Application of Oxovanadium Complex Stabilized by N,N,N,N-Chelating Ligand in Air-Drying Paints
Abstract
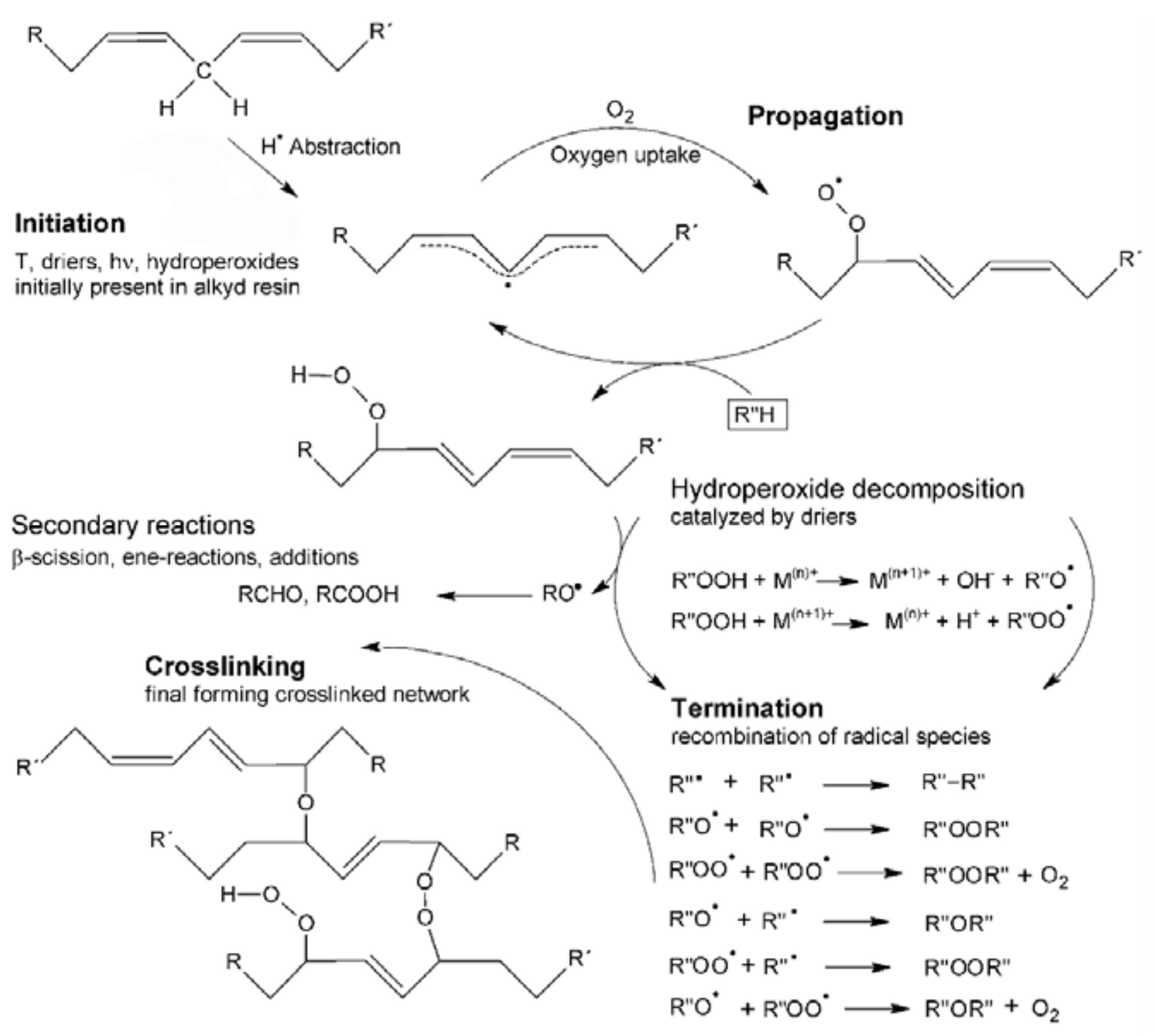
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Film Preparation
2.3. Film Drying Time
2.4. Determination of Film Hardness
2.5. FTIR Spectroscopy
2.6. EPR Spectroscopy
3. Results and Discussions
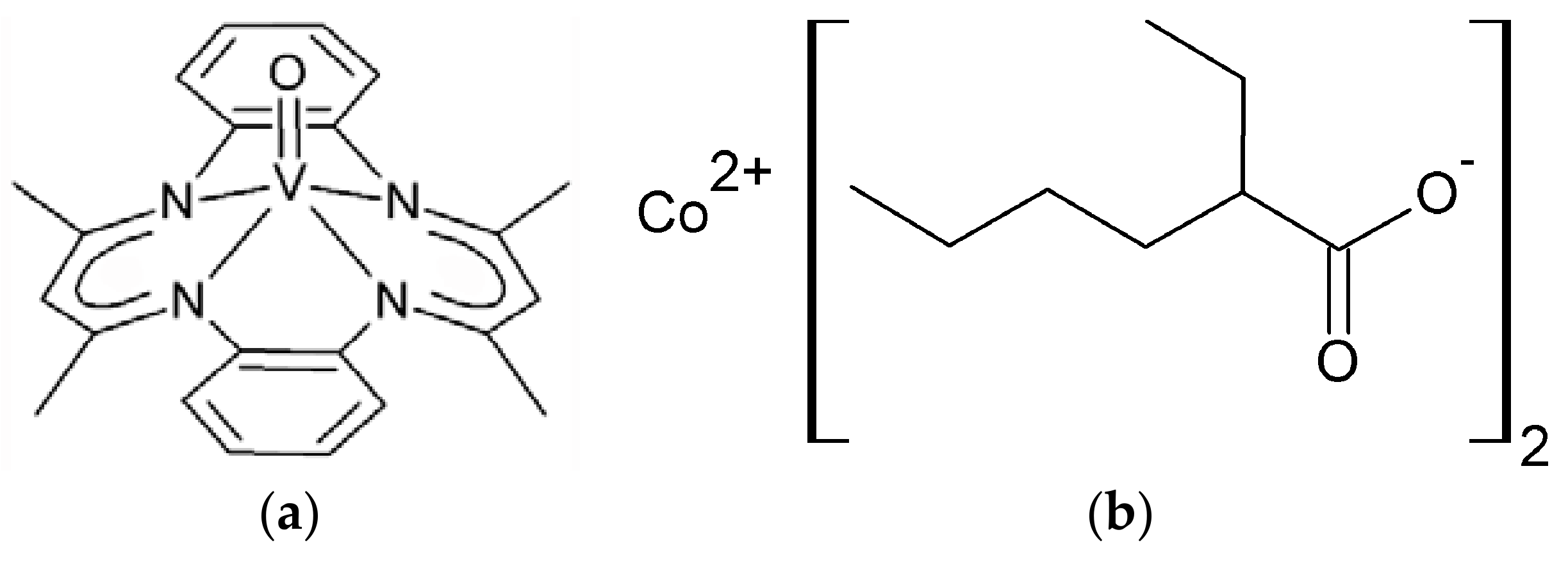
3.1. Synthesis and Characterization
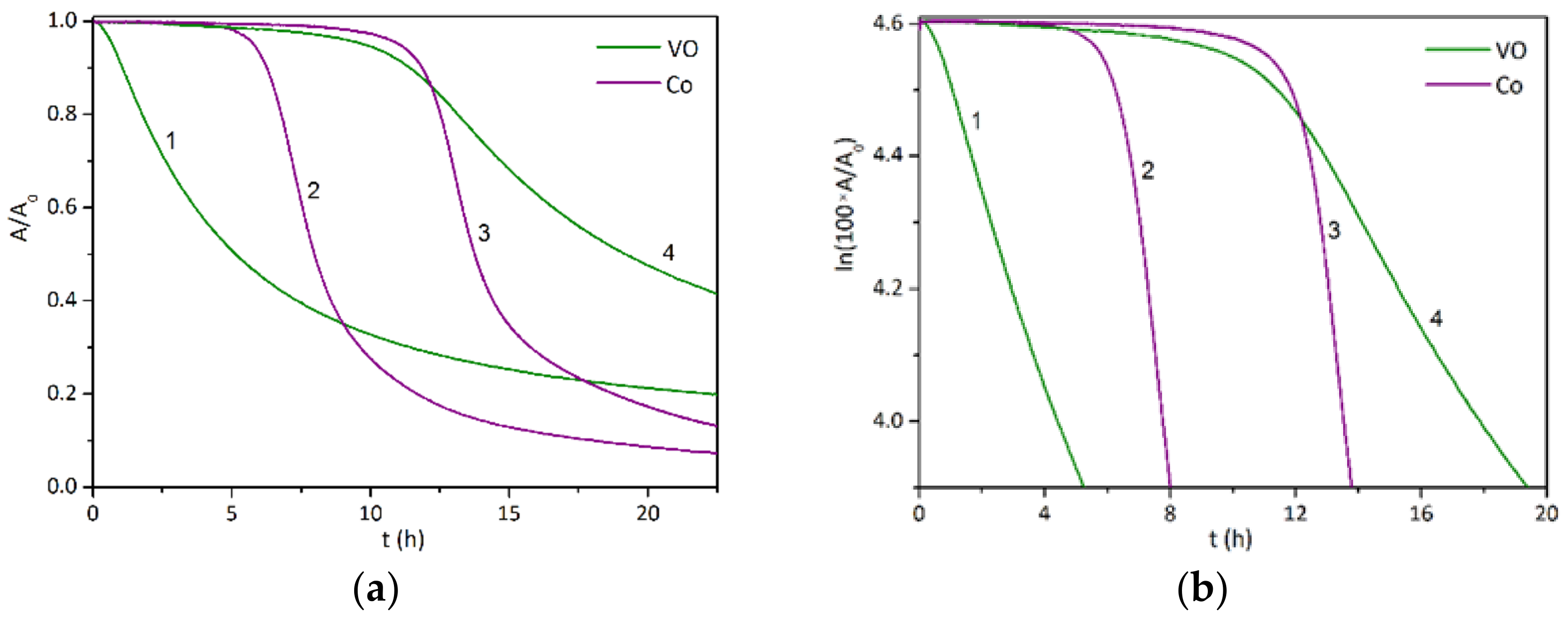
3.2. Drying Activity
3.3. Time-Resolved FTIR Spectroscopy
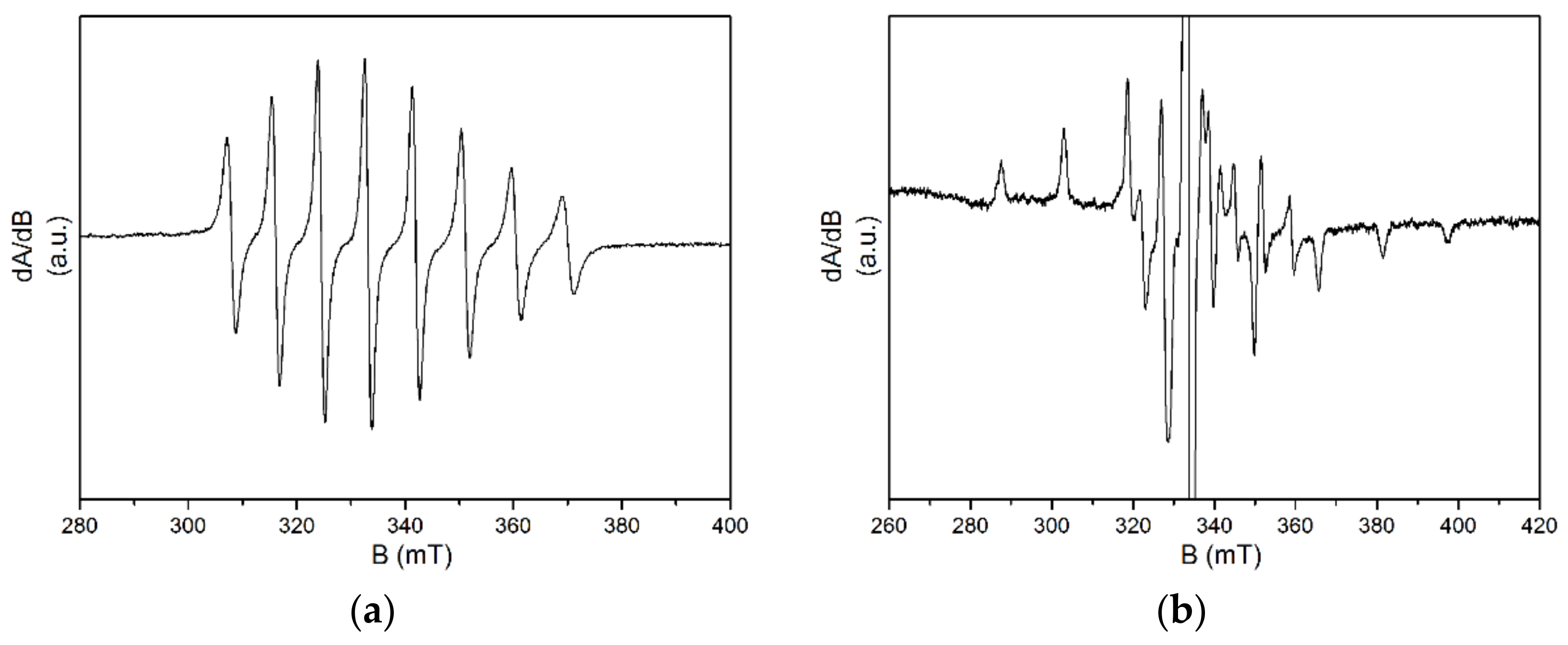
3.4. EPR Spectra of Cured Films
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jones, F.N. Alkyd resins. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012; pp. 429–444. [Google Scholar] [CrossRef]
- Soucek, M.D.; Khattab, T.; Wu, J. Review of autoxidation and driers. Prog. Org. Coat. 2012, 73, 435–454. [Google Scholar] [CrossRef]
- Hofland, A. Alkyd resins: From down and out to alive and kicking. Prog. Org. Coat. 2012, 73, 274–282. [Google Scholar] [CrossRef]
- Deffar, D.; Soucek, M.D. Synergistic effect of driers on soybean oil-based ceramer coatings. J. Coat. Technol. 2001, 73, 95–104. [Google Scholar] [CrossRef]
- Van Gorkum, R.; Bouwman, E. The oxidative drying of alkyd paint catalysed by metal complexes. Coord. Chem. Rev. 2005, 249, 1709–1728. [Google Scholar] [CrossRef]
- Hubert, J.C.; Venderbosch, R.A.M.; Muizebelt, W.J.; Klaasen, R.P.; Zabel, K.H. Mechanistic study of drying of alkyd resins using (Z,Z)- and (E,E)-3,6-nonadiene as model substances. Prog. Org. Coat. 1997, 31, 331–340. [Google Scholar] [CrossRef]
