Nanochannelar Topography Positively Modulates Osteoblast Differentiation and Inhibits Osteoclastogenesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Preparation and Characterization
2.2. Cell Culture Model
2.3. Cell Morphology
2.4. Cell Viability and Proliferation
2.5. MC3T3-E1 Cell Differentiation
2.6. Osteoclast Differentiation
2.7. Statistical Analysis
3. Results and Discussion
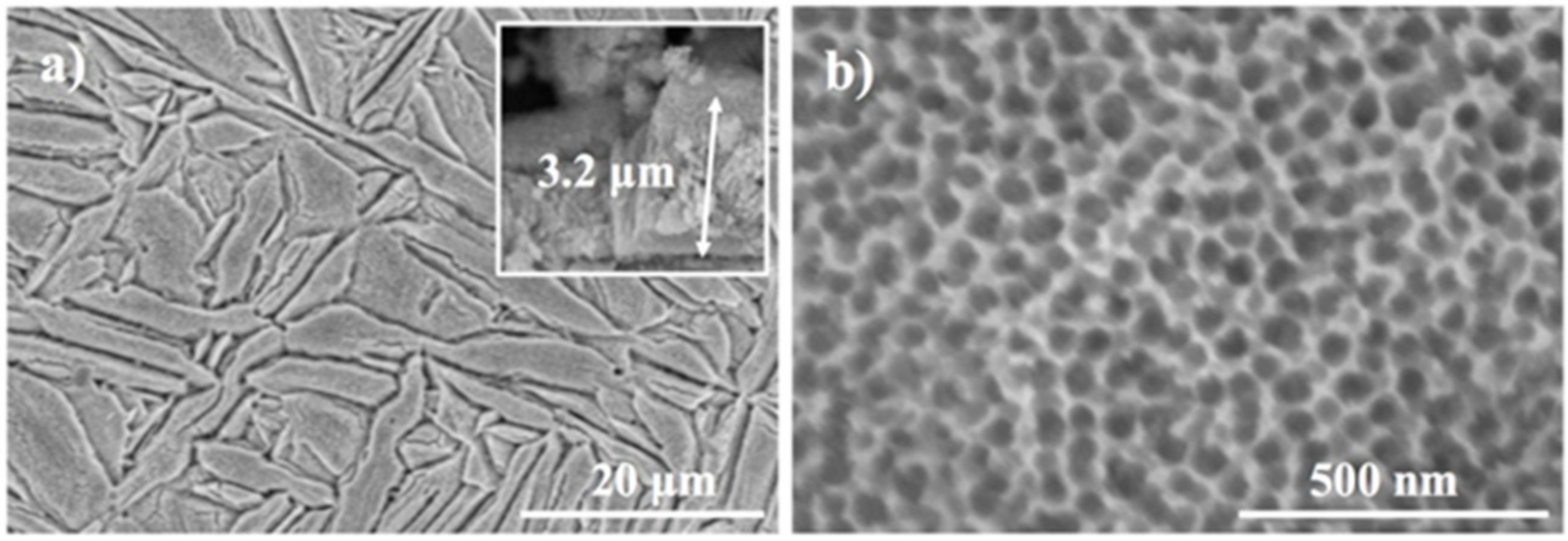
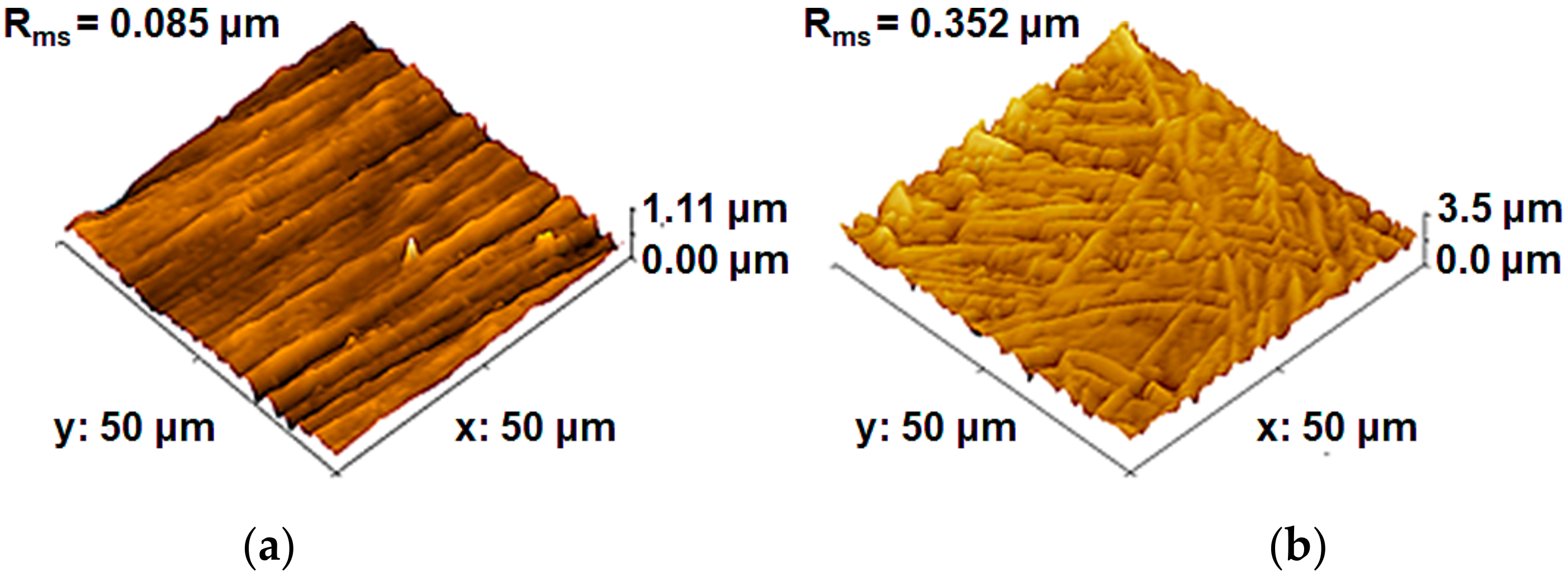
3.1. Materials Characterization
3.2. In Vitro Osteoblast Behavior
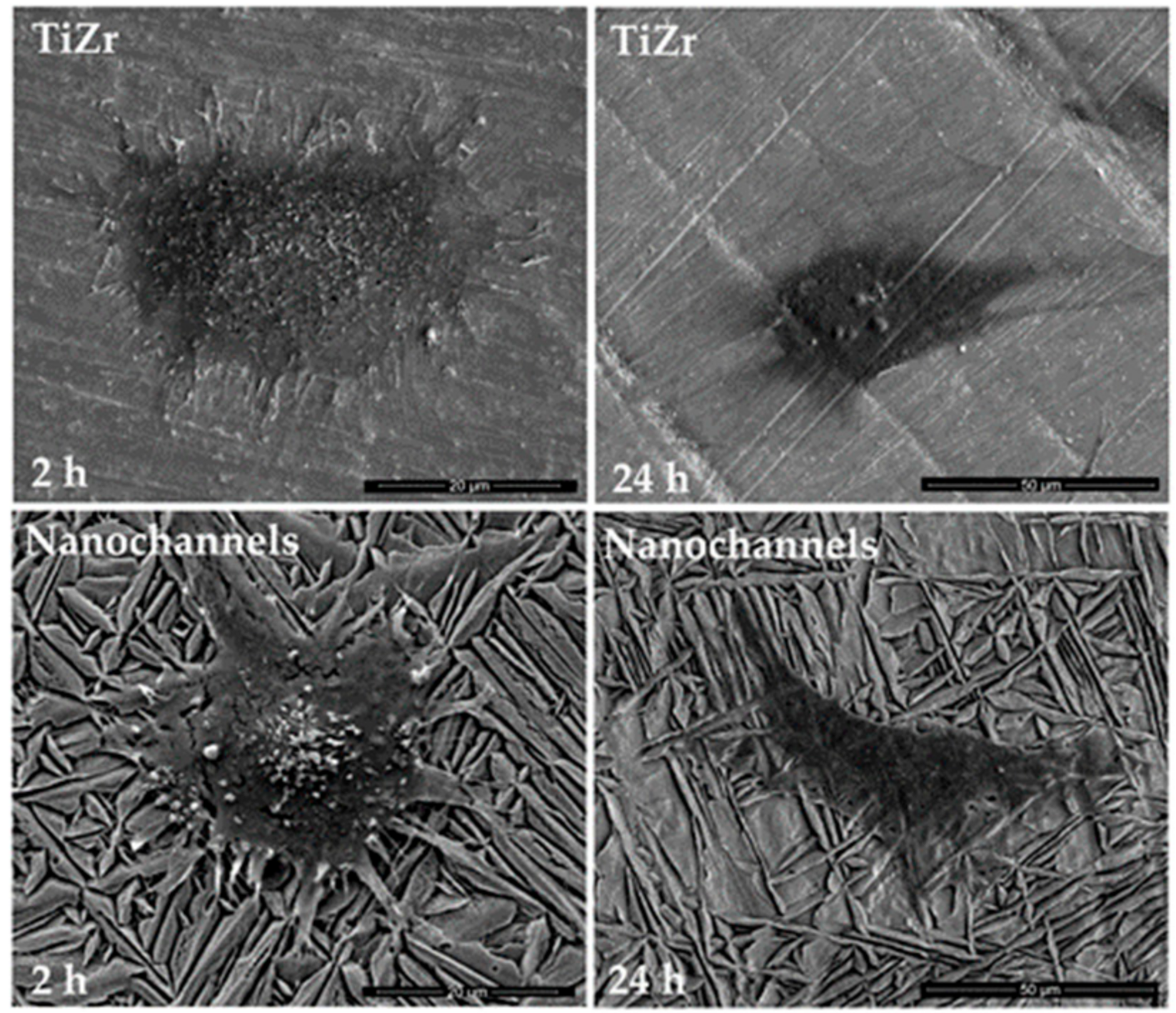
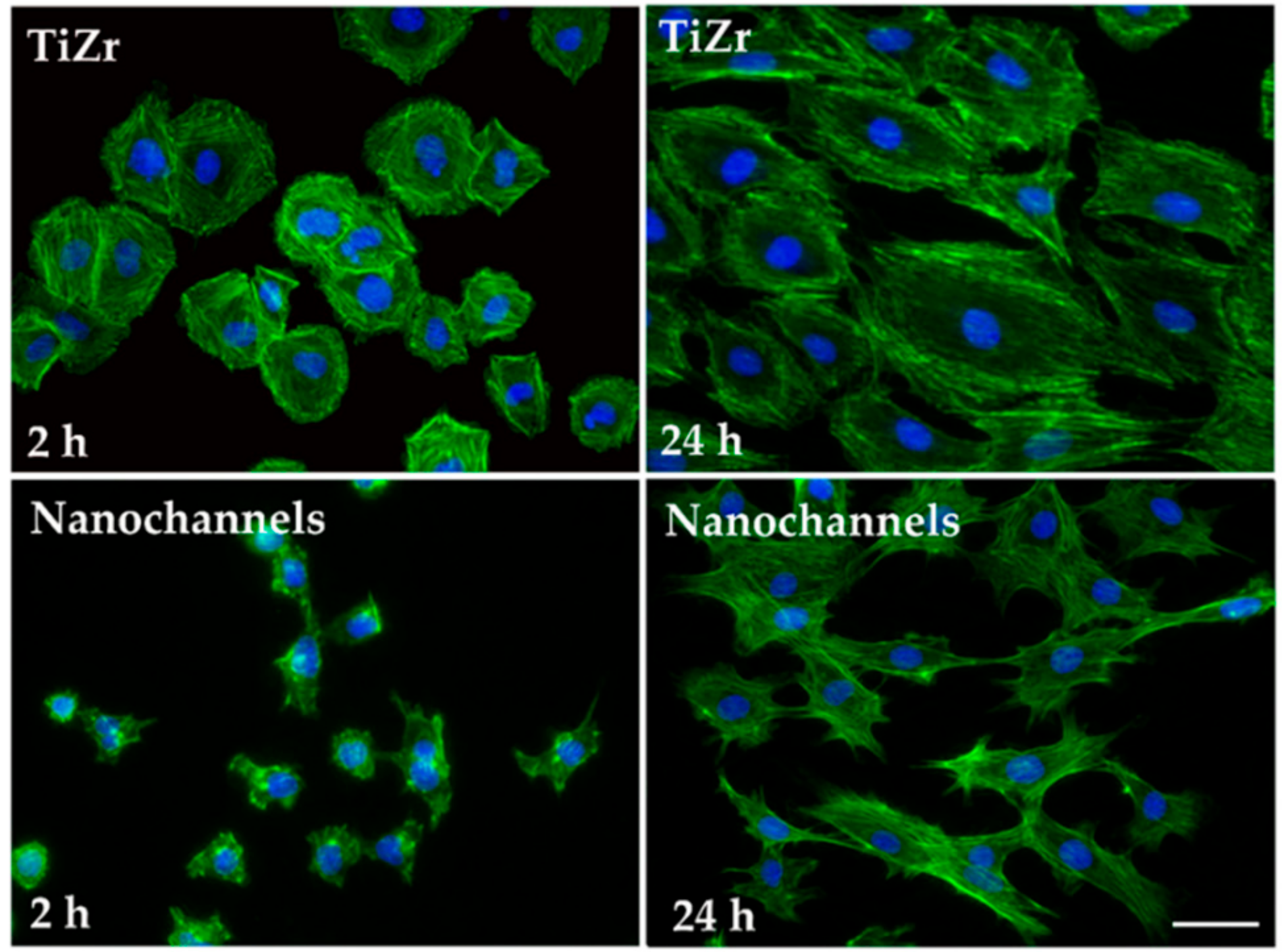
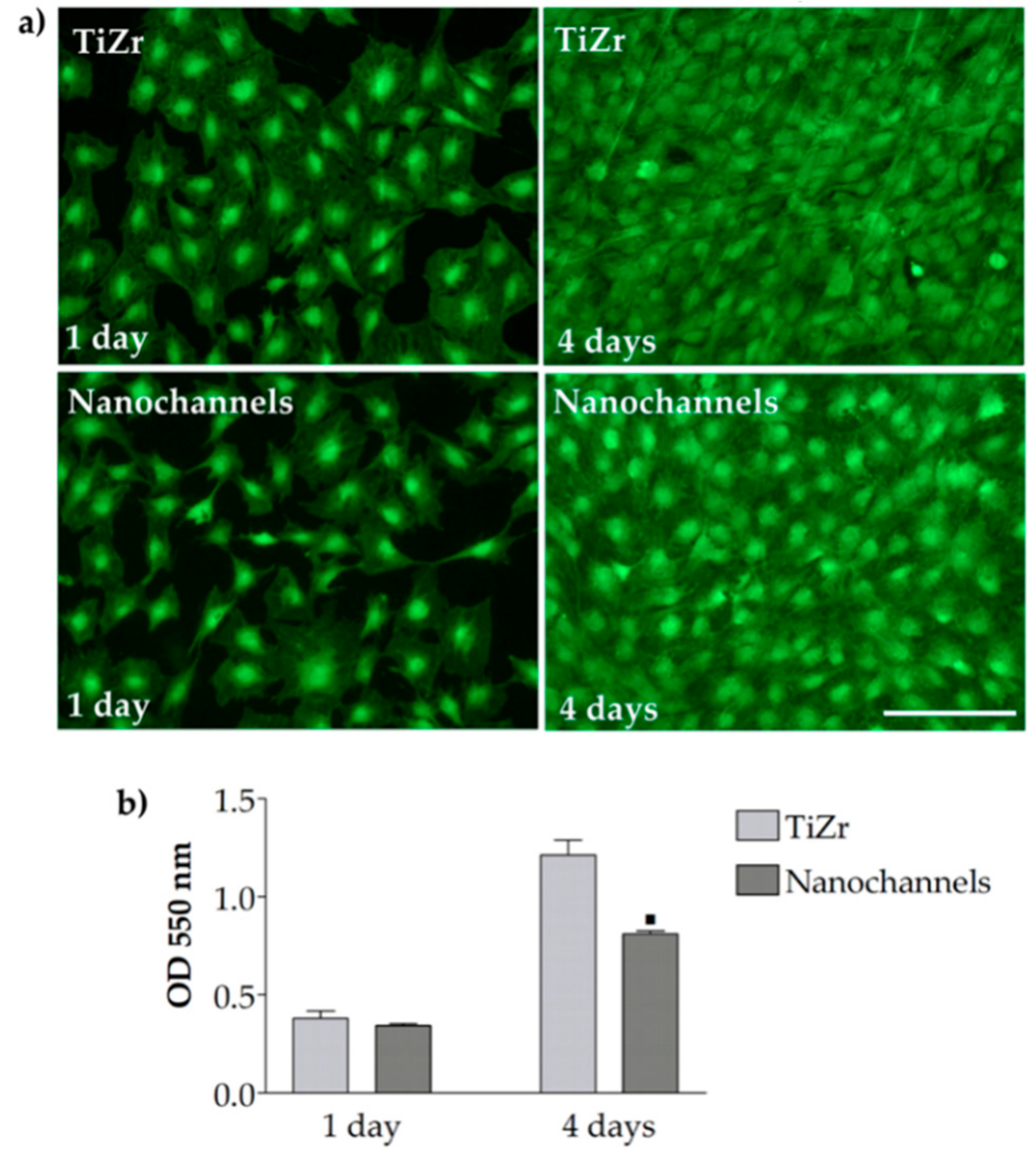
3.2.1. Cell Morphology
3.2.2. Cell Viability and Proliferation
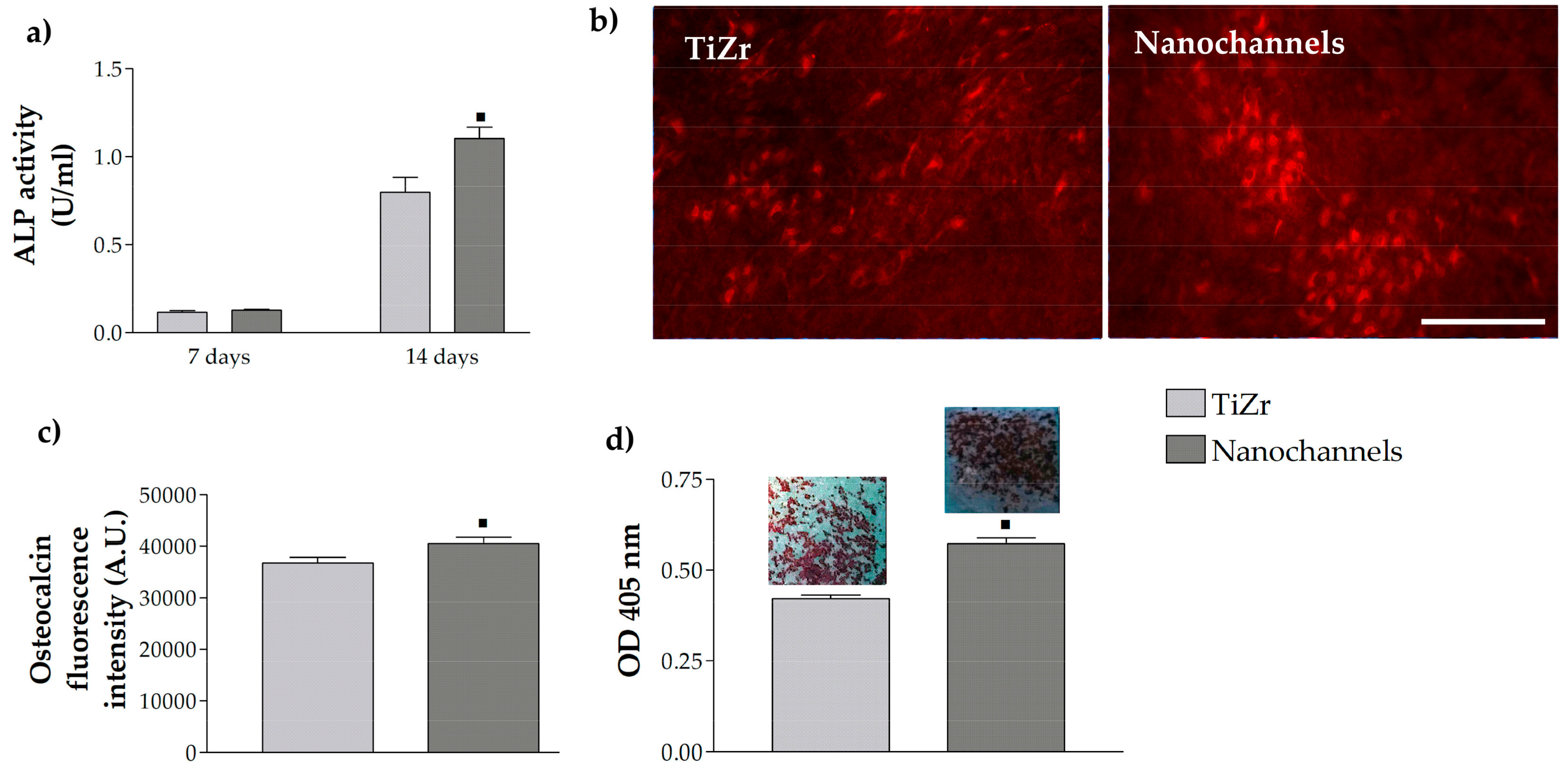
3.2.3. Pre-Osteoblast Cell Differentiation
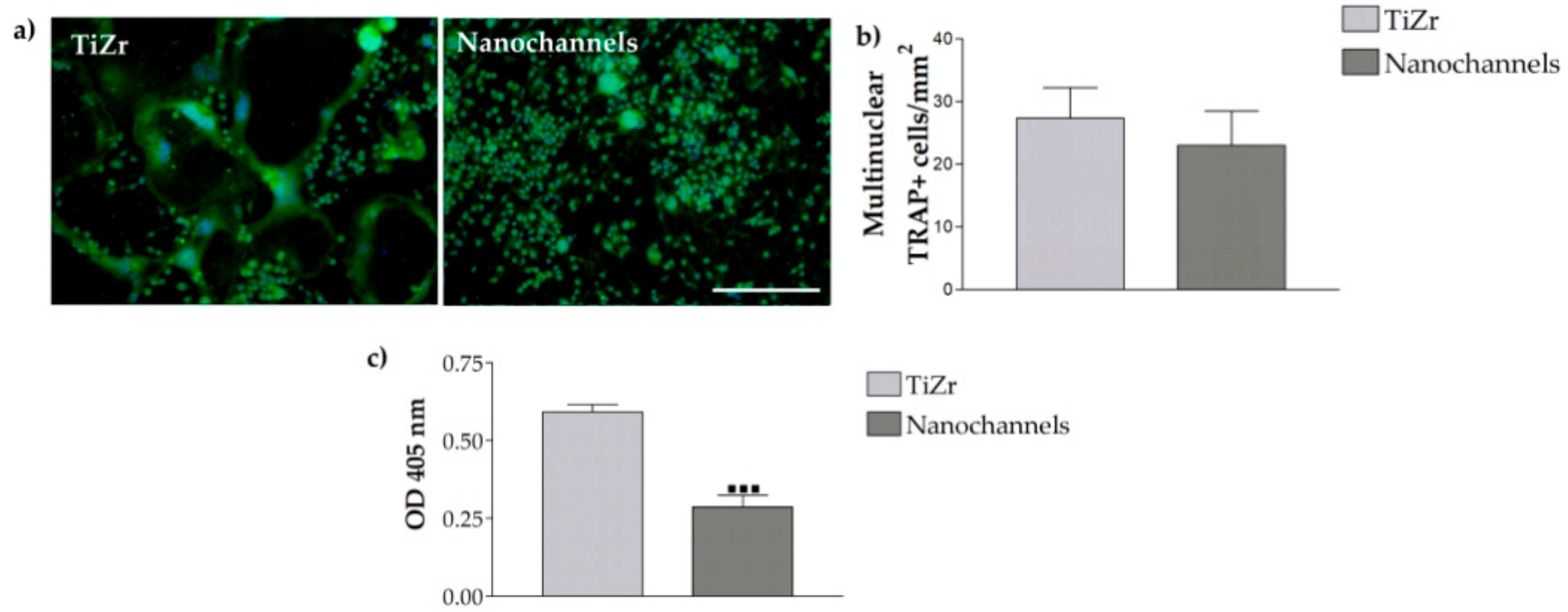
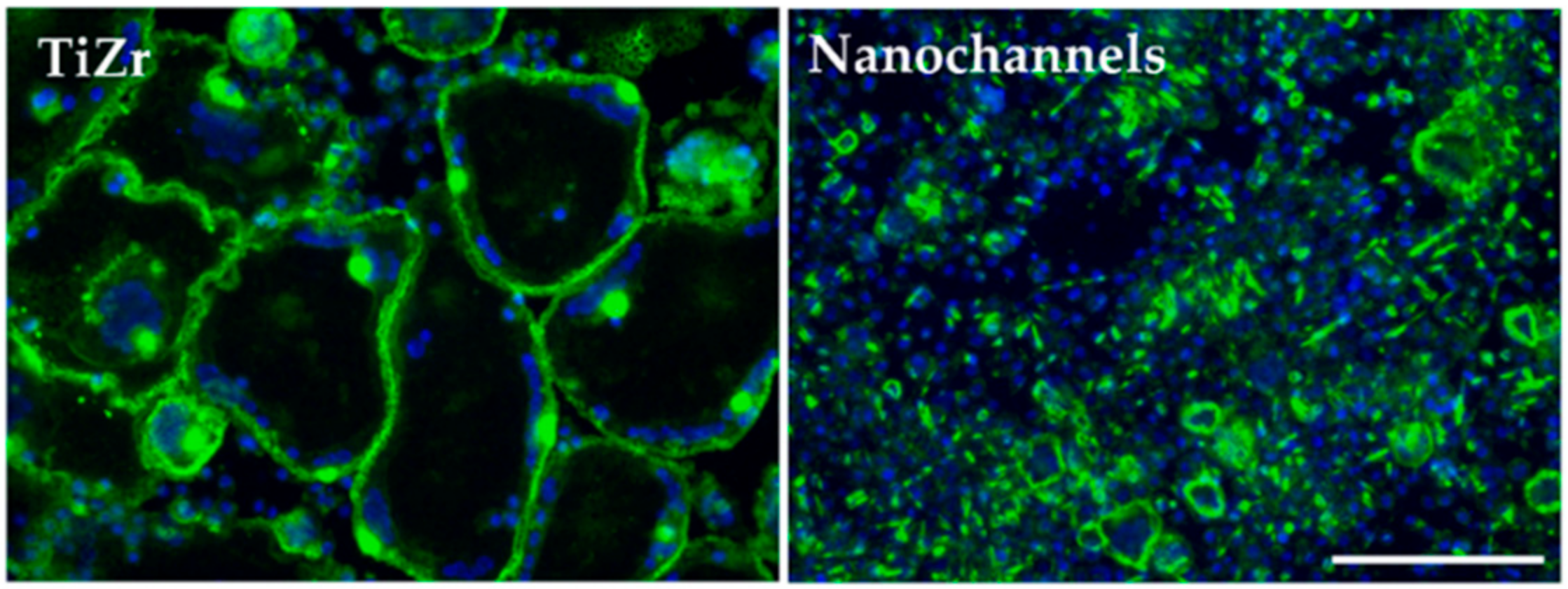
3.2.4. Osteoclast Differentiation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Karazisis, D.; Ballo, A.M.; Petronis, S.; Agheli, H.; Emanuelsson, L.; Thomsen, P.; Omar, O. The role of well-defined nanotopography of titanium implants on osseointegration: Cellular and molecular events in vivo. Int. J. Nanomed. 2016, 11, 1367–1382. [Google Scholar]
- Kulkarni, M.; Mazare, A.; Park, J.; Gongadze, E.; Killian, M.S.; Kralj, S.; von der Mark, K.; Iglic, A.; Schmuki, P. Protein interactions with layers of TiO2 nanotube and nanopore arrays: Morphology and surface charge influence. Acta Biomater. 2016, 45, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Luo, R.; Tan, J.; Wang, J.; Lu, X.; Qu, S.; Weng, J.; Feng, B. Osteoblast behaviors on titania nanotube and mesopore layers. Regen. Biomater. 2017, 4, 81–87. [Google Scholar] [CrossRef]
