3.2. Raman Spectroscopy and PCA Model
The fingerprint region of Raman spectra for different SMI/oil nanoparticle coatings on paper is illustrated in
Figure 3 (see also
Table 1 for band assignments in
Supplementary Materials). For clarity of presentation, only the spectra of the thick coatings are represented, while the thin coatings give similar results within the fingerprint region (of course, with higher intensity of substrate-related cellulose bands).
The characteristic Raman bands for coating moieties (styrene, imide, and oil) and substrate (cellulose fibers) are clearly separated and allow for accurate characterization. The spectra have been normalized by integration of the region corresponding to the cellulose bands at 1170 to 1050 cm
−1. The C=O ester bands for imide (1765 cm
−1) and oil (1750 cm
−1) are clearly resolved as distinct peaks. The band at 1655 cm
−1 represents cis conformation (C=C) of the fatty acids and naturally occurs in vegetable oils, in contrast with the trans-isomers at around 1680–1670 cm-
−1 in synthetic systems [
23]. For fatty acids with the same chain length, the intensity at 1655 cm
−1 increases in parallel with the degree of unsaturation [
24], as follows: HCO (saturated fatty acids obtained after hydrogenation); SfO, CaO, RO (monounsaturated fatty acids); CO, SO (polyunsaturated fatty acids).
The imide content and amount of free oil can be quantified from the ratios of integrated Raman band areas of imide I (1765 cm
−1), oil (1655 cm
−1) and styrene (1602 cm
−1), after calibration with a fully imidized pure SMI coating (no oil), as calculated in
Table 2. Based on the SMA grade with 26 mol % maleic anhydride, the calculated imide content should be compared to a theoretical maximum imide content (relative to styrene parts) of 35%. Depending on the oil type, the imide content is lower than the theoretical maximum, which can be explained by the interference of oil with the imidization reaction (reactive C=C oil) and consequently remaining ammonolyzed maleic anhydride. A progressively higher amount of unreacted or ‘free’ oil is present in the coating as the imide content lowers: the amount of free oil is low for polyunsaturated oil (SMI/SO, SMI/CO), intermediate for monounsaturated oils (SMI/RO, SMI/SfO, SMI/CaO), and extremely high for saturated oils (SMI/HCO).
A model for the classification of the paper coatings based on principal component analysis (PCA) of the Raman spectra is presented in
Figure 4. The analysis is used to define parameters that describe the variation in Raman spectra between the different coating types and illustrate major trends. The number of principal components (PC) included in the model is selected from a scree plot (
Figure 4a). The variation in data points is most effectively described by a model with nearly zero residual variance and as few PCs as possible: the three principal components account for 97% of total variation (PC-1: 78%, PC-2: 12%, and PC-3: 7%), and the residual variance is very small for the higher-order components. The calibration model was evaluated by a leverage and residual analysis against PC-1, PC-2, and PC-3 to detect eventual outlier samples (
Figure 4b). The samples with higher leverage are positioned further away from the mean value and have different characteristics: e.g., the leverage variation between the coating samples is somewhat larger for PC-1, while the leverage for PC-2 and PC-3 is almost the same. PC-1 consequently takes into account differences in oil type, discriminating between polyunsaturated, monounsaturated, and saturated oil. Only samples with high leverage and high residues would damage the model and should be removed from the model: as none of the samples simultaneously indicate high leverage and high residues, all samples fit into the model and each sample adds novel information to the model. The meaning of the different components in relation to the original Raman spectral bands can be recognized form the loading for individual PCs (
Figure 4c): (i) the PC-1 (82% of the variation) includes specific characteristics related to the oil type (1655 cm
−1); (ii) the PC-2 (10% of the variation) includes characteristics of the substrate (cellulose: 1120–1095 cm
−1); and (iii) the PC-3 (4% of the variation) includes the characteristics of the organic phase (styrene: 1602, 1583, 1452, 1032, 1000, 620 cm
−1; imide: 1765, 1329 cm
−1). As such, single Raman bands make a major contribution to the individual PCs and can be used for statistical discrimination between the coatings. The two-dimensional score plots of individual PCs can be used for classification of the coated papers within the 95% confidence interval (
Figure 4d). Test results of the graph include data for some representative samples from the calibration set (red points) and the validation set (blue points) with good agreement. From the score plot of PC-1 versus PC-2, the coated papers are classified according to the coating type in PC-1 and the coating thickness in PC-2. The discrimination of thick and thin coatings is related to the intensity of the Raman bands corresponding to the cellulose substrate, as reflected in PC-2. From the score plot PC-1 versus PC-3, the coated papers are classified according to the oil type, with polyunsaturated oils (SO, CO) having the highest PC values, monounsaturated oils (CaO, RO, SfO) having intermediate PC values, and the hydrogenated oils (HCO) having the lowest PC values. The latter classification is based on the relatively small variations in the organic composition of the coating (similar styrene content and slightly variable imide content), as reflected in PC-3.
