Improvement of Corrosion Resistance of Hastelloy-N Alloy in LiF-NaF-KF Molten Salt by Laser Cladding Pure Metallic Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Coating Preparation
2.3. Characterization
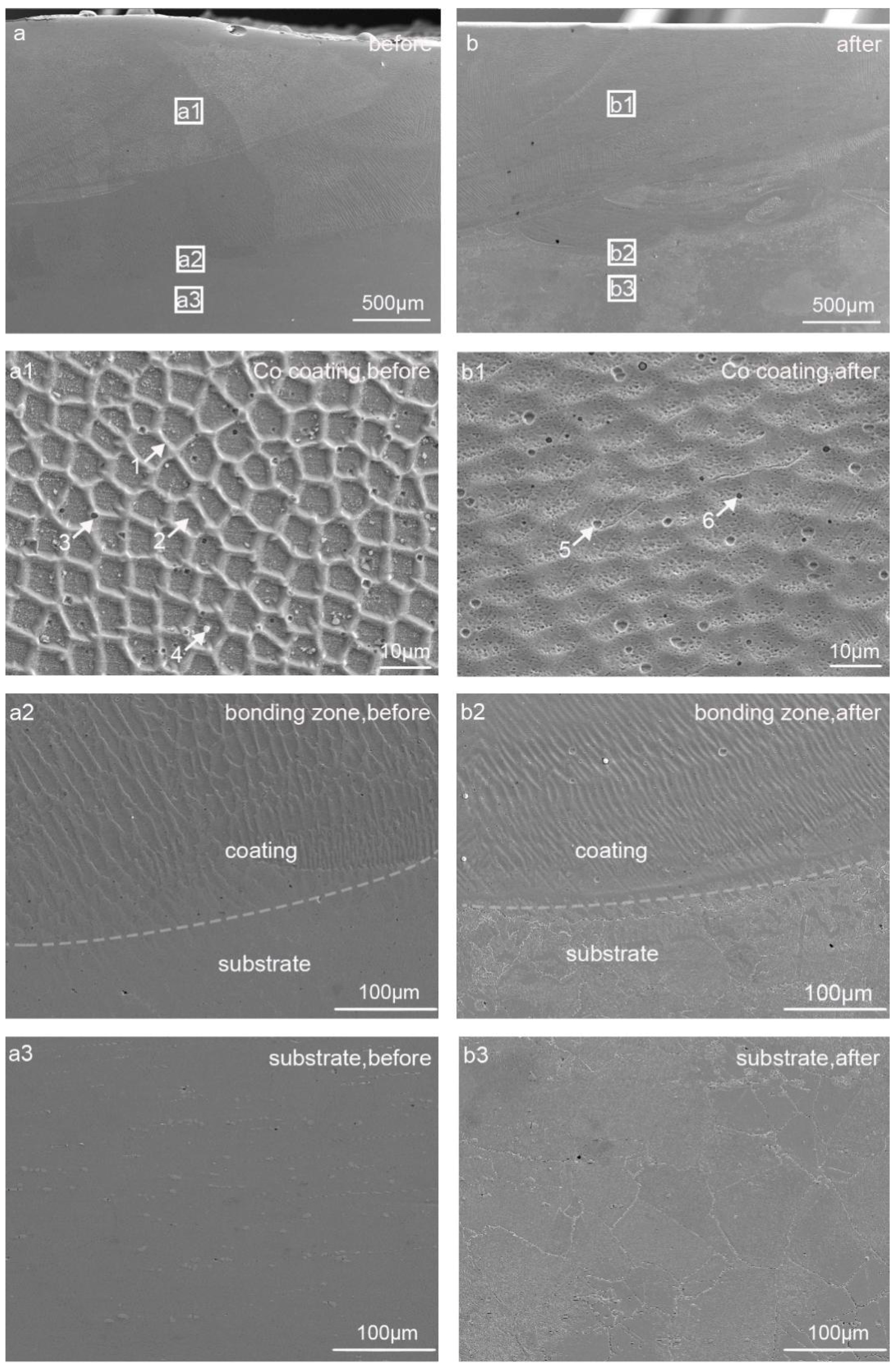
3. Results and Discussion
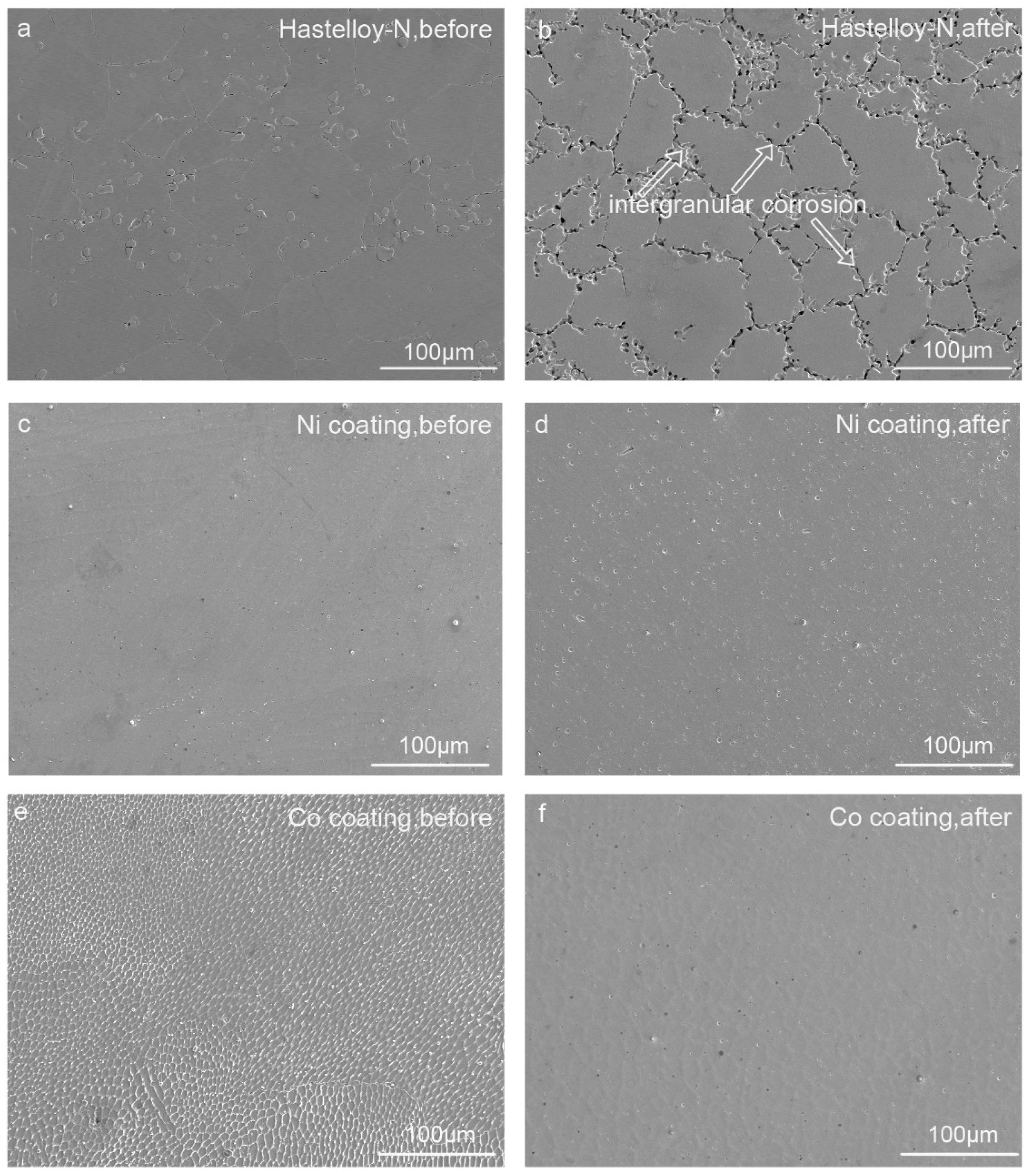
3.1. Corrosion Behavior
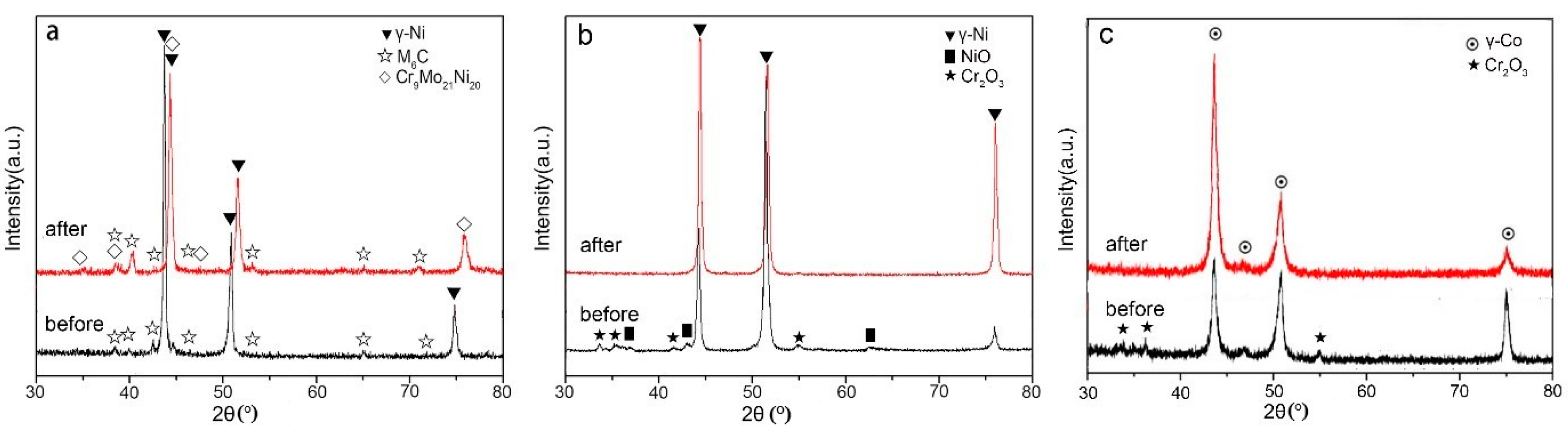
3.2. Phase Analysis
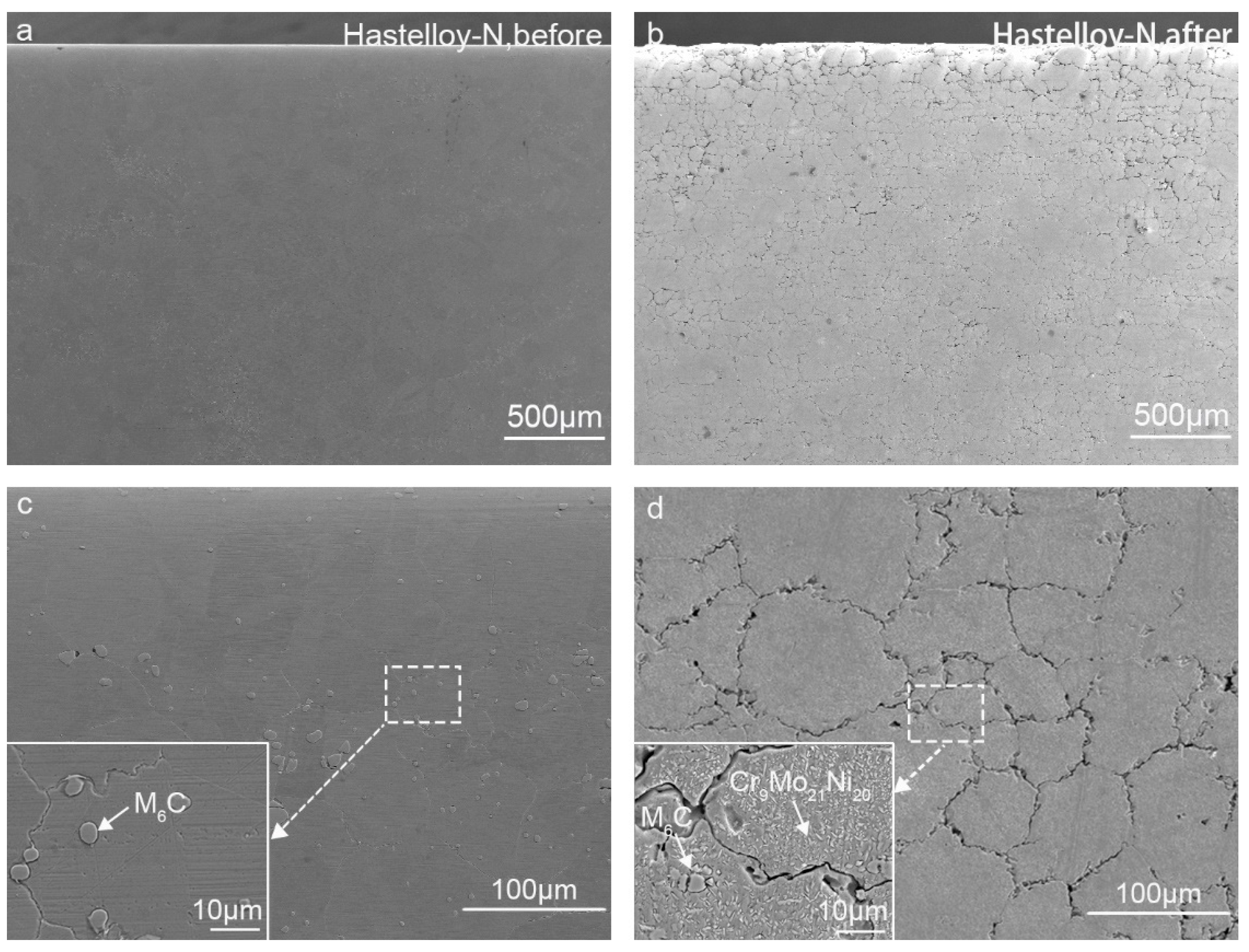