- Erben, M.; Veselý, D.; Vinklárek, J.; Honzíček, J. Acyl-substituted ferrocenes as driers for solvent-borne alkyd paints. J. Mol. Catal. A Chem. 2012, 353–354, 13–21. [Google Scholar] [CrossRef]
- Muizebelt, W.J.; Hubert, J.C.; Venderbosch, R.A.M. Mechanistic study of drying of alkyd resins using ethyl linoleate as a model substance. Prog. Org. Coat. 1994, 24, 263–279. [Google Scholar] [CrossRef]
- De Boer, J.W.; Wesenhagen, P.V.; Wenker, E.C.M.; Maaijen, K.; Gol, F.; Gibbs, H.; Hage, R. The quest for cobalt-free alkyd paint driers. Eur. J. Inorg. Chem. 2013, 21, 3581–3591. [Google Scholar] [CrossRef]
- Dubrulle, L.; Lebeuf, R.; Thomas, L.; Fressancourt-Collinet, M.; Nardello-Rataj, V. Catalytic activity of primary and secondary driers towards the oxidation and hydroperoxide decomposition steps for the chemical drying of alkyd resin. Prog. Org. Coat. 2017, 104, 141–151. [Google Scholar] [CrossRef]
- Clennan, E.L.; Pace, A. Advances in singlet oxygen chemistry. Tetrahedron 2005, 61, 6665–6691. [Google Scholar] [CrossRef]
- Erich, S.J.F.; Gezici-Koç, Ö.; Michel, M-E.B.; Thomas, C.A.A.M.; van der Ven, L.G.J.; Huinink, H.P.; Flapper, J.; Duivenvoorde, F.L.; Adan, O.C.G. The influence of calcium and zirconium based secondary driers on drying solvent borne alkyd coatings. Polymer 2017, 121, 262–273. [Google Scholar] [CrossRef]
- Lison, D.; De Boeck, M.; Verougstraete, V.; Kirsch-Volders, M. Update on the genotoxicity and carcinogenicity of cobalt compounds. Occup. Environ. Med. 2001, 58, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Magaye, R.; Zhao, J.; Bowman, L.; Ding, M. Genotoxicity