Torsional Fretting Wear Properties of Thermal Oxidation-Treated Ti3SiC2 Coatings
Abstract
:1. Introduction
2. Materials and Experimental Methodology
2.1. Materials and Thermal Oxidation of Ti3SiC2 Coatings
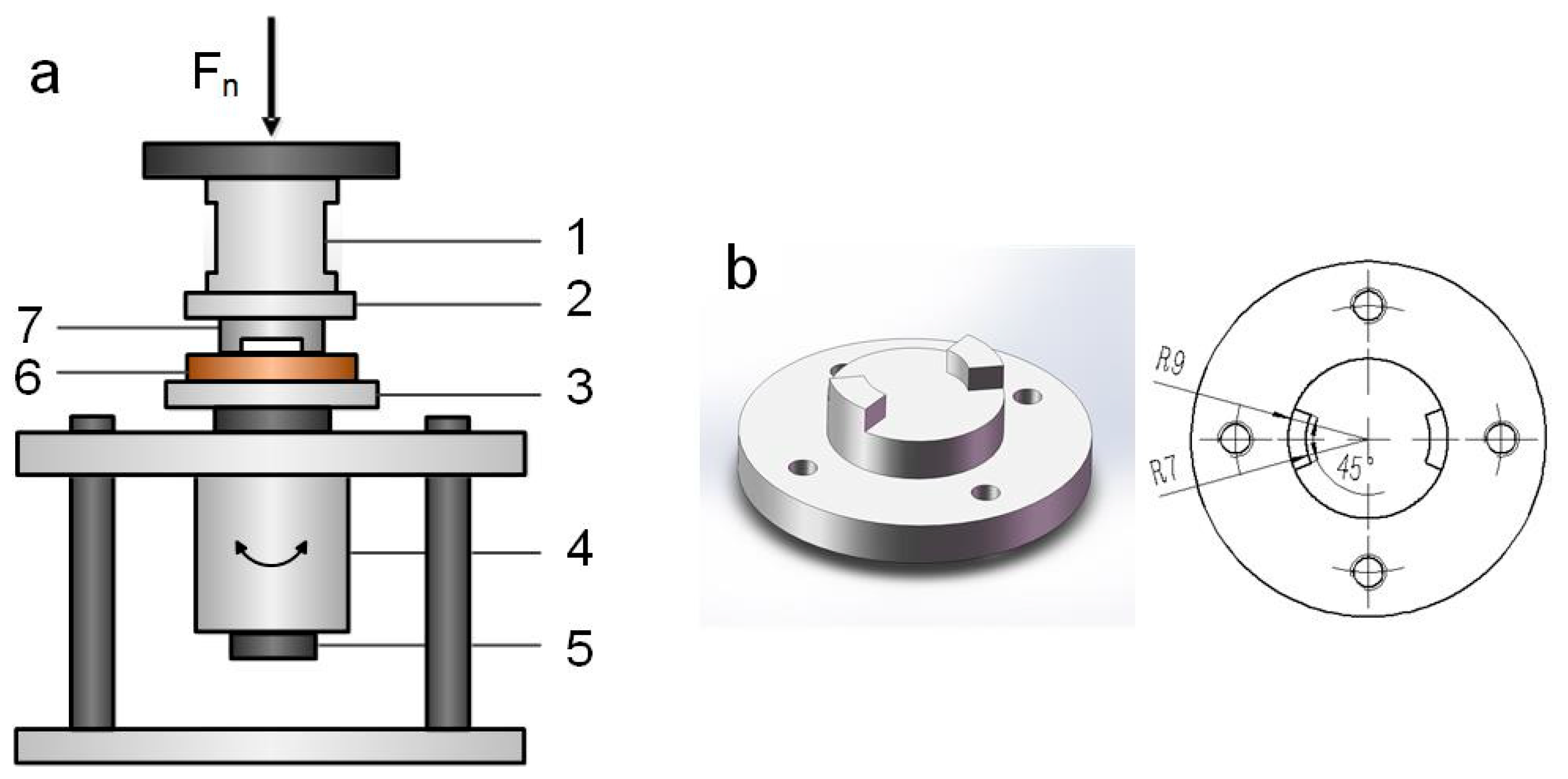
2.2. Torsional Fretting Test
2.3. Analysis
3. Experimental Results and Discussion
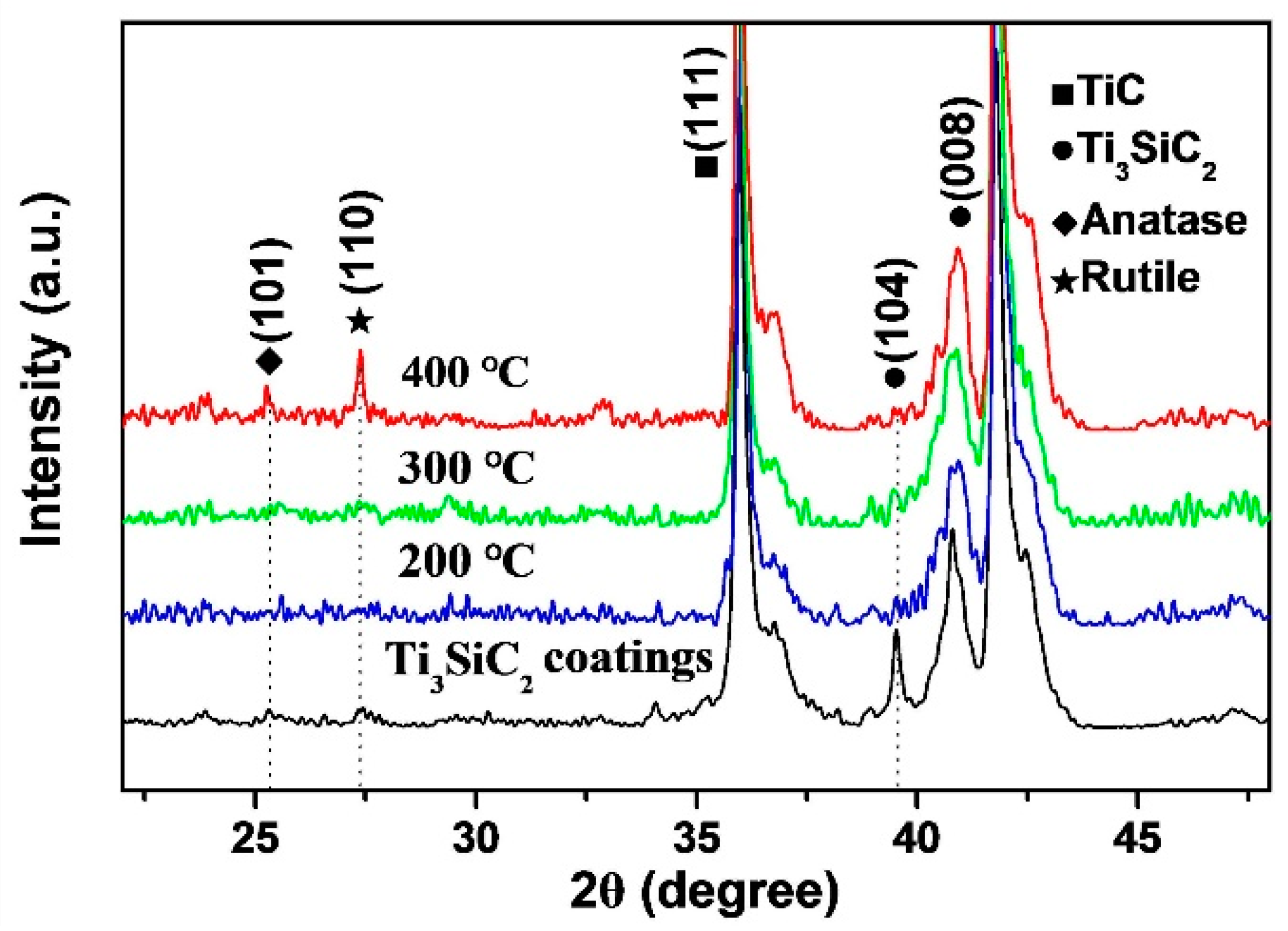
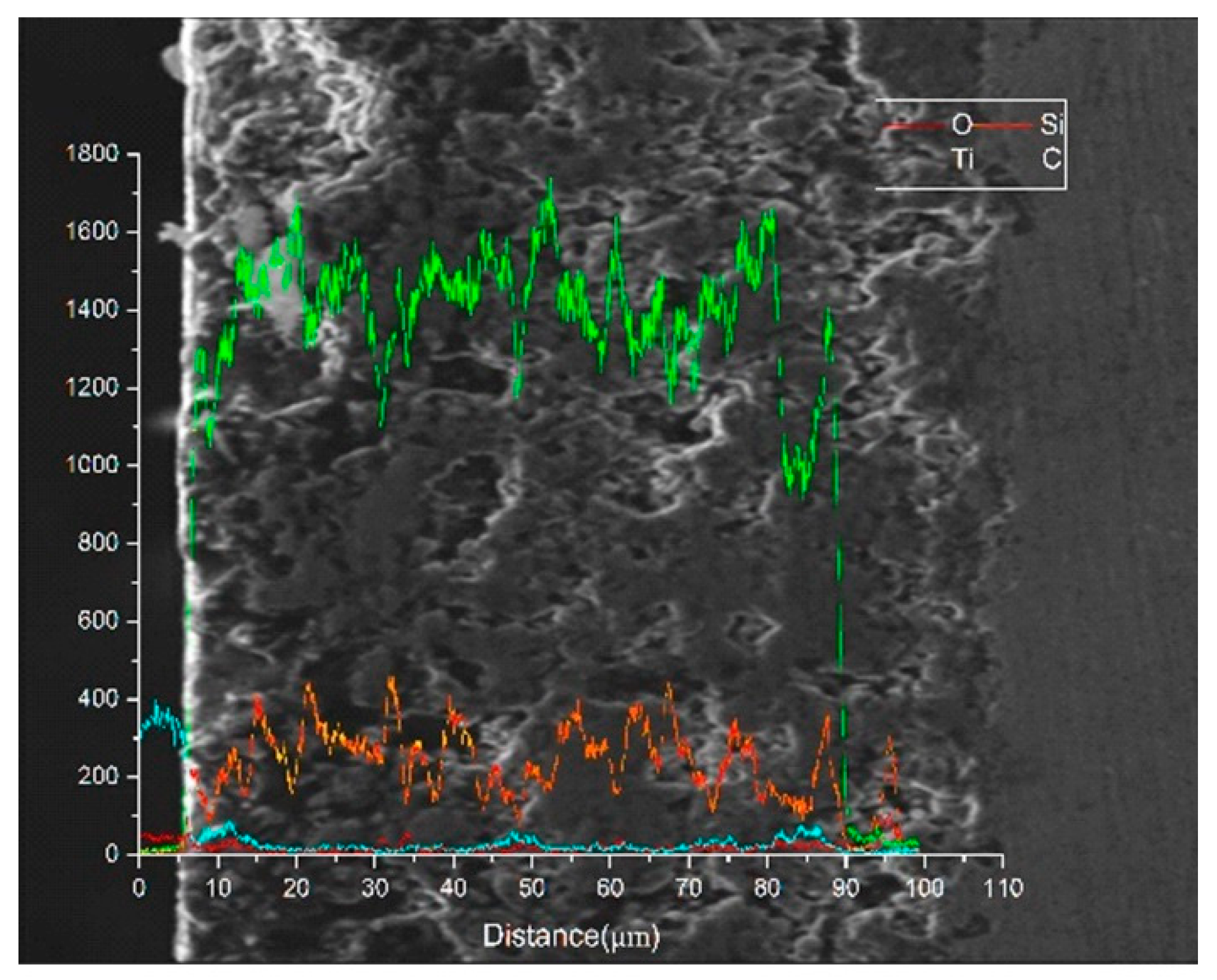