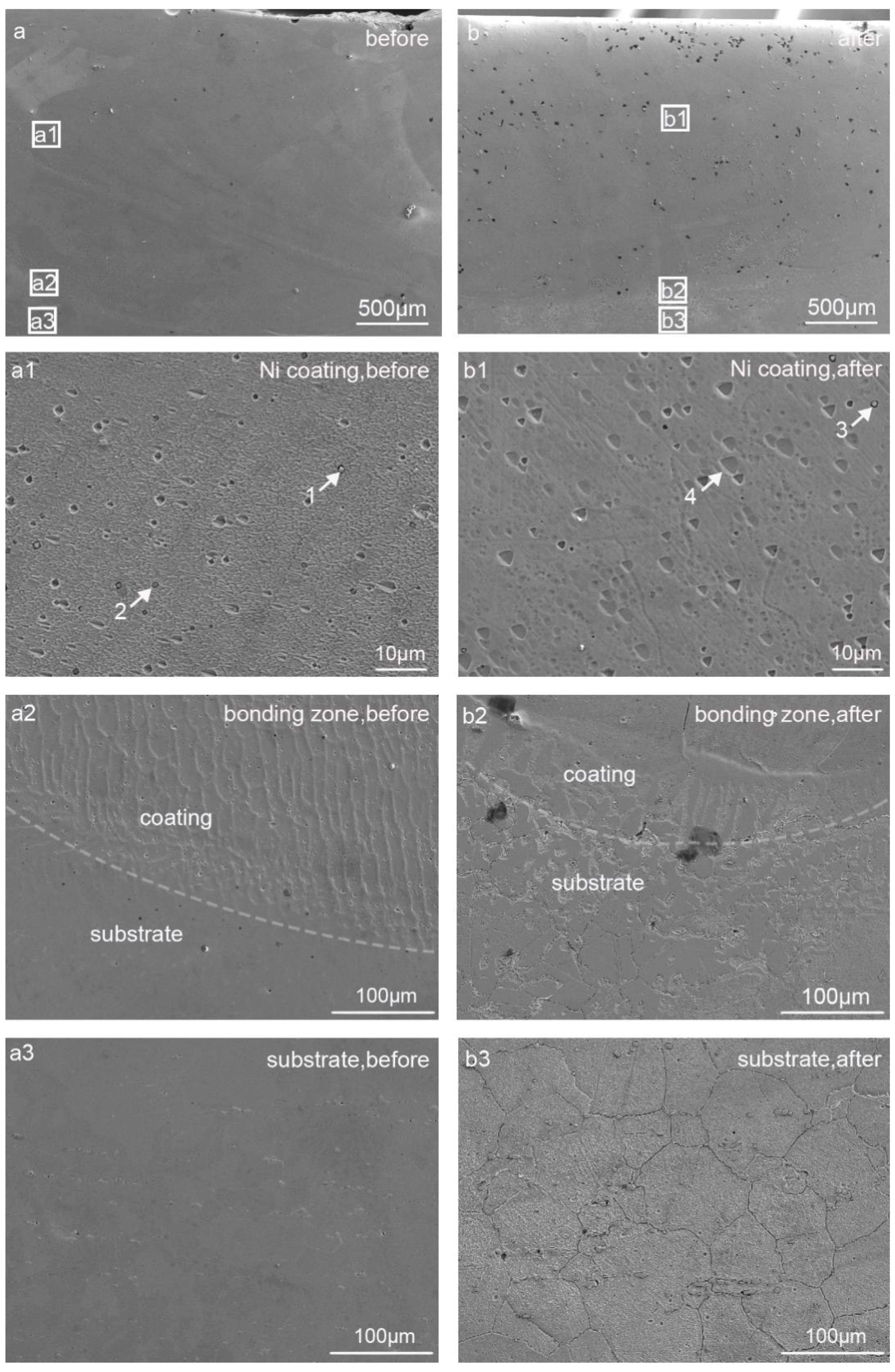
3.3. Microstructural Characterization
3.4. Corrosion Mechanisms
4. Conclusions
- Superior corrosion resistance caused by pure Ni and Co metallic coatings. Pure metallic coatings can effectively improve the corrosion resistance of Hastelloy-N alloy in molten fluoride salt, which was confirmed by the much lower corrosion rate of the pure Ni- and Co-coated specimens than that of Hastelloy-N alloy. Significantly, the