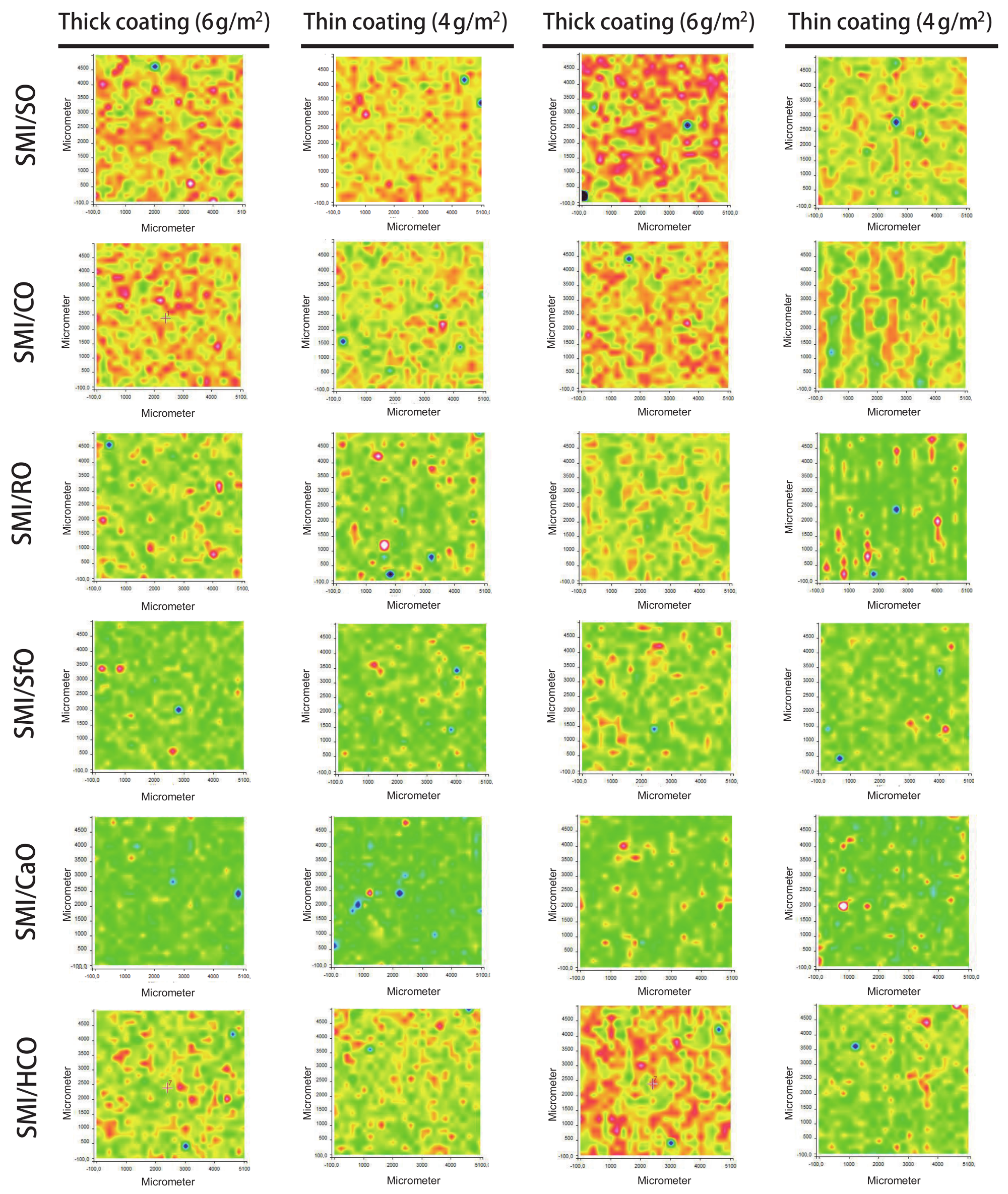
3.3. Raman Chemical Mapping (Average Intensities)
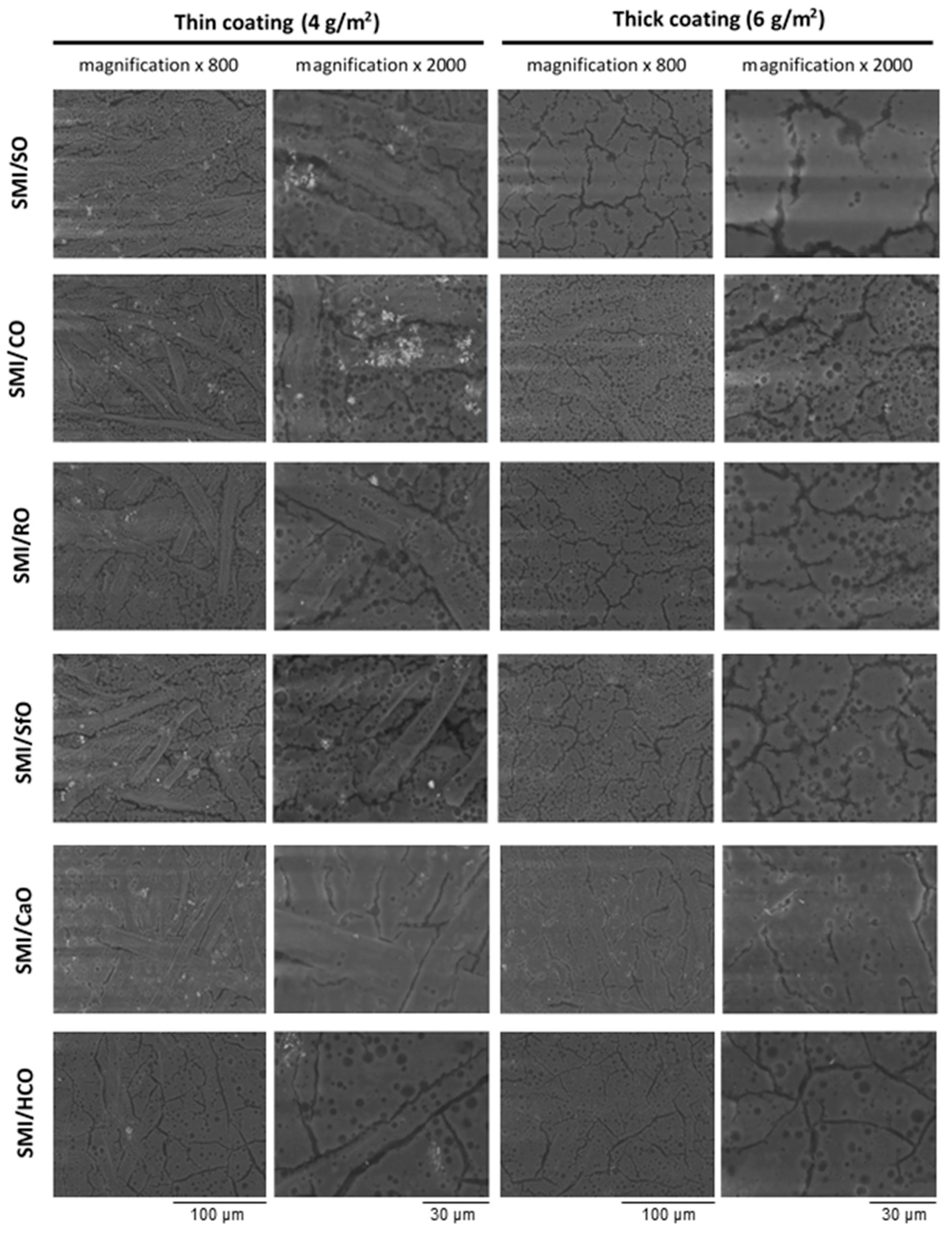
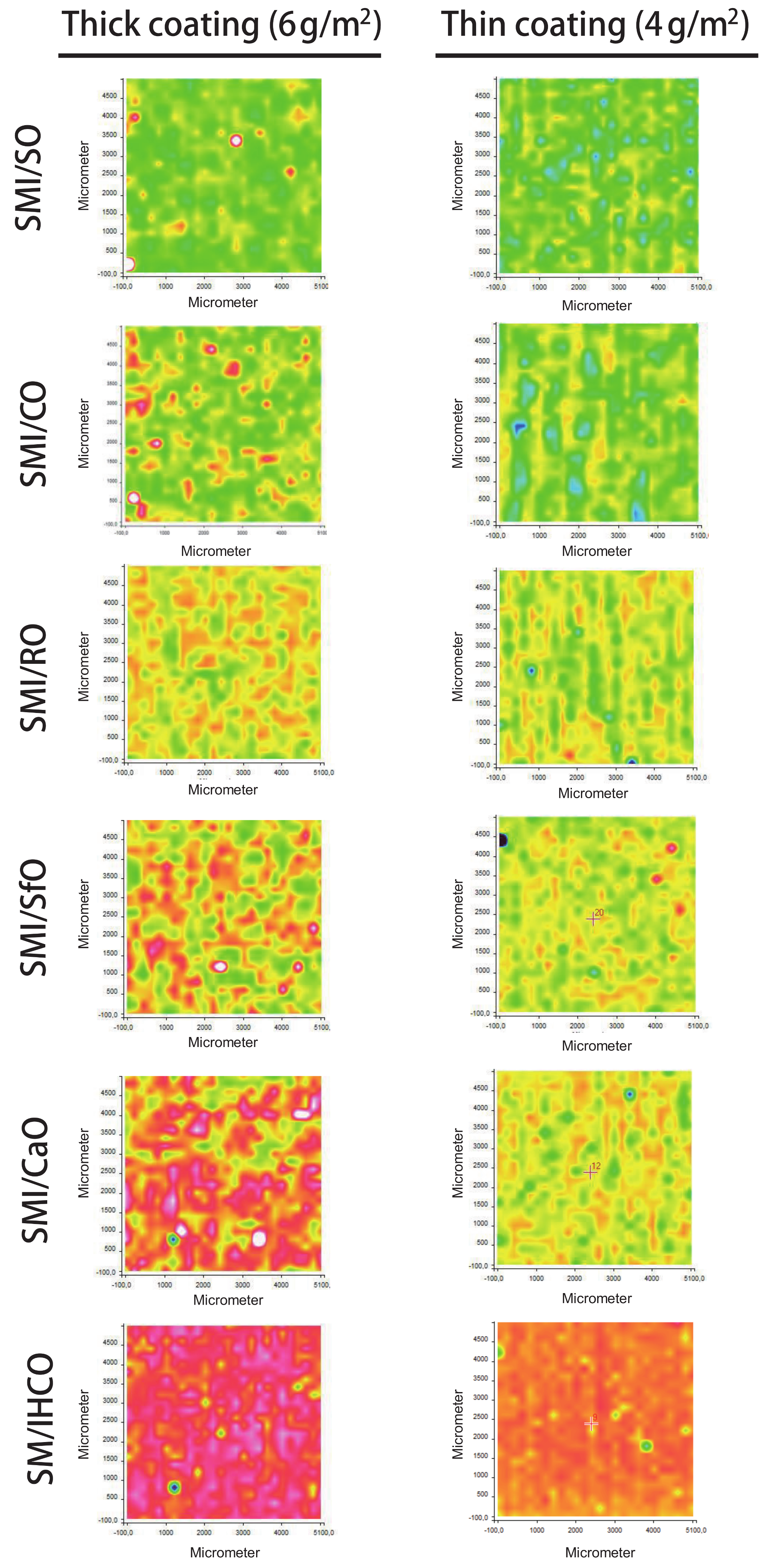
The chemical surface maps of coated papers (top view on 5 × 5 mm2 surface area) with different SMI/oil compositions were recorded to study the surface coverage, homogeneity, and distribution of different chemical moieties within the coating layer.
The average intensity maps for thick and thin paper coatings are illustrated in
Figure 5, with all maps having the same ordinate scale to make valid comparison between all SMI/oil coating types. The ordinate values are calculated as an average intensity value from the spectrum recorded at each (
x,
y) point. The surface maps consequently include data related to the intensities of the dominant Raman bands and illustrate the lateral homogeneity of the coating. As confirmed by the following more detailed analysis of single Raman bands, the ordinate intensities of average intensity maps can be related to the lateral distribution of organic coating moieties, with (i) high intensities (yellow to red) representing thick deposits of coating species and, (ii) low intensities (blue) corresponding to thin coating deposits and poor coverage. The surface areas with low average intensities consequently represent almost uncoated paper. For the thin coatings, inhomogeneities are recognized with striations parallel to the direction of the bar-coating. The average intensities are higher for thick coatings than for thin coatings as an indication for the surface coverage. The contrast in average intensities illustrate local heterogeneities: the coatings with high imide content and low amount of free oil are obviously most homogeneous, while the presence of a high amount of free oil is visualized as an island-like coating phase. The observations for average intensity maps can consequently be related to a combination of coating coverage and homogeneity, which depend on the imide content and amount of free oil. In order to confirm the significance of the average intensity maps and locations of oil, imide and styrene deposits, the features observed in the general surface maps are analyzed below in relation with specific Raman bands for the coating and paper substrate.