- Lv, L.; Liu, Y.; Zhang, P.; Zhang, X.; Liu, J.; Chen, T.; Su, P.; Li, H.; Zhou, Y. The nanoscale geometry of TiO2 nanotubes influences the osteogenic differentiation of human adipose-derived stem cells by modulating h3k4 trimethylation. Biomaterials 2014, 39, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Son, W.W.; Zhu, X.; Shin, H.I.; Ong, J.L.; Kim, K.H. In vivo histological response to anodized and anodized/hydrothermally treated titanium implants. J. Biomed. Mater. Res. Part B Appl. Biomater. 2003, 66, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Bauer, S.; Schlegel, K.A.; Neukam, F.W.; von der Mark, K.; Schmuki, P. TiO2 nanotube surfaces: 15 nm—An optimal length scale of surface topography for cell adhesion and differentiation. Small 2009, 5, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Bauer, S.; von der Mark, K.; Schmuki, P. Nanosize and vitality: TiO2 nanotube diameter directs cell fate. Nano Lett. 2007, 7, 1686–1691. [Google Scholar] [CrossRef] [PubMed]
- Su, E.P.; Justin, D.F.; Pratt, C.R.; Sarin, V.K.; Nguyen, V.S.; Oh, S.; Jin, S. Effects of titanium nanotubes on the osseointegration, cell differentiation, mineralisation and antibacterial properties of orthopaedic implant surfaces. Bone Jt. J. 2018, 100, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, L.M.; Brammer, K.S.; Johnston, G.W.; Chien, S.; Jin, S. Macrophage inflammatory response to TiO2 nanotube surfaces. J. Biomater. Nanotechnol. 2011, 2, 293–300. [Google Scholar] [CrossRef]
- Ma, Q.L.; Zhao, L.Z.; Liu, R.R.; Jin, B.Q.; Song, W.; Wang, Y.; Zhang, Y.S.; Chen, L.H.; Zhang, Y.M. Improved implant osseointegration of a nanostructured titanium surface via mediation of macrophage polarization. Biomaterials 2014, 35, 9853–9867. [Google Scholar] [CrossRef] [PubMed]
- Neacsu, P.; Mazare, A.; Cimpean, A.; Park, J.; Costache, M.; Schmuki, P.; Demetrescu, I. Reduced inflammatory activity of RAW 264.7 macrophages on titania nanotube modified Ti surface. Int. J. Biochem. Cell Biol. 2014, 55, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Feng, X.; Li, W.; Wang, L.-N.; Wang, X. Regulation of RAW 264.7 macrophages behavior on anodic TiO2 nanotubular arrays. Front. Mater. Sci. 2017, 11, 318–327. [Google Scholar] [CrossRef]