firstly-reported Co-coated specimen exhibits the optimal stability in the molten fluoride salt without any discernible corrosion.
- Unique microstructure for treated Hastelloy-N alloy. The Hastelloy-N alloy is composed of γ-Ni and M6C, and a new Cr9Mo21Ni20 precipitate after corrosion. In contrast, the pure Ni coating is mainly composed of γ-Ni, NiO, and Cr2O3, while the pure Co coating is mainly composed of γ-Co and Cr2O3. After corrosion in molten fluoride salt, only γ-Ni phase or γ-Co phase exists in the pure metallic coatings, respectively.
- Mechanism understanding on the corrosion behavior. The elemental Cr in Hastelloy-N diffuses and dissolves into the molten fluoride salt, leading to an elemental depleted layer on the alloy surface with severe intergranular corrosion. For pure metal-coated specimens, in contrast, only metal oxides formed during laser cladding process dissolve into the molten fluoride salt. The dense pure metal (Ni or Co) coatings can effectively hinder the penetration of the molten fluoride and thus improve the corrosion resistance of the substrate remarkably.
Author Contributions
Funding
Conflicts of Interest
References
- Holcomb, D.E.; Cetiner, S.M. An Overview of Liquid Fluoride Salt Heat Transport Systems; Report No. ORNL/TM-2010/156; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2010.