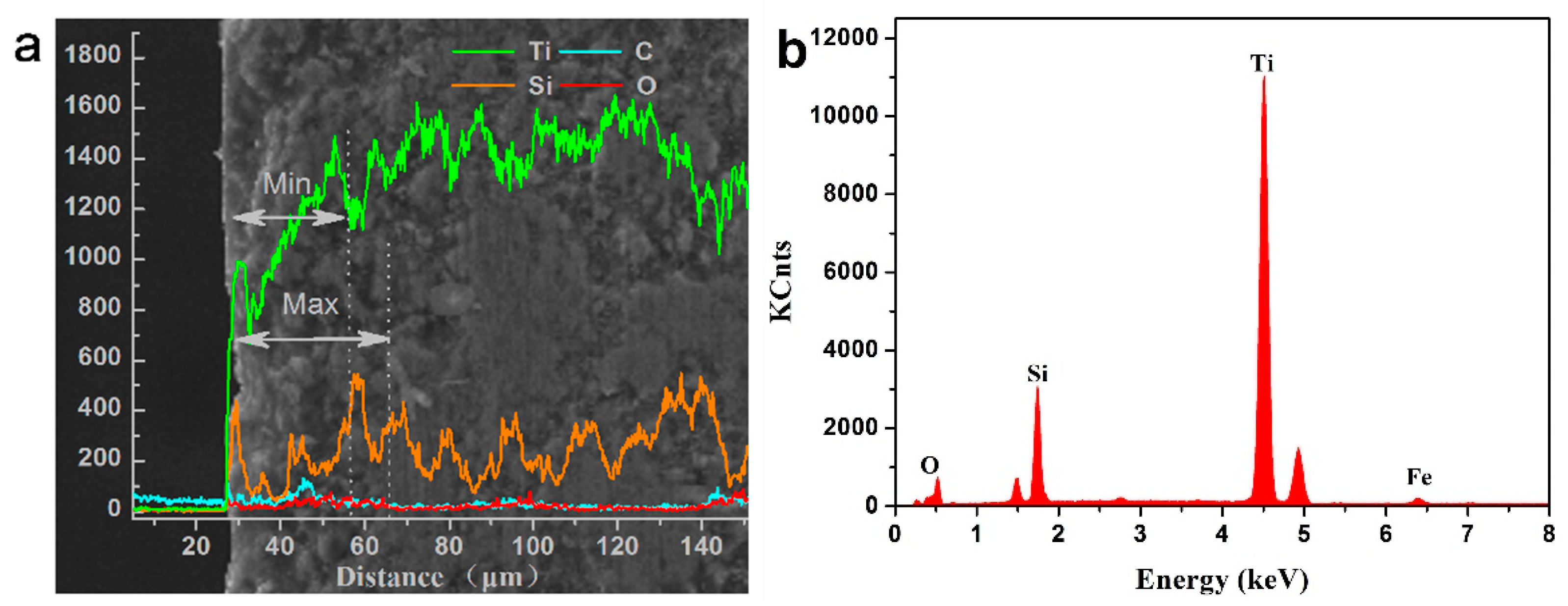
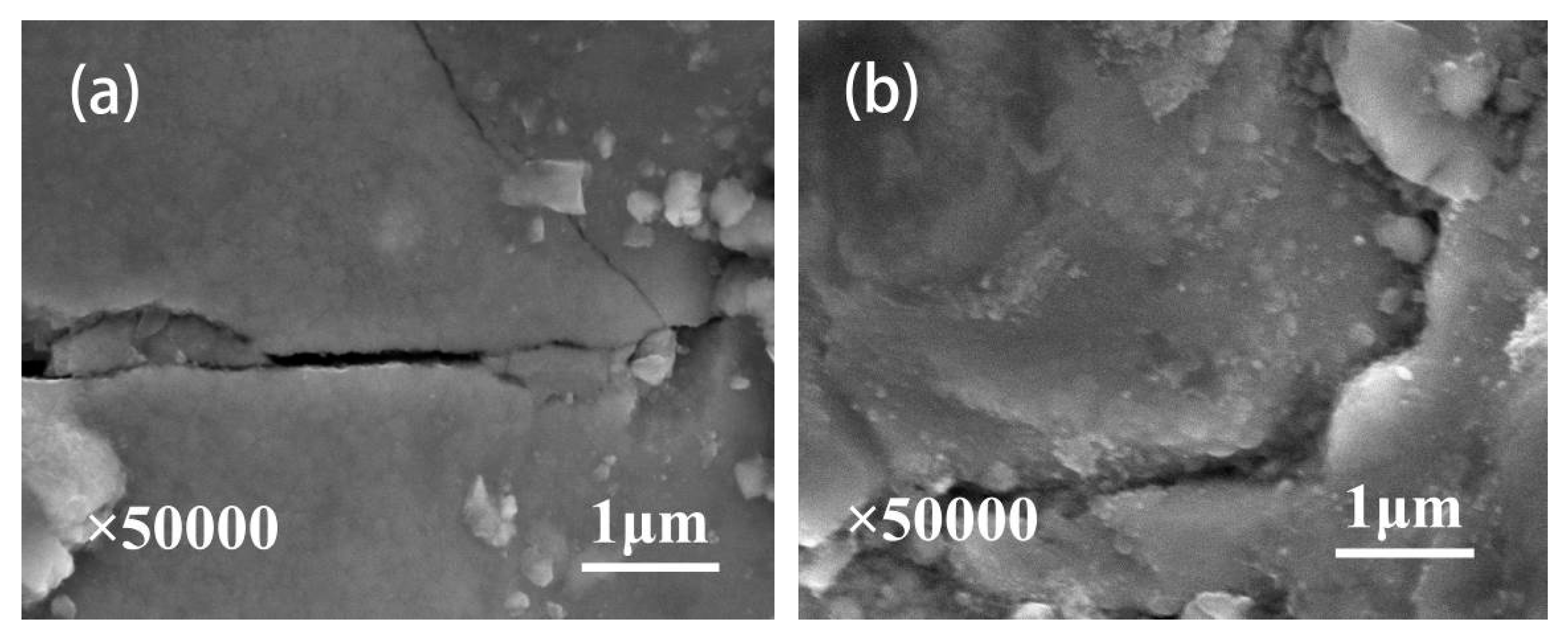
3.1. Composition and Characterization of Thermal Oxidation-Treated Coatings
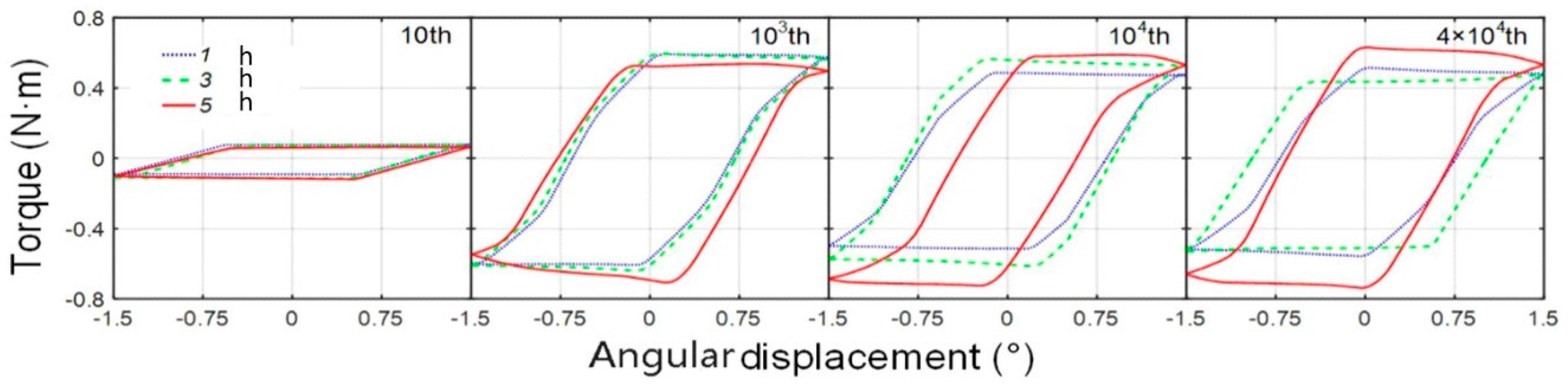
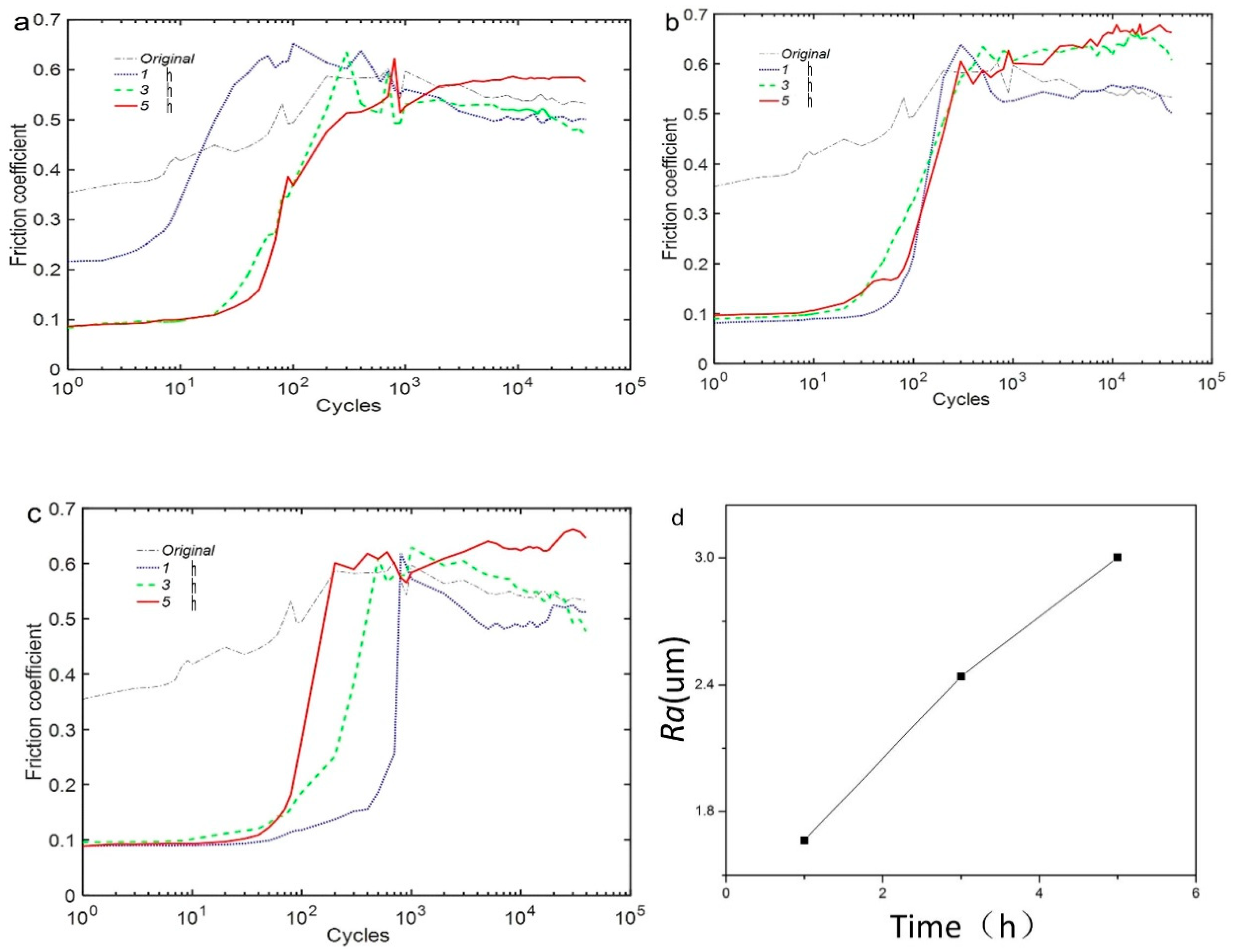
3.2. Friction Kinetics Behavior
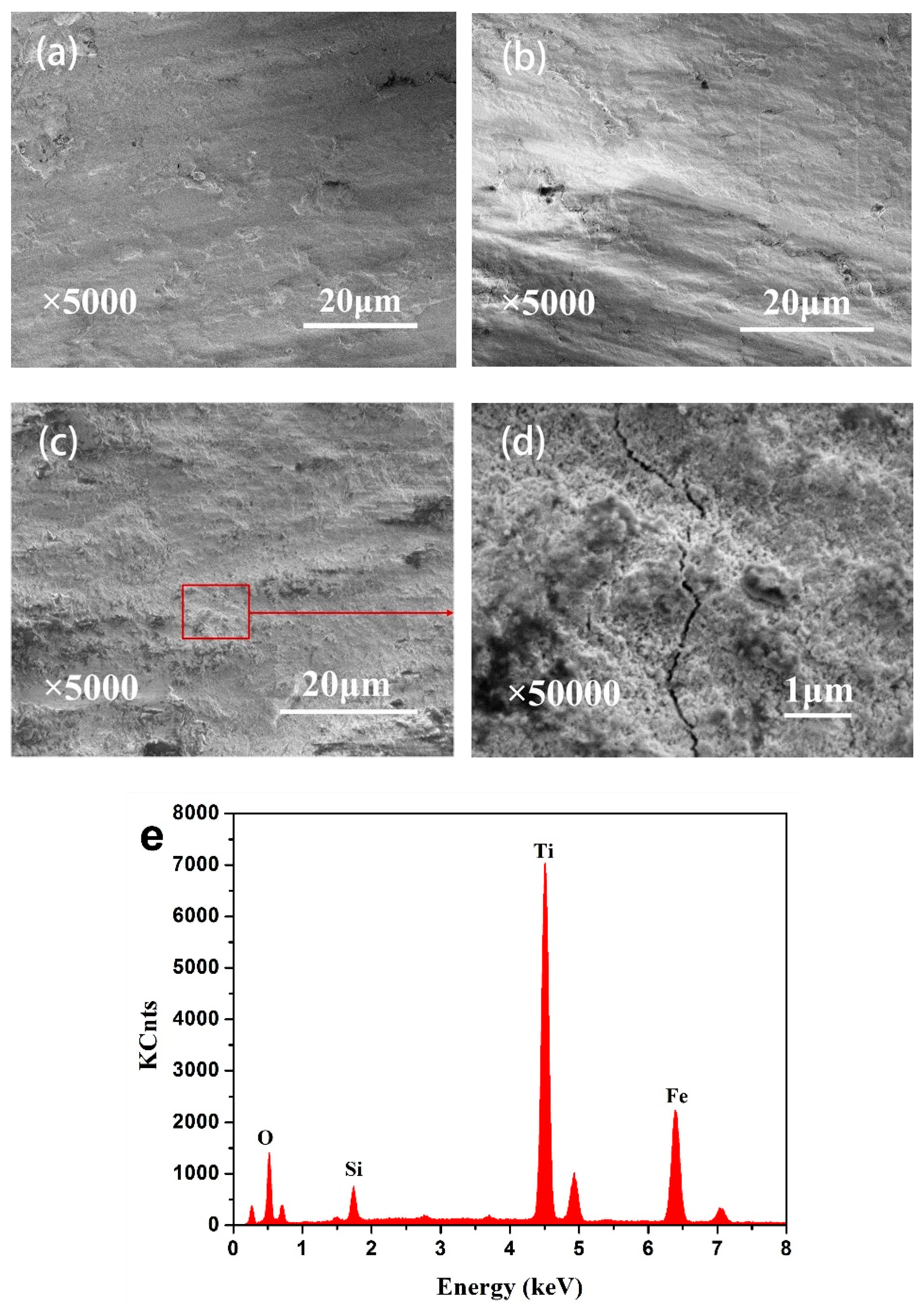
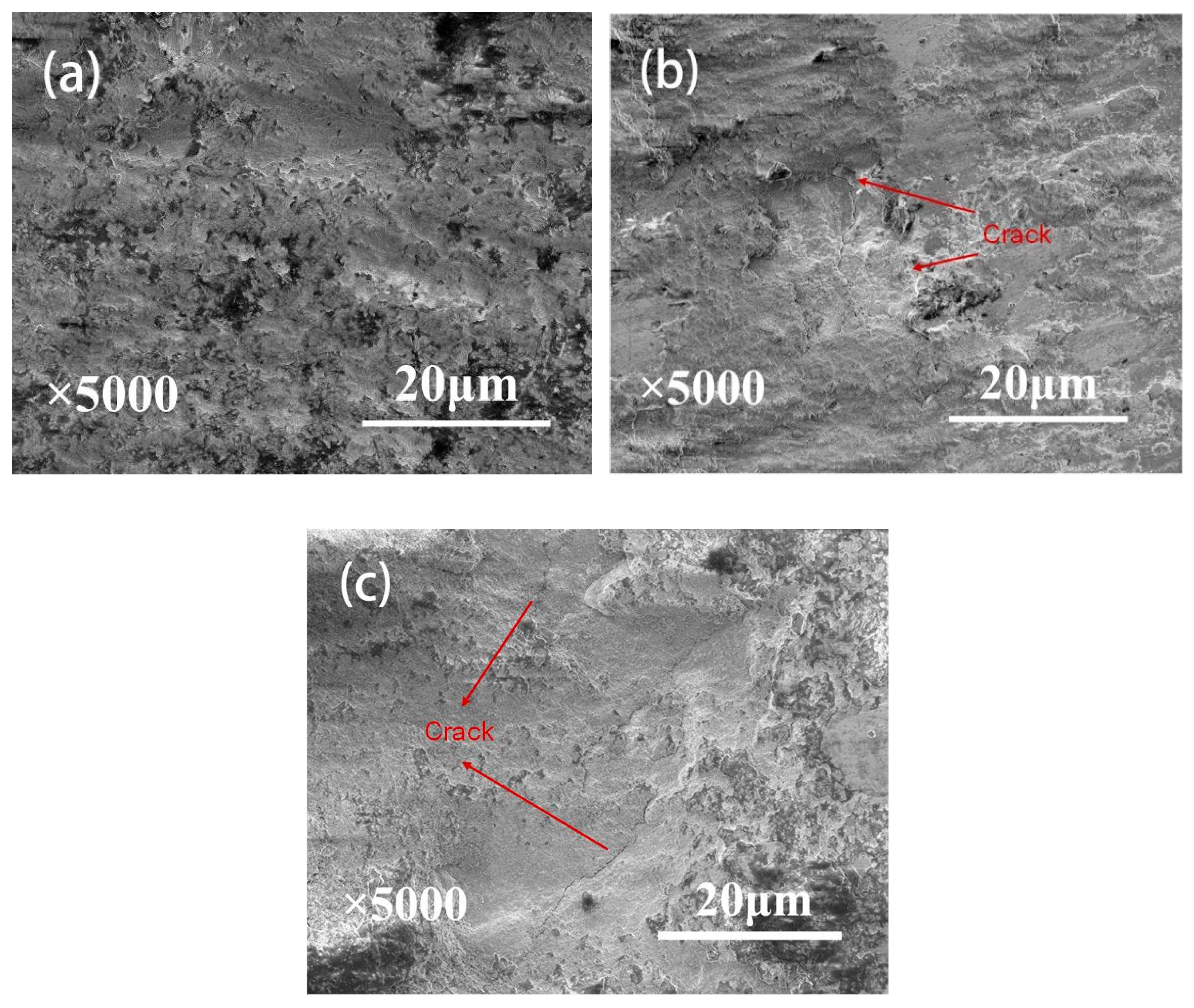
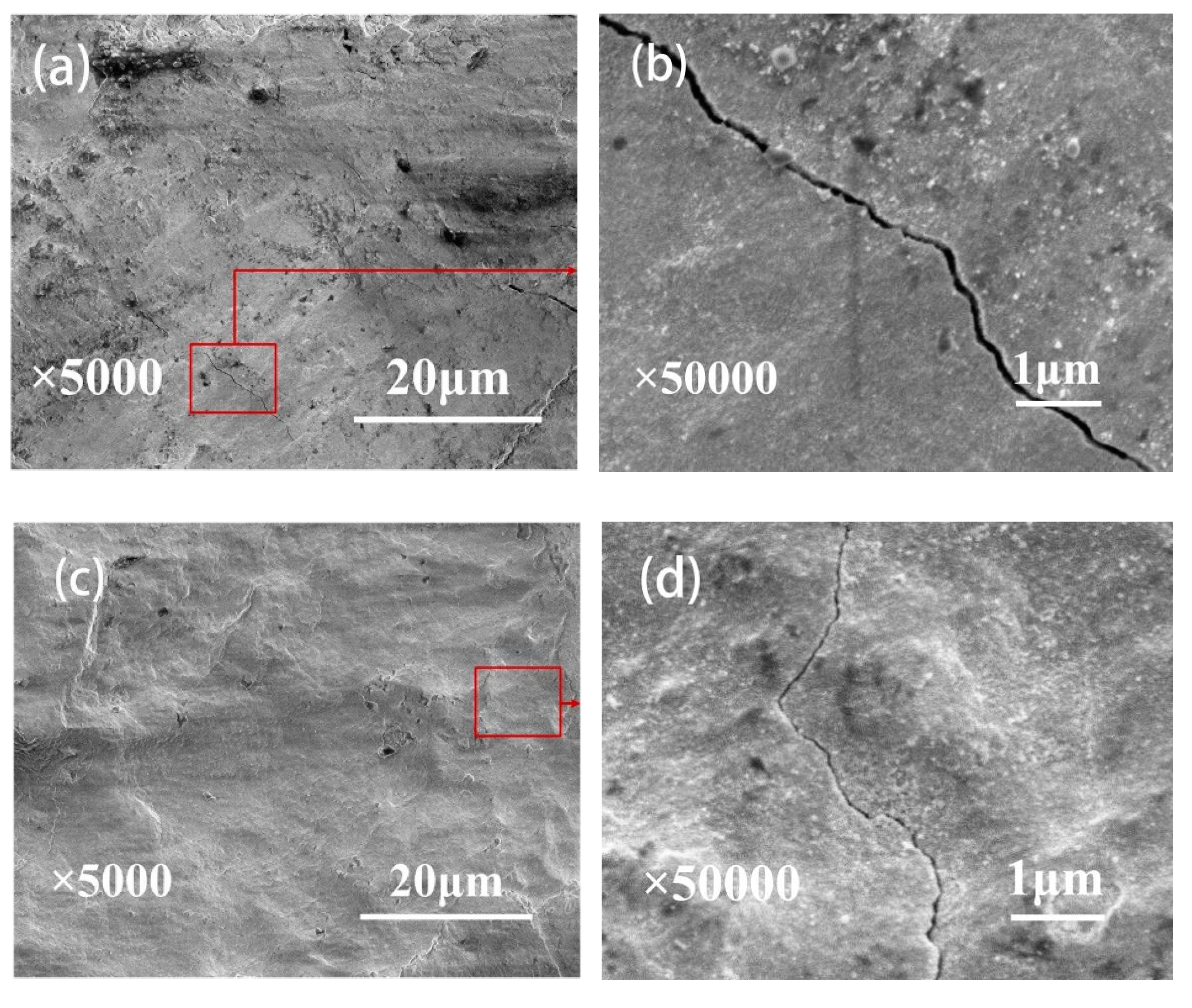
3.3. Wear Scar Observation
4. Conclusions