- Ercan, B.; Taylor, E.; Alpaslan, E.; Webster, T.J. Diameter of titanium nanotubes influences anti-bacterial efficacy. Nanotechnology 2011, 22, 295102. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, S.; Nguyen, N.T.; Mazare, A.; Hahn, R.; Cerri, I.; Schmuki, P. Fast growth of TiO2 nanotube arrays with controlled tube spacing based on a self-ordering process at two different scales. Electrochem. Commun. 2017, 77, 98–102. [Google Scholar] [CrossRef]
- Lu, H.; Fan, H.; Jin, R.; Chong, B.; Shen, X.; Yan, S.; Zhu, X. Formation and morphology evolution of anodic TiO2 nanotubes under negative pressure. Electrochim. Acta 2016, 215, 380–387. [Google Scholar] [CrossRef]
- Kapusta-Kołodziej, J.; Syrek, K.; Pawlik, A.; Jarosz, M.; Tynkevych, O.; Sulka, G.D. Effects of anodizing potential and temperature on the growth of anodic TiO2 and its photoelectrochemical properties. Appl. Surf. Sci. 2017, 396, 1119–1129. [Google Scholar] [CrossRef]
- Kowalski, D.; Kim, D.; Schmuki, P. TiO2 nanotubes, nanochannels and mesosponge: Self-organized formation and applications. Nano Today 2013, 8, 235–264. [Google Scholar] [CrossRef]
- Ion, R.; Stoian, A.B.; Dumitriu, C.; Grigorescu, S.; Mazare, A.; Cimpean, A.; Demetrescu, I.; Schmuki, P. Nanochannels formed on TiZr alloy improve biological response. Acta Biomater. 2015, 24, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Ion, R.; Vizireanu, S.; Stancu, C.E.; Luculescu, C.; Cimpean, A.; Dinescu, G. Surface plasma functionalization influences macrophage behavior on carbon nanowalls. Mater. Sci. Eng. C 2015, 48, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Ion, R.; Drob, S.I.; Ijaz, M.F.; Vasilescu, C.; Osiceanu, P.; Gordin, D.M.; Cimpean, A.; Gloriant, T. Surface characterization, corrosion resistance and in vitro biocompatibility of a new Ti-Hf-Mo-Sn alloy. Materials 2016, 9, 818. [Google Scholar] [CrossRef] [PubMed]