and carcinogenicity of cobalt-, nickel- and copper-based nanoparticles. Exp. Ther. Med. 2012, 4, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Sosa, A.D.; Echeverría, M.D. Surface alterations produced in grinding of austempered ductile iron. Procedia. Mater. Sci. 2015, 8, 155–161. [Google Scholar] [CrossRef]
- Bouwman, E.; van Gorkum, R. A study of new manganese complexes as potential driers for alkyd paints. J. Coat. Technol. Res. 2007, 4, 491–503. [Google Scholar] [CrossRef]
- Oyman, Z.O.; Minga, W.; van der Linde, R.; van Gorkum, R.; Bouwman, E. Effect of [Mn(acac)3] and its combination with 2,2’-bipyridine on the autoxidation and oligomerisation of ethyl linoleate. Polymer 2005, 46, 1731–1738. [Google Scholar] [CrossRef]
- Warzeska, S.T.; Zonneveld, M.; van Gorkum, R.; Muizebelt, W.J.; Bouwman, E.; Reedijk, J. The influence of bipyridine on the drying of alkyd paints: a model study. Prog. Org. Coat. 2002, 44, 243–248. [Google Scholar] [CrossRef]
- Honzíček, J.; Vinklárek, J. Chemical curing of alkyd resin catalyzed by benzoylferrocene: Performance, kinetics, and thickness effects. J. Appl. Polym. Sci. 2018, 135, 46184. [Google Scholar] [CrossRef]
- Miccichè, F.; Oostveen, E.; van Haveren, J.; van der Linde, R. The combination of reducing agents/iron as environmentally friendlier alternatives for Co-based driers in the drying of alkyd paints. Prog. Org. Coat. 2005, 53, 99–105. [Google Scholar] [CrossRef]
- Pirš, B.; Znoj, B.; Skale, S.; Zabret, J.; Godnjavec, J.; Venturini, P. Iron as an alternative drier for curing of high-solid alkyd coatings. J. Coat. Technol. Res. 2015, 12, 965–974. [Google Scholar] [CrossRef]