- A combination of XRD and EDS patterns demonstrated that the products formed during oxidation treatment consisted mainly of TiO2, which was transformed mostly by the oxidation reaction of Ti3SiC2 phases. The average composition of element O as well as the thickness of oxide layers increased with increasing temperature. Well-adhering phases of TiO2 and some other productions healed the original cracks on the plasma-sprayed coatings with the increased oxidation temperature of 400 °C.
- According to the T-θ curves and damage morphologies, the torsional fretting was expected to run in the gross slip regime, and the main wear mechanisms were delamination and abrasive wear.
- The friction coefficient was expected to have a relatively lower value with decreased oxidation time. The morphologies of wear scars with increased oxidation time were rougher due to the deformation in the fretting wear process.
- Compared with the as-deposited Ti3SiC2 coatings, the wear volume exhibited an appreciable decrease after oxidation treatment due to the lubrication of TiO2 and the healing of microcracks generated by the oxidizing reaction of Ti3SiC2. The wear volume of coatings showed a decrease with the increase in oxidation time under the oxidation treatment at 200 °C, whereas there was an increase with increasing oxidation time under the oxidation temperature of 300 and 400 °C. Despite better original crack healing at 400 °C, the higher number of cracks was caused on the initial surface due to the oxide formation process and the brittleness of the oxide during wear. The preferable tribological performances were obtained under the oxidation temperature of 300 °C and the oxidation time of 1 h.