- Neacsu, P.; Staras, A.I.; Voicu, S.I.; Ionascu, I.; Soare, T.; Uzun, S.; Cojocaru, V.D.; Pandele, A.M.; Croitoru, S.M.; Miculescu, F. Characterization and in vitro and in vivo assessment of a novel cellulose acetate-coated Mg-based alloy for orthopedic applications. Materials 2017, 10, 686. [Google Scholar] [CrossRef] [PubMed]
- Mitran, V.; Dinca, V.; Ion, R.; Cojocaru, V.D.; Neacsu, P.; Dinu, C.Z.; Rusen, L.; Brajnicov, S.; Bonciu, A.; Dinescu, M.; et al. Graphene nanoplatelets-sericin surface-modified gum alloy for improved biological response. RSC Adv. 2018, 8, 18492–18501. [Google Scholar] [CrossRef]
- Ion, R.; Gordin, D.M.; Mitran, V.; Osiceanu, P.; Dinescu, S.; Gloriant, T.; Cimpean, A. In vitro bio-functional performances of the novel superelastic beta-type Ti–23Nb–0.7Ta–2Zr–0.5N alloy. Mater. Sci. Eng. C 2014, 35, 411–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owen, T.A.; Aronow, M.; Shalhoub, V.; Barone, L.M.; Wilming, L.; Tassinari, M.S.; Kennedy, M.B.; Pockwinse, S.; Lian, J.B.; Stein, G.S. Progressive development of the rat osteoblast phenotype in vitro: Reciprocal relationships in expression of genes associated with osteoblast proliferation and differentiation during formation of the bone extracellular matrix. J. Cell Physiol. 1990, 143, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Biggs, M.J.; Richards, R.G.; Gadegaard, N.; McMurray, R.J.; Affrossman, S.; Wilkinson, C.D.; Oreffo, R.O.; Dalby, M.J. Interactions with nanoscale topography: Adhesion quantification and signal transduction in cells of osteogenic and multipotent lineage. J. Biomed. Mater. Res. A 2008, 91, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Biggs, M.J.P.; Dalby, M.J. Focal