- Romatoski, R.R.; Hu, L.W. Fluoride salt coolant properties for nuclear reactor applications: A review. Ann. Nucl. Energy 2017, 109, 635–647. [Google Scholar] [CrossRef]
- Qiu, J.; Zou, Y.; Yu, G.J.; He, S.M.; Liu, W.G.; Jia, Y.Y.; Li, Z.J.; Xu, H.J. Speciation study of chromium corrosion product in molten LiF-NaF-KF salt. Nucl. Sci. Tech. 2015, 26, 60602. [Google Scholar]
- Ouyang, F.Y.; Chang, C.H.; Kai, J.J. Long-term corrosion behaviors of Hastelloy-N and Hastelloy-B3 in moisture-containing molten FLiNaK salt environments. J. Nucl. Mater. 2014, 446, 81–89. [Google Scholar] [CrossRef]
- Wang, Y.L.; Zeng, C.L.; Li, W.H. The influence of temperature gradient on the corrosion of materials in molten fluorides. Corros. Sci. 2018, 136, 180–187. [Google Scholar] [CrossRef]
- Ai, H.; Hou, J.; Ye, X.X.; Zeng, C.L.; Sun, H.; Li, X.; Yu, G.Y.; Zhou, X.T.; Wang, J.Q. Influence of graphite-alloy interactions on corrosion of Ni-Mo-Cr alloy in molten fluorides. J. Nucl. Mater. 2018, 503, 116–123. [Google Scholar] [CrossRef]
- Kuravi, S.; Trahan, J.; Goswami, D.Y.; Rahman, M.M.; Stefanakos, E.K. Thermal energy storage technologies and systems for concentrating solar power plants. Prog. Energy Combust. Sci. 2013, 39, 285–319. [Google Scholar] [CrossRef]
- Yin, H.Q.; Qiu, J.; Liu, H.J.; Liu, W.G.; Wang, Y.; Fei, Z.J.; Zhao, S.F.; An, X.H.; Cheng, J.H.; Chen, T.; et al. Effect of CrF3 on the corrosion behaviour of Hastelloy-N and 316L stainless steel alloys in FLiNaK molten salt. Corros. Sci. 2018, 131, 355–364. [Google Scholar] [CrossRef]
- Gu, Y.F.; Liu, J.X.; Wang, Y.; Xue, J.X.; Wang, X.G.; Zhang, H.B.; Xu, F.F.; Zhang, G.J. Corrosion behavior of TiC-SiC composite ceramics in molten FLiNaK salt. J. Eur. Ceram. Soc. 2017, 37, 2575–2582. [Google Scholar] [CrossRef]
- Cheng, H.W.; Leng, B.; Chen, K.; Jia, Y.Y.; Dong, J.S.; Li, Z.J.; Zhou, X.T. EPMA and TEM characterization of intergranular tellurium corrosion of Ni-16Mo-7Cr-4Fe superalloy. Corros. Sci. 2015, 97, 1–6. [Google Scholar] [CrossRef]
- Ye, X.X.; Ai, H.; Guo, Z.; Huang, H.F.; Jiang, L.; Wang, J.Q.; Li, Z.J.; Zhou, X.T. The high-temperature corrosion of Hastelloy N alloy (UNS N10003) in molten fluoride salts analysed by STXM, XAS, XRD, SEM, EPMA, TEM/EDS. Corros. Sci. 2016, 106, 249–259. [Google Scholar] [CrossRef]
- Cao, W.; Xia, S.; Bai, Q.; Zhang, W.Z.; Zhou, B.X.; Li, Z.J.; Jiang, L. Effects of initial microstructure on the grain boundary network during grain boundary engineering in Hastelloy N alloy. J. Alloys Compd. 2017, 704, 724–733. [Google Scholar] [CrossRef]
- Olson, L.; Sridharan, K.; Anderson, M.; Allen, T. Nickel-plating for active metal dissolution resistance in molten fluoride salts. J. Nucl. Mater. 2011, 411, 51–59. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Liu, Y.H.; Zhou, Z.J.; Zheng, M.M.; Kong, S.Y.; Xia, H.H.; Li, H.L. Research on Protective Coating on Inner Surface of Alloy Tube. IOP Conf. Ser.: Mater. Sci. Eng. 2017, 230, 012018. [Google Scholar] [CrossRef] [Green Version]
- Muralidharan, G.; Wilson, D.F.; Walker, L.R.; Santella, M.L.; Holcomb, D.E. Cladding Alloys for Fluoride Salt Compatibility Final Report; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2011.
