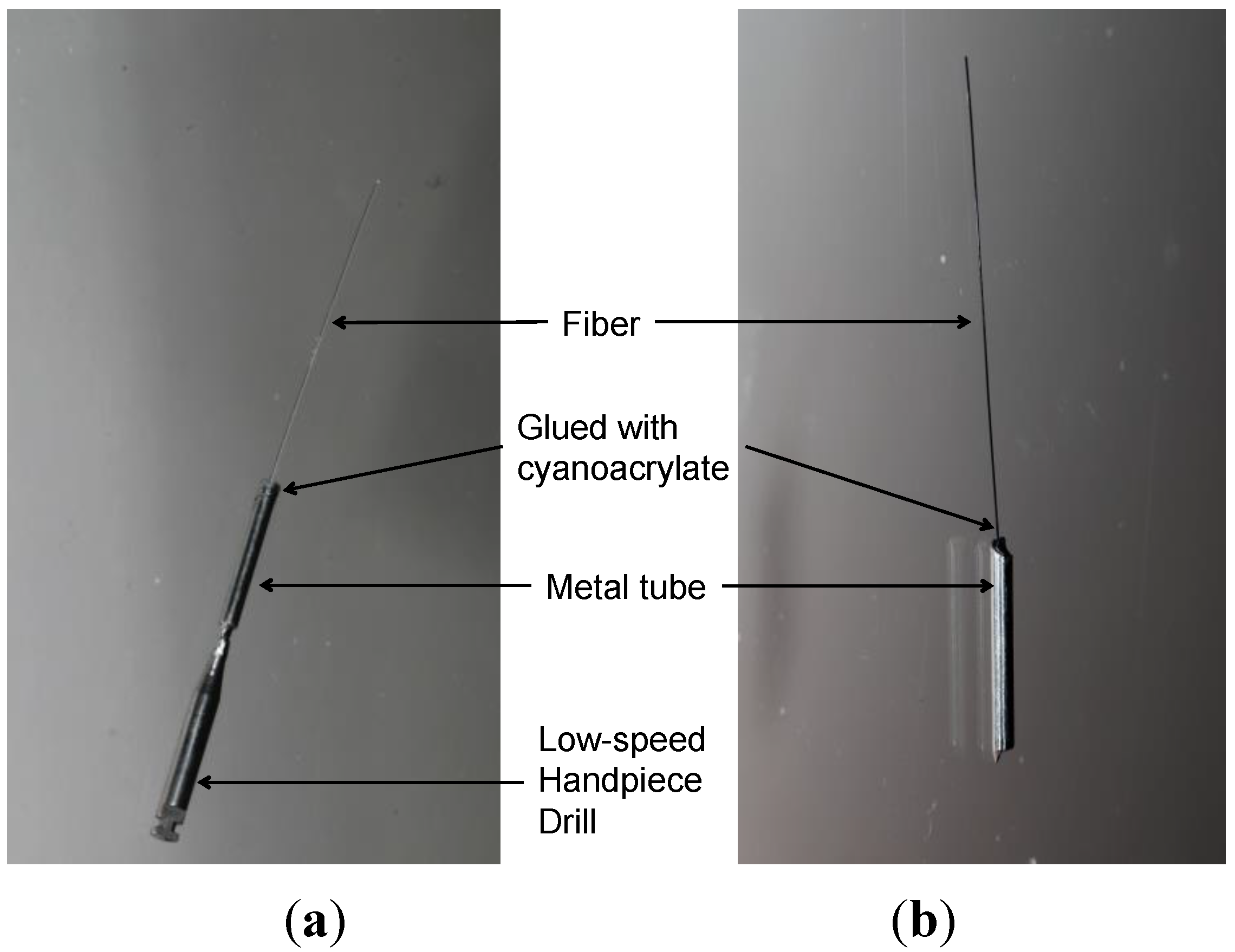
Two hand-pieces were used in this study, a low-speed hand-piece (NSK, Tokyo, Japan, denoted as LH) and a reciprocating EVA hand-piece (ProFin™, W&H, St Albans, UK, denoted as RH). A single fiber with an exposed length of 20.0 mm was fixed, using a cyanoacrylate glue (Aron Alpha, Toagosei, Japan), in a stainless steel metal tube, which had the same outer diameter (2.0 mm) as the hand-pieces (
Figure 1). Then, the files were inserted into the hand-piece directly and then activated for 5 min prior to use in a cleaning test to check if they were workable and stable enough.
Before cleaning with the fiber files, a #10 K file was used to achieve access to the acrylic block in order to allow the soft fiber to slide in. Then, the specimens were cleansed with different fiber files with the hand-pieces, including four study groups (i) Fiber W with an RH; (ii) Fiber B with an RH; (iii) Fiber W with an LH; and (iv) Fiber B with an LH. One simulated root canal specimen was prepared using a NiTi file (ProTaper, Dentsply, Weybridge, UK) with the low-speed hand-piece, and this served as the control. Each acrylic block specimen was drilled separately, using these four hand-pieces, and the protocol was 15 s, 10 times per simulated root canal.
Figure 1.
Custom-made fiber files used in this study for the low-speed hand-piece (a) and for the reciprocating EVA hand-piece (b). Note: An adaptor was used to connect the low-speed hand-piece to the metal tube.