adhesions in osteoneogenesis. Proc. Inst. Mech. Eng. H 2010, 224, 1441–1453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yim, E.K.F.; Darling, E.M.; Kulangara, K.; Guilak, F.; Leong, K.W. Nanotopography-induced changes in focal adhesions, cytoskeletal organization, and mechanical properties of human mesenchymal stem cells. Biomaterials 2010, 31, 1299–1306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.-H.; Provenzano, P.P.; Smith, C.L.; Levchenko, A. Matrix nanotopography as a regulator of cell function. J. Cell Biol. 2012, 197, 351–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, W.L.; McDevitt, T.C.; Engler, A.J. Materials as stem cell regulators. Nat. Mater. 2014, 13, 547–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abagnale, G.; Steger, M.; Nguyen, V.H.; Hersch, N.; Sechi, A.; Joussen, S.; Denecke, B.; Merkel, R.; Hoffmann, B.; Dreser, A.; et al. Surface topography enhances differentiation of mesenchymal stem cells towards osteogenic and adipogenic lineages. Biomaterials 2015, 61, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Shi, Y.; Cao, Y.; Liu, W. Recent progress in stem cell differentiation directed by material and mechanical cues. Biomed. Mater. 2016, 11, 014109. [Google Scholar] [CrossRef] [PubMed]
- Newman, P.; Galeano Niño, J.L.; Graney, P.; Razal, J.M.; Minett, A.I.; Ribas, J.; Ovalle-Robles, R.; Biro, M.; Zreiqat, H. Relationship between nanotopographical alignment and stem cell fate with live imaging and shape analysis. Sci. Rep. 2016, 6, 37909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isaac, J.; Galtayries, A.; Kizuki, T.; Kokubo, T.; Berda, A.; Sautier, J.M. Bioengineered titanium surfaces affect the gene-expression and phenotypic response of osteoprogenitor cells derived from mouse calvarial bones. Eur. Cells Mater. 2010, 20, 178–196. [Google Scholar] [CrossRef]