- Křižan, M.; Vinklárek, J.; Erben, M.; Císařová, I.; Honzíček, J. Autoxidation of alkyd resins catalyzed by iron(II) bispidine complex: Drying performance and in-depth infrared study. Prog. Org. Coat. 2017, 111, 361–370. [Google Scholar] [CrossRef]
- Ha, D.; Joo, H.; Ahn, G.; Kim, M. J.; Bing, S.J.; An, S.; Kim, H.; Kang, K.-G.; Lim, Y.-K.; Jee, Y. Jeju ground water containing vanadium induced immune activation on splenocytes of low dose γ-rays-irradiated mice. Food Chem. Toxicol. 2012, 50, 2097–2105. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A. K. Anti-diabetic and toxic effects of vanadium compounds. Mol. Cell. Biochem. 2000, 206, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Preininger, O.; Vinklárek, J.; Honzíček, J.; Mikysek, T.; Erben, M. A promising drying activity of environmentally friendly oxovanadium(IV) complexes in air-drying paints. Prog. Org. Coat. 2015, 88, 191–198. [Google Scholar] [CrossRef]
- Preininger, O.; Charamzová, I.; Vinklárek, J.; Císařová, I.; Honzíček, J. Oxovanadium(IV) complexes bearing substituted pentane-2,4-dionate ligands: Synthesis, structure and drying activity in solvent-borne alkyd paints. Inorg. Chim. Acta 2017, 462, 16–22. [Google Scholar] [CrossRef]
- Preininger, O.; Honzíček, J.; Kalenda, P.; Vinklárek, J. Drying activity of oxovanadium(IV) 2-ethylhexanoate in solvent-borne alkyd paints. J. Coat. Technol. Res. 2016, 13, 479–487. [Google Scholar] [CrossRef]