3.4. Raman Chemical Mapping (Band Ratios)
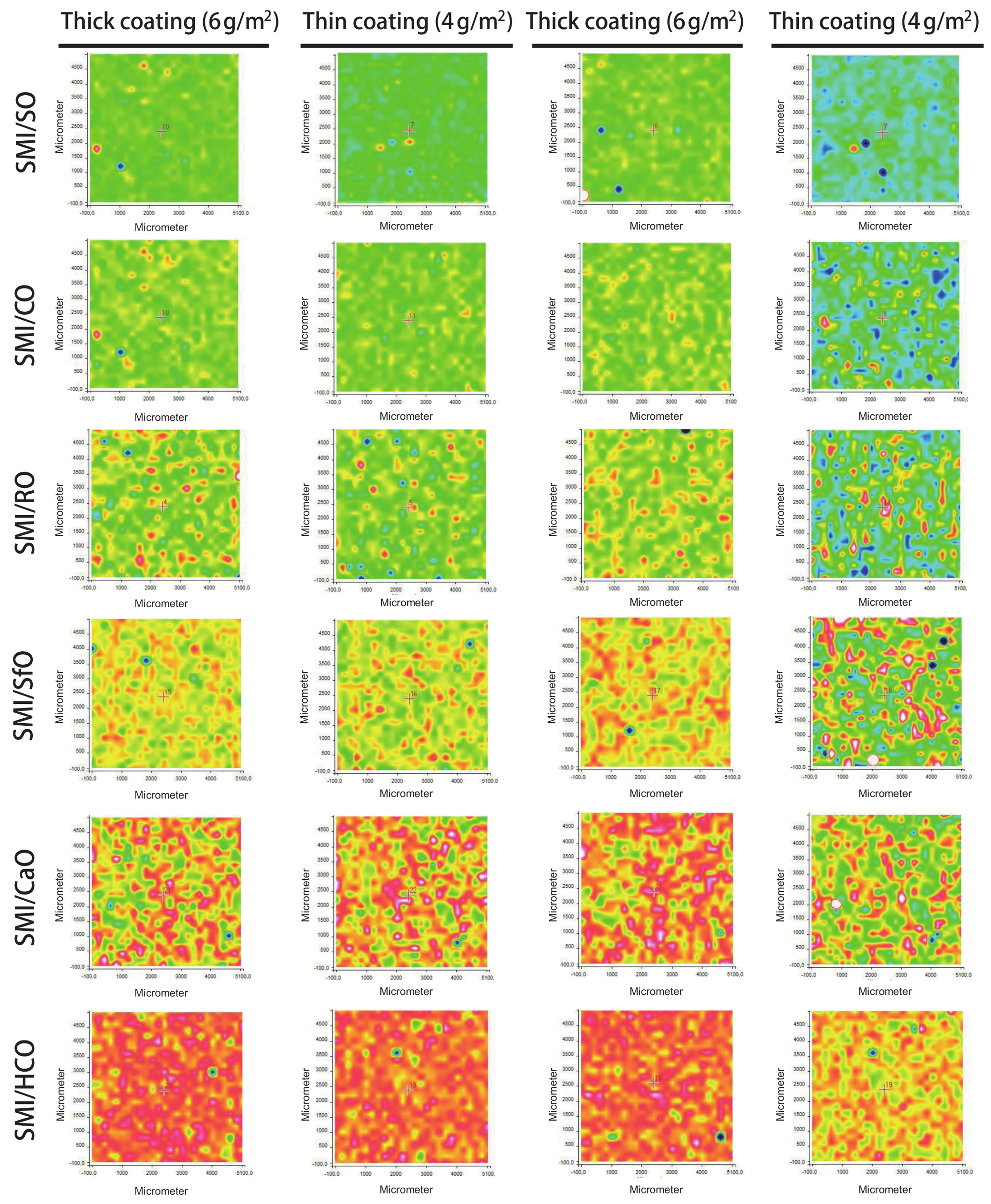
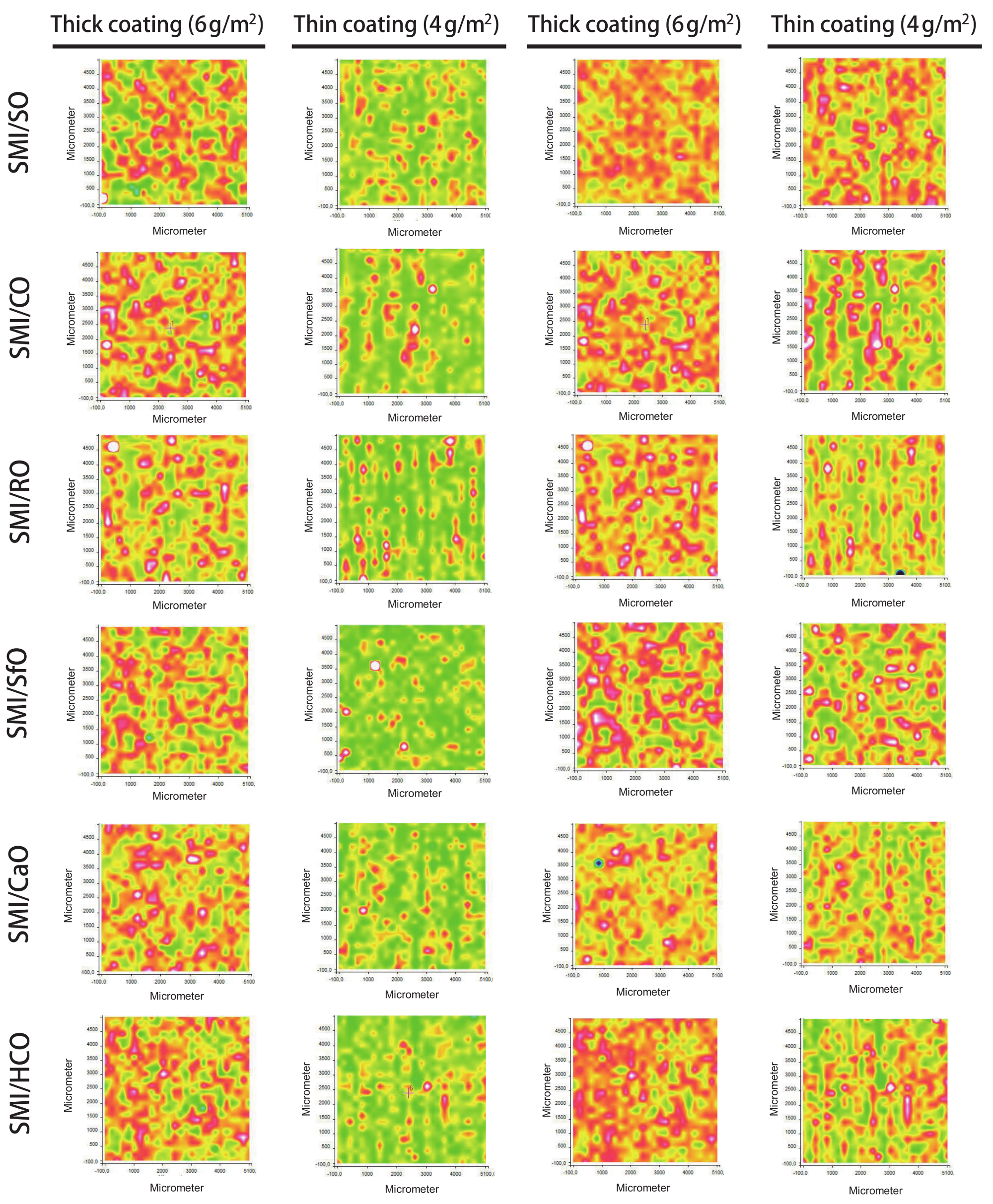
The intensity ratios of specific Raman bands related to the oil, styrene, imide, and cellulose components are further detailed to provide information on the local chemical composition and distribution of these elements over the paper coating.
The distribution of oil is evaluated from the intensity ratios of Raman bands for oil (1655 cm
−1), relative to styrene (1602 cm
−1) and cellulose (1095 cm
−1), as illustrated in
Figure 6: the styrene band is chosen as a reference band for the distribution of oil within the coating, while the cellulose band is chosen as a reference for the distribution of coating over the paper substrate. The progressive increase in oil at the surface for different SMI/oil coatings agrees with previous calculations (see
Table 2). The band intensity ratios and surface coverage of oil is comparable for thin and thick coatings when expressing the relative intensity of oil versus styrene within the coating itself: indeed, the same coating dispersions for deposition of thin and thick coatings were used. Contrarily, the differences in oil exposure between thin and thick coatings become clear when using the relative band intensity of oil versus cellulose. The surface patterns and locations covered with oil in the coating and those covered with oil are relative to the paper substrate overlap. Interestingly, the inhomogeneities for thin coatings observed in the average intensity maps are not reflected in the oil distribution over the surface. It is obvious that the inhomogeneities of the coating relative to the paper substrate are not expressed when taking the coating (styrene) as a reference. However, homogeneous distribution of the oil is also observed when taking the substrate (cellulose) as a reference. Therefore, the oil seems to be homogeneously spread over the surface and provides a continuous phase. The coating defects in thin coatings with striations can likely be attributed to the viscosity of the coating dispersions, where the presence of a free oil phase locally enhances the formation of a continuous coating due to better flow properties.