- Andrukhov, O.; Huber, R.; Shi, B.; Berner, S.; Rausch-Fan, X.; Moritz, A.; Spencer, N.D.; Schedle, A. Proliferation, behavior, and differentiation of osteoblasts on surfaces of different microroughness. Dent. Mater. 2016, 32, 1374–1384. [Google Scholar] [CrossRef] [PubMed]
- Jäger, M.; Urselmann, F.; Witte, F.; Zanger, K.; Li, X.; Ayers, D.C.; Krauspe, R. Osteoblast differentiation onto different biometals with an endoprosthetic surface topography in vitro. J. Biomed. Mater. Res. A 2007, 86, 61–75. [Google Scholar]
- Velasco-Ortega, E.; Alfonso-Rodríguez, C.A.; Monsalve-Guil, L.; España-López, A.; Jiménez-Guerra, A.; Garzón, I.; Alaminos, M.; Gil, F.J. Relevant aspects in the surface properties in titanium dental implants for the cellular viability. Mater. Sci. Eng. C 2016, 64, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dalby, M.J.; Gadegaard, N.; Tare, R.; Andar, A.; Riehle, M.O.; Herzyk, P.; Wilkinson, C.D.; Oreffo, R.O. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat. Mater. 2007, 6, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Brammer, K.S.; Li, Y.S.J.; Teng, D.; Engler, A.J.; Chien, S.; Jin, S. Stem cell fate dictated solely by altered nanotube dimension. Proc. Natl. Acad. Sci. USA 2009, 106, 2130–2135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavenus, S.; Pilet, P.; Guicheux, J.; Weiss, P.; Louarn, G.; Layrolle, P. Behaviour of mesenchymal stem cells, fibroblasts and osteoblasts on smooth surfaces. Acta Biomater. 2011, 7, 1525–1534. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.S.; Alonso, J.L.; Ostuni, E.; Whitesides, G.M.; Ingber, D.E. Cell shape provides global control of focal adhesion assembly. Biochem. Biophys. Res. Commun. 2003, 307, 355–361. [Google Scholar] [CrossRef]
- Pollard, T.D.; Cooper, J.A. Actin, a central player in cell shape and movement. Science 2009, 326, 1208–1212. [Google Scholar] [CrossRef] [PubMed]