- Brupbacher, M.C.; Zhang, D.J.; Buchta, W.M.; Graybeal, M.L.; Rhim, Y.R.; Nagle, D.C.; Spicer, J.B. Synthesis and characterization of binder-free Cr3C2 coatings on nickel-based alloys for molten fluoride salt corrosion resistance. J. Nucl. Mater. 2015, 461, 215–220. [Google Scholar] [CrossRef]
- Zhu, H.M.; Li, B.C.; Chen, M.H.; Liu, Z.L.; Tang, Z.F.; Qiu, C.J. AlN coatings on Hastelloy-N alloy offering superior corrosion resistance in LiF-KF-NaF molten salt. J. Fluorine Chem. 2018, 213, 80–86. [Google Scholar] [CrossRef]
- Wang, H.Y.; Zhang, S.; Zhang, C.H.; Wu, C.L.; Zhang, C.B.; Abdullah, A.O. Effects of V and Cr on laser-cladded Fe-based coatings. Coatings 2018, 8, 107. [Google Scholar] [CrossRef]
- Olson, L.C.; Ambrosek, J.W.; Sridharan, K.; Anderson, M.H.; Allen, T.R. Materials corrosion in molten LiF-NaF-KF salt. J. Fluorine Chem. 2009, 130, 67–73. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, J.H.; Wang, D.Z. High temperature oxidation and microstructure of MoSi2/MoB composite coating for Mo substrate. Int. J. Refract. Met. Hard Mater. 2017, 68, 60–64. [Google Scholar] [CrossRef]
- Dai, Q.L.; Ye, X.X.; Ai, H.; Chen, H.J.; Li, J.; Liang, J.P.; Yu, K.; Leng, B.; Li, Z.J.; Zhou, X.T. Corrosion of Incoloy 800H alloys with nickel cladding in FLiNaK salts at 850 °C. Corros. Sci. 2018, 133, 349–357. [Google Scholar] [CrossRef]
- Gale, W.F.; Totemeier, T.C. Thermochemical data. In Smithells Metals Reference Book, 8th ed.; Elsevier Butterworth-Heinemann: Burlington, MA, USA, 2004; pp. 24–26. [Google Scholar]
- Wang, Y.; Tang, Z.F.; Fu, Y.; Huang, S.R.; Zhao, S.F.; Zhang, P.; Xie, L.D.; Wang, X.G.; Zhang, G.J. Corrosion behavior of ZrC-SiC composite ceramics in LiF-NaF-KF molten salt at high temperatures. Ceram. Int. 2015, 41, 12996–13005. [Google Scholar] [CrossRef]
- Razik, N.A. Precise lattice constants determination of cubic crystals from X-ray powder diffractometric measurements. Appl. Phys. A 1985, 37, 187–189. [Google Scholar] [CrossRef]
- Wang, Y.L.; Liu, H.J.; Zeng, C.L. Galvanic corrosion of pure metals in molten fluorides. J. Fluorine Chem. 2014, 165, 1–6. [Google Scholar] [CrossRef]
- Watanabe, T.; Kondo, M.; Nagasaka, T.; Sagara, A. Corrosion characteristic of AlN, Y2O3, Er2O3 and Al2O3 in Flinak for molten salt blanket system. J. Plasma Fusion Res. SERIES 2010, 9, 342–347. [Google Scholar]
- Liu, L.F.; Wu, S.S.; Chen, Y.; Lu, S.L. Oxidation behavior of RE-modified nickel-based superalloy between 950 °C and 1150 °C in air. Trans. Nonferrous Met. Soc. Chin. 2016, 26, 1163–1169. [Google Scholar] [CrossRef]
- Liu, T.; Dong, J.S.; Wang, L.; Li, Z.J.; Zhou, X.T.; Lou, L.H.; Zhang, J. Effect of long-term thermal exposure on microstructure and stress rupture properties of GH3535 superalloy. J. Mater. Sci. Technol. 2015, 31, 269–279. [Google Scholar] [CrossRef]