The distribution of organic coating moeities is evaluated from the intensity ratios of Raman bands for imide (1765 cm
−1), relative to styrene (1602 cm
−1) and cellulose (1095 cm
−1), as represented in
Figure 7. The relative intensity of imide progressively decreases for different SMI/oil coatings, in agreement with previous calculations (see
Table 2). The relative intensity of imide is comparable for thick and thin coatings when expressed against styrene, while the differences in imide between thick and thin coatings become clear relative to the paper substrate: the thick coatings are relatively homogeneous with even distribution of the imide, while thin coatings show striations at the same locations as observed in the average intensity maps. Therefore, the distribution of the organic imide phase plays a major role in the coating homogeneity. The imide phase is likely more rigid and viscous compared to the oil phase, with more risk of coating defects. There is good agreement in the surface maps of oil/cellulose versus imide/cellulose for the thin coatings, confirming that an amount of free oil is present between the imide phase and contributes to the formation of a homogeneous coating layer by ‘filling’ the non-imide zones as a binder. The complementarity of the oil and imide phases is visible for thin coatings with poly- and monounsaturated oils (e.g., SMI/CO, SMI/RO), where the calculated amounts of free oil are relatively small. The latter is less visible for thin coatings with polyunsaturated oils (e.g., SMI/SO) as there is only a limited amount of free oil, while it is partly visible for the coatings with unsaturated oil (e.g., SMI/HCO) as the oil coverage is much larger than the zones in between the imide phase.