- Lee, S.; Floriani, C.; Chiesi-Villa, A.; Guastini, C. Oxovanadium(IV) and dioxomolybdenum(VI) complexes: Synthesis from the corresponding acetylacetonato complexes and X-ray structures. J. Chem. Soc. Dalton Trans. 1989, 145–149. [Google Scholar] [CrossRef]
- ASTM International. ASTM D5895-03 Standard Test Methods for Evaluating Drying or Curing During Film Formation of Organic Coating Using Mechanical Recorders; ASTM International: West Conshohocken, PA, USA, 2008. [Google Scholar]
- International Organization for Standardization. ISO 1522:2006 Paints and Varnishes—Pendulum Damping Test; International Organization for Standardization: Geneva, Switzerland, 2007. [Google Scholar]
- Niewahner, J.H.; Walters, A.K.; Wagner, A. Improved synthesis of Geodken’s macrocycle through the synthesis of the dichloride salt. J. Chem. Educ. 2007, 84, 477–479. [Google Scholar] [CrossRef]
- Wu, X.; Huang, T.; Lekich, T.T.; Sommer, R.D.; Weare, W.W. Synthesis of unsupported d1 − dx oxido-bridged heterobimetallic complexes containing VIV: A new direction for metal-to-metal charge transfer. Inorg. Chem. 2015, 54, 5322–5328. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.S., II; LoBrutto, R.; Pecoraro, V.L. Paramagnetic spectroscopy of vanadyl complexes and its applications to biological systems. Coord. Chem. Rev. 2002, 228, 1–18. [Google Scholar] [CrossRef]
- Schumann, H. Zur Redoxchemie und Struktur von Oxovanadium(IV,V)-Komplexen mit substituierten Dibenzotetraaza[14]annulen Liganden. Z. Naturforsch 1995, 50b, 1494–1504. (In German) [Google Scholar]

| Conc. (wt %) | Drier/Alkyd | τ2 a (h) | τ3 b (h) | Hrel,10c (%) | Hrel,100d (%) | Drier/Alkyd | τ2 a (h) | τ3 b (h) | Hrel,10c (%) | Hrel,100d (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.1 | VO/S401 | 0.6 | 6.2 | 32.1 | 58.0 | VO/S471 | 4.5 | 8.1 | 23.9 | 53.1 |