- Mendez, M.G.; Kojima, S.-I.; Goldman, R.D. Vimentin induces changes in cell shape, motility, and adhesion during the epithelial to mesenchymal transition. FASEB J. 2010, 24, 1838–1851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damania, D.; Subramanian, H.; Tiwari, A.K.; Stypula, Y.; Kunte, D.; Pradhan, P.; Roy, H.K.; Backman, V. Role of cytoskeleton in controlling the disorder strength of cellular nanoscale architecture. Biophys. J. 2010, 99, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Rudolf, E.; Cervinka, M.; Cerman, J.; Schroterova, L. Hexavalent chromium disrupts the actin cytoskeleton and induces mitochondria-dependent apoptosis in human dermal fibroblasts. Toxicol. In Vitro 2005, 19, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Vetterkind, S.; Illenberger, S.; Kubicek, J.; Boosen, M.; Appel, S.; Naim, H.Y.; Scheidtmann, K.-H.; Preuss, U. Binding of par-4 to the actin cytoskeleton is essential for Par-4/Dlk-mediated apoptosis. Exp. Cell Res. 2005, 305, 392–408. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, C.; Nakamura, N.; Yoshikawa, H.; Itoh, K. Transient dynamic actin cytoskeletal change stimulates the osteoblastic differentiation. J. Bone Miner. Metab. 2009, 27, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Kirstein, B.; Chambers, T.J.; Fuller, K. Secretion of tartrate-resistant acid phosphatase by osteoclasts correlates with resorptive behavior. J. Cell. Biochem. 2006, 98, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Halleen, J.M.; Alatalo, S.L.; Suominen, H.; Cheng, S.; Janckila, A.J.; Vaananen, H.K. Tartrate-resistant acid phosphatase 5b: A novel serum marker of bone resorption. J. Bone Miner. Res. 2000, 15, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Takito, J.; Otsuka, H.; Yanagisawa, N.; Arai, H.; Shiga, M.; Inoue, M.; Nonaka, N.; Nakamura, M. Regulation of osteoclast multinucleation by the actin cytoskeleton signaling network. J. Cell. Physiol. 