The distribution of organic coating moieties from the intensity ratios of Raman bands for styrene (1602 cm
−1 and 1000 cm
−1), relative to cellulose (1095 cm
−1), are shown in
Figure 8. The maximum intensity of styrene bands is comparable for all SMI/oil coating types and does not strongly differ with the oil type, because all copolymers contain a fixed amount of 74 mol % styrene that remains inert during the synthesis of the SMI/oil nanoparticles. The differences in intensities of styrene therefore mainly correspond to variations in coating coverage. In particular, the effects of thin and thick coatings are visualized. The locations for coverage of styrene components represented by Raman bands at 1602 cm
−1 and 1000 cm
−1 are in agreement, but slight differences in intensity may be attributed to the specific orientation of the styrene parts. The styrene-related absorption bands are characteristic for either the aromatic C=C stretch (1602 cm
−1) or the aromatic C–H bending (1000 cm
−1). Their intensities depend on the bonding length of the π-conjugation between the vinyl group and benzene ring and change with local conformation of the aromatic styrene groups [
25]. The styrene orientation in the SMI copolymer is induced by a specific molecular structure of the high-molecular-weight SMA precursor and induces nanoparticle formation through self-assembly [
26]. Therefore, the variations in ratio of both styrene bands at 1602 and 1000 cm
−1 can eventually be attributed to configuration or orientation effects: the influences of segment orientation in styrene bloc copolymers with polarized Raman scattering were detected [
27]. In particular, the locations corresponding to the aromatic parts (1602 cm
−1) are more confined that the aliphatic parts of the styrene, as the aromatic styrene groups are likely more influenced by self-organization within the nanoparticles.
In conclusion, the locations for styrene coverage strongly overlap with the previous locations for imide coverage (see
Figure 6). The chemical mapping for the band ratios of organic coating moieties (imide and styrene) generally corresponds with the chemical mapping based on average intensities: the areas with high intensity in styrene (see
Figure 7) overlap with the areas of high average intensity (see
Figure 4). In case of coatings with polyunsaturated oils (SMI/SO, SMI/CO, SMI/SfO), the surface coverage with high intensities of band ratios for oil moieties (see
Figure 5) overlap with the areas of styrene coverage (see
Figure 7), as the oil is largely encapsulated in the organic parts. For coatings with monounsaturated oils (e.g., SMI/CaO), the surface areas with high intensities of oil moieties (see
Figure 5) are complementary to the surface areas with high intensities of styrene moieties (see
Figure 7), as there is a large amount of free oil in between the organic coating phase.
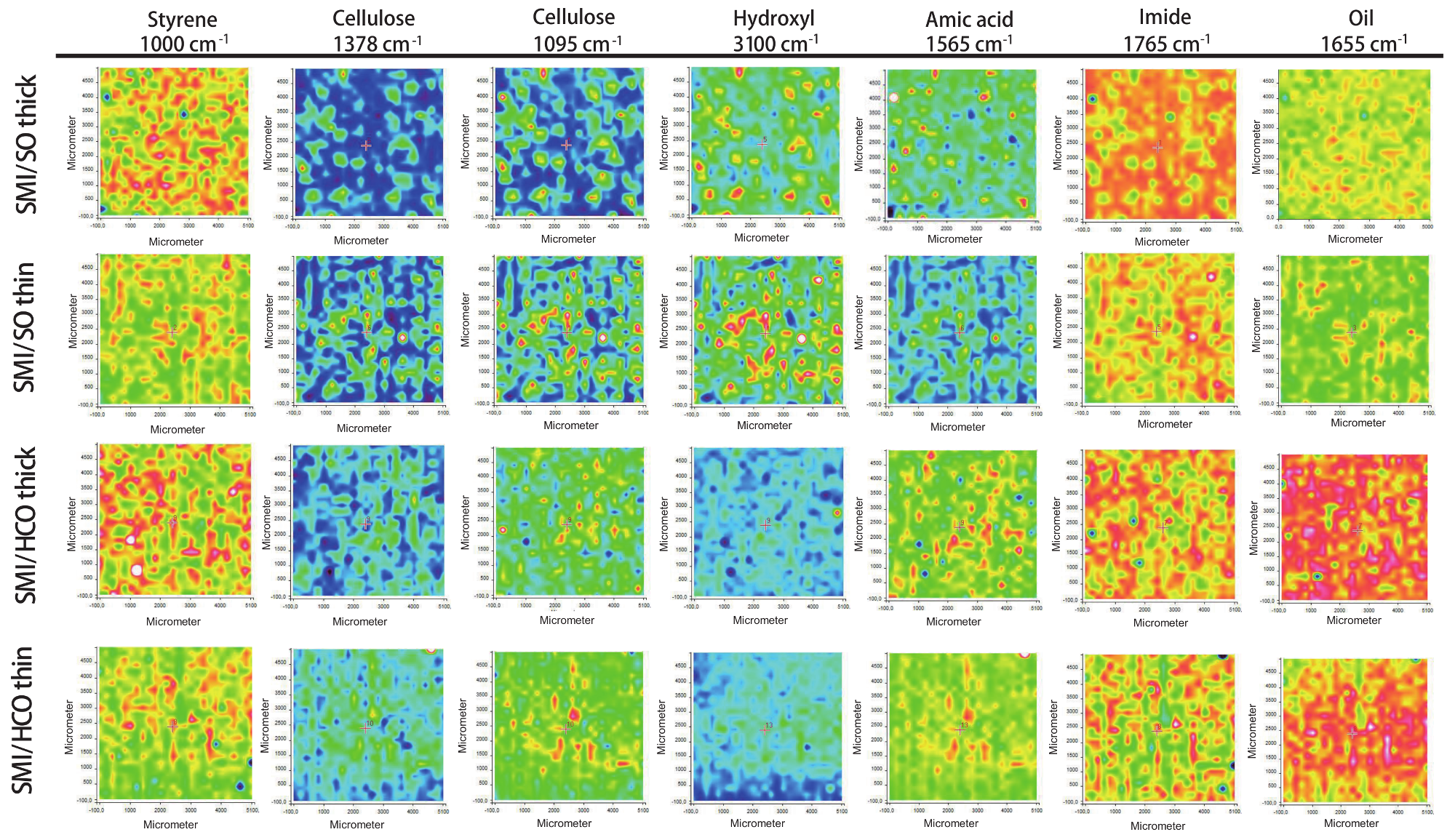
3.5. Raman Chemical Mapping (Single Bands)