Author Contributions
Funding
Conflicts of Interest
References
- Barsoum, M.W. The Mn+1AXn phases: A new class of solids: Thermodynamically stable nanolaminates. Prog. Solid State Chem. 2000, 28, 201–281. [Google Scholar] [CrossRef]
- Zhan, X.; Li, Z.; Gao, Z.; Zheng, L.; Wang, X.; Wang, J.; Zhou, Y. Strengthening Ti3AlC2 by in situ synthesizing Ti3AlC2-Y4Al2O9 composites. J. Am. Ceram. Soc. 2012, 95, 2314–2321. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Wan, P.; Zhan, X.; Zhou, Y. Insights into high-temperature uniaxial compression deformation behavior of Ti3AlC2. J. Am. Ceram. Soc. 2015, 98, 3332–3337. [Google Scholar] [CrossRef]
- Myhra, S.; Summers, J.W.B.; Kisi, E.H. Ti3SiC2—A layered ceramic exhibiting ultra-low friction. Mater. Lett. 1999, 39, 6–11. [Google Scholar] [CrossRef]
- Zhai, W.; Lu, W.; Zhang, P.; Wang, J.; Liu, X.; Zhou, L. Wear-triggered self-healing nanocrystalline nickel aluminum bronze/Ti3SiC2. Appl. Surf. Sci. 2018, 436, 1038–1049. [Google Scholar] [CrossRef]
- Shao, Z.; Sun, Y.; Liu, W.; Zhang, X.; Jiang, X. Effects of multi-phase reinforcements on microstructures, mechanical and tribological properties of Cu/Ti3SiC2/C/BN/GNPs nanocomposites sintered by vacuum hot-pressing and hot isostatic pressing. Metals 2016, 6, 324. [Google Scholar] [CrossRef]
- Chen, X.; Bei, G. Toughening mechanisms in nanolayered MAX phase ceramics—A review. Materials 2017, 10, 366. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Meng, J.; Lu, J.; Yang, S. Tribological behavior of Ti3SiC2 sliding against Ni-based alloys at alevated temperatures. Tribol. Lett. 2008, 31, 129–137. [Google Scholar] [CrossRef]
- Zhang, P.; Lu, W.; Liu, X.; Zhai, W.; Zhou, M.; Zeng, W. Torsional fretting and torsional sliding wear behaviors of CuNiAl against 42CrMo4 under dry condition. Tribol. Int. 2018, 118, 11–19. [Google Scholar] [CrossRef]
- Shi, W.; Luo, X.; Zhang, Z.; Liu, Y.; Lu, W. Influence of external load on the frictional characteristics of rotary model using a molecular dynamics approach. Comput. Mater. Sci. 2016, 122, 201–209. [Google Scholar] [CrossRef]
- Lu, W.; Zhang, G.; Liu, X.; Zhou, L.; Chen, L.; Jiang, X. Prediction of surface topography at the end of sliding running-in wear based on areal surface parameters. Tribol. Trans. 2014, 57, 553–560. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, X.; Lu, W. A parameter prediction model of runing-in based on surface topography. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2013, 227, 1047–1055. [Google Scholar] [CrossRef]
- Hai, W.; Ren, S.; Meng, J.; Lu, J. Tribo-oxidation of self-mated Ti3SiC2 at elevated temperatures and low speed. Tribol. Lett. 2012, 48, 425–432. [Google Scholar] [CrossRef]
- Zhai, W.; Shi, X.; Yang, K.; Huang, Y.; Zhou, L.; Lu, W. Mechanical and tribological behaviors of the tribo-layer of the nano crystalline sturcture during sliding contact: Experiments and model assessment. Compos. Part B Eng. 2017, 108, 354–363. [Google Scholar] [CrossRef]
- Zhang, R.; Feng, K.; Meng, J.; Liu, F.; Ren, S.; Hai, W.; Zhang, A. Tribological behavior of Ti3SiC2 and Ti3SiC2/Pb composites sliding against Ni-based alloys at elevated temperatures. Cerim. Int. 2016, 42, 7107–7117. [Google Scholar] [CrossRef]
- Zhou, M.; Lu, W.; Liu, X.; Zhai, W.; Zhang, P.; Zhang, G. Fretting wear properties of plasma-sprayed Ti3SiC2 coatings with oxidative crack-healing feature. Tribol. Int. 2017, 118, 196–207. [Google Scholar] [CrossRef]
- Zhai, H.; Huang, Z.; Zhou, Y.; Zhang, Z.; Wang, Y.; Ai, M. Oxidation layer in sliding friction surface of high-purity Ti3SiC2. J. Mater. Sci. 2004, 39, 6635–6637. [Google Scholar] [CrossRef]
- Barsoum, M.W.; El-Raghy, T. Oxidation of Ti3SiC2 in air. J. Electrochem. Soc. 1997, 144, 2508–2516. [Google Scholar] [CrossRef]
- Sun, Z.; Zhou, Y.; Li, M. Cyclic-Oxidation behavior of Ti3SiC2-base material at 1100 °C. Oxidation Met. 2002, 57, 379–394. [Google Scholar] [CrossRef]
- Lee, D.B.; Park, S.W. Oxidation of Ti3SiC2 between 900 and 1200 °C in air. Oxid. Met. 2007, 67, 51–66. [Google Scholar] [CrossRef]
- Król, S.; Ptacek, L.; Zalisz, Z.; Hepner, M. Friction and wear properties of titanium and oxidised titanium in dry sliding against hardened C45 steel. J. Mater. Process. Technol. 2004, 157–158, 364–369. [Google Scholar] [CrossRef]
- Krishna, D.S.R.; Brama, Y.L.; Sun, Y. Thick rutile layer on titanium for tribological applications. Tribol. Int. 2007, 40, 329–334. [Google Scholar]
- Ren, S.; Meng, J.; Wang, J.; Lu, J.; Yang, S. Friction and wear of thermal oxidation-treated Ti3SiC2. Tribol. Lett. 2010, 37, 59–67. [Google Scholar] [CrossRef]
- Basu, S.N.; Ye, G.; Gevelber, M.; Wroblewski, D. Microcrack formation in plasma sprayed thermal barrier coatings. Int. J. Refract. Met. Hard Mater. 2005, 23, 335–343. [Google Scholar] [CrossRef]
- Yang, Z.C.; Zhong, Y.C.; Zhou, C.S. Quantitative assessment of the surface crack density in thermal barrier coatings. Acta Mech. Sin. 2014, 30, 167–174. [Google Scholar] [CrossRef]
- Song, G.M.; Pei, Y.T.; Sloof, W.G.; Li, S.B.; De Hosson, J.T.M.; Van der Zwaag, S. Oxidation-induced crack healing in Ti3AlC2 ceramics. Scr. Mater. 2008, 58, 13–16. [Google Scholar] [CrossRef]
- Farle, A.S.; Kwakernaak, C.; van der Zwaag, S.; Sloof, W.G. A conceptual study into the potential of Mn+1AXn-phase ceramics for self-healing of crack damage. J. Eur. Ceram. Soc. 2015, 35, 37–45. [Google Scholar] [CrossRef]