2014, 230, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, F.; Zhang, C.; Gao, B.; Tan, P.; Mi, B.; Zhang, Y.; Cheng, H.; Liao, H.; Huo, K.; et al. The dimension of titania nanotubes influences implant success for osteoclastogenesis and osteogenesis patients. J. Nanosci. Nanotechnol. 2015, 15, 4136–4142. [Google Scholar] [CrossRef] [PubMed]
- Silverwood, R.K.; Fairhurst, P.G.; Sjöström, T.; Welsh, F.; Sun, Y.; Li, G.; Yu, B.; Young, P.S.; Su, B.; Meek, R.D.M.; et al. Analysis of osteoclastogenesis/osteoblastogenesis on nanotopographicaltitania surfaces. Adv. Healthc. Mater. 2016, 5, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Sommer, B.; Felix, R.; Sprecher, C.; Leunig, M.; Ganz, R.; Hofstetter, W. Wear particles and surface topographies are modulators of osteoclastogenesis in vitro. J. Biomed. Mater. Res. A 2004, 72, 67–76. [Google Scholar]
- Brinkmann, J.; Hefti, T.; Schlottig, F.; Spencer, N.D.; Hall, H. Response of osteoclasts to titanium surfaces with increasing surface roughness: An in vitro study. Biointerphases 2012, 7, 34. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ion, R.; Mazare, A.; Dumitriu, C.; Pirvu, C.; Schmuki, P.; Cimpean, A. Nanochannelar Topography Positively Modulates Osteoblast Differentiation and Inhibits Osteoclastogenesis. Coatings 2018, 8, 294. https://doi.org/10.3390/coatings8090294
Ion R, Mazare A, Dumitriu C, Pirvu C, Schmuki P, Cimpean A. Nanochannelar Topography Positively Modulates Osteoblast Differentiation and Inhibits Osteoclastogenesis. Coatings. 2018; 8(9):294. https://doi.org/10.3390/coatings8090294
Chicago/Turabian StyleIon, Raluca, Anca Mazare, Cristina Dumitriu, Cristian Pirvu, Patrick Schmuki, and Anisoara Cimpean. 2018. "Nanochannelar Topography Positively Modulates Osteoblast Differentiation and Inhibits Osteoclastogenesis" Coatings 8, no. 9: 294. https://doi.org/10.3390/coatings8090294