- Liu, T.; Dong, J.S.; Xie, G.; Wang, L.; Lou, L.H. Effect of silicon on microstructure and stress rupture properties of a corrosion resistant Ni-based superalloy during long term thermal exposure. Mater. Sci. Eng. A. 2016, 656, 75–83. [Google Scholar] [CrossRef]



| Element | Ni | Cr | Mo | Fe | Mn | Al | W | Si | C |
| Mass Fraction (wt.%) | Balance | 7.01 | 16.80 | 4.16 | 0.52 | 0.28 | 0.20 | 0.36 | 0.06 |
| Specimens | Corrosion Weight Loss (mg/mm2) |
|---|---|
| Hastelloy-N Alloy | 0.3164 ± 0.0002 |
| Ni-Coated Specimen | 0.1263 ± 0.0003 |
| Co-Coated Specimen | 0.1129 ± 0.0001 |
| Oxides | Reactions for Oxide Formation during the Laser Cladding Process | Gibbs Free Energy of Formation of Oxides | Reactions and Gibb’s Free Energy in 900 °C for Oxide Dissolution in the Molten Fluoride Salt |
|---|---|---|---|
| Cr2O3 | 2Cr + 1.5O2 = Cr2O3 | = −1056.62 + 0.25349T | Cr2O3 + 6HF = 2CrF3 + 3H2O, ΔG = −106.254 kJ/mol |
| NiO | Ni + 0.5O2 = NiO | = −210.04 + 0.08467T | NiO + 2HF = NiF2 + H2O, ΔG = −55.779 kJ/mol |
| CoO | Co + 0.5O2 = CoO | = −210.17 + 0.06485T | CoO + 2HF = CoF2 + H2O, ΔG = −68.379 kJ/mol |
| Particles | Element (at.%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| C | O | Ni | Cr | Si | Fe | Mo | Mn | Al | |
| 1 (Cr2O3) | 0.07 | 65.10 | 5.60 | 17.53 | 3.98 | – | – | 4.96 | 2.76 |
| 2 (NiO) | 0.05 | 52.60 | 29.49 | 2.01 | 7.84 | – | 0.72 | 0.72 | 6.60 |
| 3 (Cr2O3) | 0.06 | 66.22 | 2.52 | 23.42 | – | – | – | – | 7.77 |
| 4 (γ-Ni) | 0.08 | – | 95.16 | 1.90 | – | 1.81 | 1.05 | – | – |
| Particles | Element (at.%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | O | Co | Ni | Cr | Si | Fe | Mo | Mn | Al | |
| 1 (γ-Co) | 0.06 | – | 57.74 | 31.41 | 2.86 | – | 1.60 | 6.09 | – | – |
| 2 (γ-Co) | 0.15 | – | 58.58 | 31.43 | 3.28 | – | 1.97 | 4.59 | – | – |
| 3 (Cr2O3) | 0.06 | 68.89 | 2.81 | 1.05 | 11.72 | 9.83 | – | 0.42 | 3.57 | 1.65 |
| 4 | 0.04 | 61.98 | 4.85 | 2.40 | 4.98 | 16.03 | – | 0.81 | 5.34 | 3.56 |
| 5 (γ-Co) | 0.10 | – | 56.61 | 33.47 | 2.66 | – | 1.96 | 5.19 | – | – |
| 6 (Cr2O3) | 0.03 | 55.80 | 11.59 | 6.57 | 7.71 | 12.53 | 0.64 | 0.99 | 3.61 | 0.54 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, H.; Li, B.; Chen, M.; Qiu, C.; Tang, Z. Improvement of Corrosion Resistance of Hastelloy-N Alloy in LiF-NaF-KF Molten Salt by Laser Cladding Pure Metallic Coatings. Coatings 2018, 8, 322. https://doi.org/10.3390/coatings8090322
Zhu H, Li B, Chen M, Qiu C, Tang Z. Improvement of Corrosion Resistance of Hastelloy-N Alloy in LiF-NaF-KF Molten Salt by Laser Cladding Pure Metallic Coatings. Coatings. 2018; 8(9):322. https://doi.org/10.3390/coatings8090322
Chicago/Turabian StyleZhu, Hongmei, Baichun Li, Minghui Chen, Changjun Qiu, and Zhongfeng Tang. 2018. "Improvement of Corrosion Resistance of Hastelloy-N Alloy in LiF-NaF-KF Molten Salt by Laser Cladding Pure Metallic Coatings" Coatings 8, no. 9: 322. https://doi.org/10.3390/coatings8090322