Figure 1.
Custom-made fiber files used in this study for the low-speed hand-piece (a) and for the reciprocating EVA hand-piece (b). Note: An adaptor was used to connect the low-speed hand-piece to the metal tube.
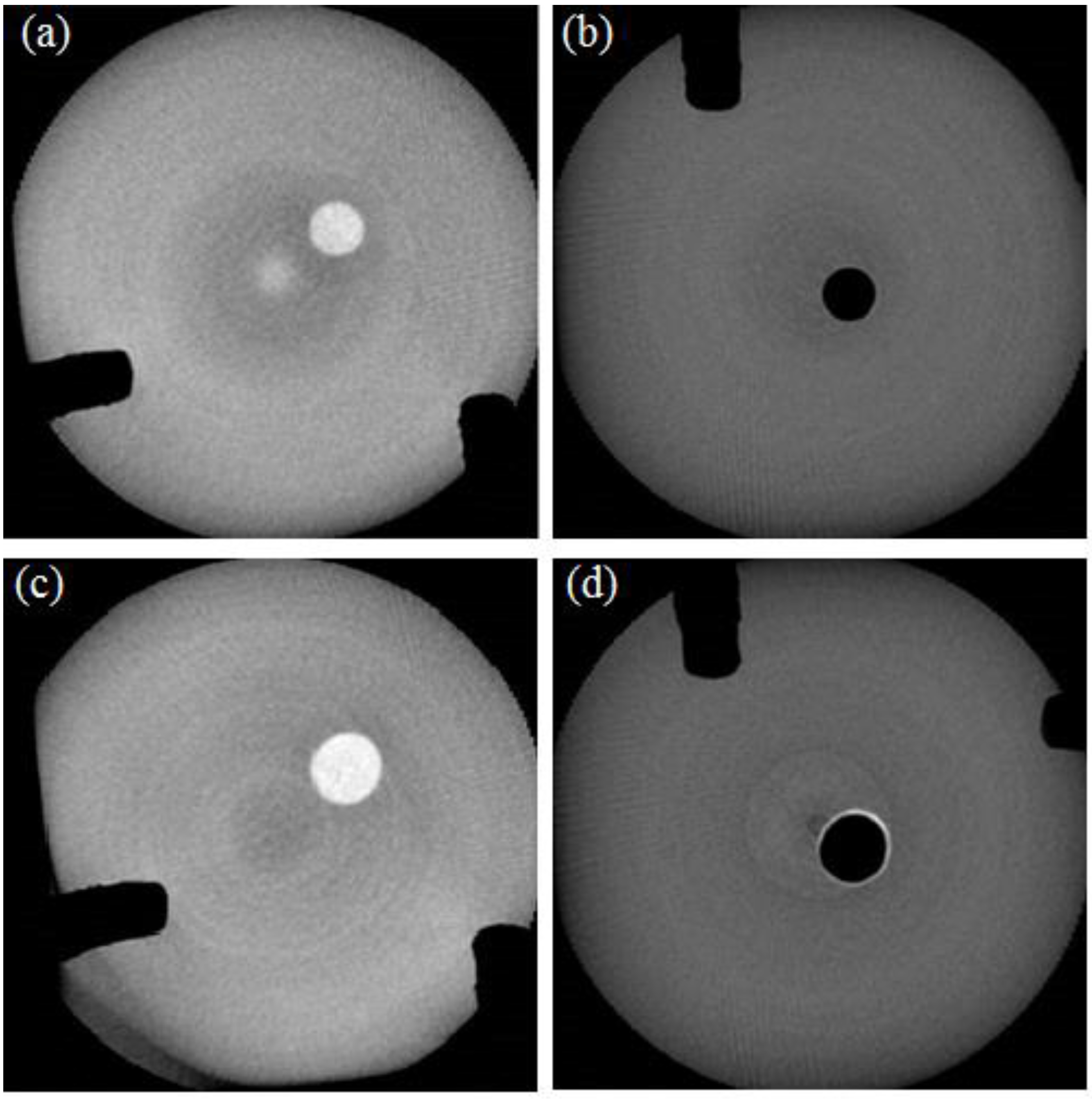
Cross-sectional images of the cleaned simulated root canal specimens were obtained by scanning using a micro-CT (Skyscan 1172, Bruker, Brussels, Belgium) at the apical third, the medium third, and the coronal third parts. It was observed that 24 to 48 h was needed to scan each specimen. Then, the remaining nail-polish-derived dye in the canal blocks was cleansed and washed out using acetone and the blocks were prepared for the next test.
The dye was expressed as a white color in the image, before and after the cleansing, because of the light reflection. After the cleansing, when the dye was removed, no reflection of light occurred and, thus, a black color was observed. All the black and white areas were evaluated by using the micro-CT software, before and after the cleansing. Then, the cleansing efficiency (CE), expressed in percentage, was evaluated by:
Student’s t-test was used to analyze whether the types of fiber and hand-pieces, respectively, might give any statistical significance to the cleansing. One-way ANOVA was used to analyze the average cleansing efficiency between the cleansing areas. All the descriptive statistics and statistical analyses were done at α = 0.05 using Microsoft Excel 2010 (Microsoft, Redmond, WA, USA).