- Pei, R.; McDonald, S.A.; Shen, L.; van der Zwaag, S.; Sloof, W.G.; Withers, P.J.; Mummery, P.M. Crack healing behaviour of Cr2AlC MAX phase studied by X-ray tomography. J. Eur. Ceram. Soc. 2017, 37, 441–450. [Google Scholar] [CrossRef]
- Li, S.; Xiao, L.; Song, G.; Wu, X.; Sloof, W.G.; van der Zwaag, S. Oxidation and crack healing behavior of a fine-grained Cr2AlC ceramic. J. Am. Ceram. Soc. 2013, 96, 892–899. [Google Scholar] [CrossRef]
- Shen, L.; Eichner, D.; van der Zwaag, S.; Leyens, C.; Sloof, W.G. Reducing the erosive wear rate of Cr2AlC MAX phase ceramic by oxidative healing of local impact damage. Wear 2016, 358–359, 1–6. [Google Scholar] [CrossRef]
- Sun, Z.; Zhou, Y.; Li, M. High temperature oxidation behavior of Ti3SiC2-based material in air. Acta Mater. 2001, 49, 4347–4353. [Google Scholar] [CrossRef]
- Sun, Z.M.; Zhou, Y.C.; Li, M.S. Oxidation behaviour of Ti3SiC2-based ceramic at 900–1300 °C in air. Corros. Sci. 2001, 43, 1095–1109. [Google Scholar] [CrossRef]
- Zhang, H.B.; Zhou, Y.C.; Bao, Y.W.; Wang, J.Y. Oxidation behavior of bulk Ti3SiC2 at intermediate temperatures in dry air. J. Mater. Res. 2006, 21, 402–408. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, X.; Lu, W.; Zhai, W.; Zhou, M.; Wang, J. Fretting wear behavior of CuNiAl against 42CrMo4 under different lubrication conditions. Tribol. Int. 2018, 117, 59–67. [Google Scholar] [CrossRef]
- Lu, W.; Zhang, P.; Liu, X.; Zhai, W.; Zhou, M.; Luo, J.; Zeng, W.; Jiang, X.J. Influence of surface topography on torsional fretting wear under flat-on-flat contact. Tribol. Int. 2017, 109, 367–372. [Google Scholar] [CrossRef]
- Zhai, W.; Lu, W.; Liu, X.; Zhou, L. Nanodiamond as an effective additive in oil to dramatically reduce friction and wear for fretting steel/copper interfaces. Tribol. Int. 2019, 129, 75–81. [Google Scholar] [CrossRef]
- Zhang, P.; Lu, W.; Liu, X.; Zhai, W.; Zhou, M.; Jiang, X.J. A comparative study on torsional fretting and torsional sliding wear of CuNiAl under different lubricated conditions. Tribol. Int. 2018, 117, 78–86. [Google Scholar] [CrossRef]
- Zhai, W.; Lu, W.; Chen, Y.; Liu, X.; Zhou, L.; Lin, D. Gas-atomized copper-based particles encapsulated in graphene oxide for high wear-resistance performance. Compos. Part B Eng. 2019, 157, 131–139. [Google Scholar] [CrossRef]
- Cai, Z.B.; Zhu, M.H.; Zheng, J.F.; Jin, X.S.; Zhou, Z.R. Torsional fretting behaviors of LZ50 steel in air and nitrogen. Tribol. Int. 2009, 42, 1676–1683. [Google Scholar] [CrossRef]
- Quan, H.; Gao, S.; Zhu, M.; Yu, H. Comparison of the torsional fretting behavior of three porous titanium coatings for biomedical applications. Tribol. Int. 2015, 92, 29–37. [Google Scholar] [CrossRef]
- Cai, Z.B.; Zhu, M.H.; Zhou, Z.R. An experimental study torsional fretting behaviors of LZ50 steel. Tribol. Int. 2010, 43, 361–369. [Google Scholar] [CrossRef]
- Zhai, W.; Lu, W.; Zhang, P.; Zhou, M.; Liu, X.; Zhou, L. Microstructure, mechanical and tribological properties of nickel-aluminium bronze alloys developed via gas-atomization and spark plasma sintering. Mater. Sci. Eng. A 2017, 707, 325–336. [Google Scholar] [CrossRef]
- Zhang, P.; Lu, W.; Liu, X.; Zhou, M.; Zhai, W.; Zhang, G.; Zeng, W.; Jiang, X.J. Torsional fretting wear behavior of CuNiAl against 42CrMo4 under flat on flat contact. Wear 2017, 380–381, 6–14. [Google Scholar] [CrossRef]
- Shen, M.X.; Zhu, M.H.; Cai, Z.B.; Xie, X.Y.; Zuo, K.C. Dual-rotary fretting wear behavior of 7075 aluminum alloy. Tribol. Int. 2012, 48, 162–171. [Google Scholar] [CrossRef]
- Tobi, A.M.; Sun, W.; Shipway, P.H. Evolution of plasticity-based wear damage in gross sliding fretting of a Ti-6Al-4V non-conforming contact. Tribol. Int. 2017, 113, 474–486. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.F.; Luo, J.; Mo, J.L.; Peng, J.F.; Jin, X.S.; Zhu, M.H. Fretting wear behaviors of a railway axle steel. Tribol. Int. 2010, 43, 906–911. [Google Scholar] [CrossRef]
- Lan, P.; Gheisari, R.; Meyer, J.L.; Polycarpou, A.A. Tribological performance of aromatic thermosetting polyester (ATSP) coatings under cryogenic conditions. Wear 2018, 398, 47–55. [Google Scholar] [CrossRef]
- Lan, P.; Zhang, Y.; Dai, W.; Polycarpou, A.A. A phenomenological elevated temperature friction model for viscoelastic polymer coatings based on nanoindentation. Tribol. Int. 2018, 119, 299–307. [Google Scholar] [CrossRef]
- Economou, S.; De Bonte, M.; Celis, J.P.; Smith, R.W.; Lugscheider, E. Tribological behaviour at room temperature and at 550 °C of TiC-based plasma sprayed coatings in fretting gross slip conditions. Wear 2000, 244, 165–179. [Google Scholar] [CrossRef]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Luo, X.; Sun, Y. Torsional Fretting Wear Properties of Thermal Oxidation-Treated Ti3SiC2 Coatings. Coatings 2018, 8, 324. https://doi.org/10.3390/coatings8090324
Wang J, Luo X, Sun Y. Torsional Fretting Wear Properties of Thermal Oxidation-Treated Ti3SiC2 Coatings. Coatings. 2018; 8(9):324. https://doi.org/10.3390/coatings8090324
Chicago/Turabian StyleWang, Jian, Xiaohui Luo, and Yanhua Sun. 2018. "Torsional Fretting Wear Properties of Thermal Oxidation-Treated Ti3SiC2 Coatings" Coatings 8, no. 9: 324. https://doi.org/10.3390/coatings8090324