| 0.06 | 1.5 | 6.5 | 22.2 | 50.8 | 5.7 | 9.3 | 19.5 | 53.7 | ||
| 0.03 | 1.5 | 10.8 | 29.8 | 55.4 | 5.8 | 10.6 | 21.1 | 52.4 | ||
| 0.01 | 2.3 | 12.3 | 32.1 | 54.0 | 7.7 | 17.7 | 20.5 | 51.4 | ||
| 0.005 | 2.7 | 16.9 | 34.1 | 53.7 | 13.8 | >24 | 35.7 | 50.6 | ||
| 0.1 | Co/S401 | 5.5 | 7.6 | 25.9 | 61.2 | Co/S471 | 5.2 | 6.7 | 20.7 | 54.1 |
| 0.06 | 2.7 | 6.9 | 35.3 | 58.5 | 7.7 | 9.5 | 20.6 | 53.7 | ||
| 0.03 | 5.5 | 15.4 | 35.8 | 57.4 | 19.2 | >24 | 22.4 | 49.8 | ||
| 0 | S401 | >24 | >24 | 6.8 | 41.9 | S471 | >24 | >24 | 2.4 | 33.8 |
| Formulation | IT (h) | –kCH,max (h−1) | tconj (h) |
|---|---|---|---|
| VO/S401 | – | 0.17 | 9.1 |
| Co/S401 | 6.2 | 0.43 | 10.5 |
| VO/S471 | 10.5 | 0.09 | 26.6 |
| Co/S471 | 12.0 | 0.43 | 15.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charamzová, I.; Vinklárek, J.; Kalenda, P.; Honzíček, J. Application of Oxovanadium Complex Stabilized by N,N,N,N-Chelating Ligand in Air-Drying Paints. Coatings 2018, 8, 204. https://doi.org/10.3390/coatings8060204
Charamzová I, Vinklárek J, Kalenda P, Honzíček J. Application of Oxovanadium Complex Stabilized by N,N,N,N-Chelating Ligand in Air-Drying Paints. Coatings. 2018; 8(6):204. https://doi.org/10.3390/coatings8060204
Chicago/Turabian StyleCharamzová, Iva, Jaromír Vinklárek, Petr Kalenda, and Jan Honzíček. 2018. "Application of Oxovanadium Complex Stabilized by N,N,N,N-Chelating Ligand in Air-Drying Paints" Coatings 8, no. 6: 204. https://doi.org/10.3390/coatings8060204