The surface maps with intensities of single Raman bands provide direct chemical information on the surface composition of coated papers. As only a single band is considered, the spectra are first normalized (cellulose region 1170–1050 cm
−1) and baseline-corrected before selecting a specific band. An example of chemical surface maps for different single Raman bands of thick and thin coatings of SMI/SO and SMI/HCO is shown in
Figure 9 (the maps for other coating types are given in
Figures S2–S5 in Supplementary Materials). The selected Raman bands provide complementary information on the surface coverage of different chemical components.
The surface areas covered with high intensities of the styrene band (1000 cm
−1) are similar to those covered with high intensities of the imide band (1765 cm
−1). The same surface areas have also been covered by high intensities in previous surface maps with band intensity ratios of organic coating moieties. The surface maps of the cellulose bands (1378 cm
−1 and 1095 cm
−1) are complementary to the holes observed in the surface maps with organic coating deposits, indicating that the paper is locally penetrating through the coating at those locations. Both cellulose bands cover the same surface areas and exposure of cellulose bands at the surface is more intense for thin coatings than for thick coatings. The intensity of the single band related to hydroxyl groups (3100 cm
−1) is highest at locations where cellulose fibers are exposed and lowest at locations covered by the coating. The exposure of cellulose hydroxyl groups at the surface is evidently more intense for thin coatings than for thick coatings. Therefore, interactions between the coating and the paper substrate are expected to happen through hydrogen bonding between hydroxyl groups at the cellulose fibers and the imidized SMI/oil nanoparticles. In a search for other complementary bands, the locations with low intensities in the 1765 cm
−1 band (imide) correspond to locations with high intensities of the 1565 cm
−1 band. The latter band can be assigned to the amide II band (NH bend + CN stretch) and represents amic acid groups as a remaining intermediate product after imidization (i.e., ammonolyzed maleic anhydride). The non-imidized parts obviously induce minor local defect spots and influence the formation of a fully homogeneous coating layer. These parts do not interact well with the paper substrate as they appear at similar surface areas with high intensities of hydroxyl bands. The surface areas with high intensities of the single oil band (1655 cm
−1) fully overlap with the imide zones for coatings with polyunsaturated oil (e.g., SMI/SO), corresponding to previous conclusions that oil is embedded in the organic phase and there is very little free oil. The overlap of oil coverage and imide locations is much less for coatings with monounsaturated oil (see
Figures S3–5 in Supplementary Materials). In particular, the oil coverage largely exceeds the locations with imide deposits in the case of unsaturated oil types (e.g., SMI/HCO) due to the large amount of free oil.
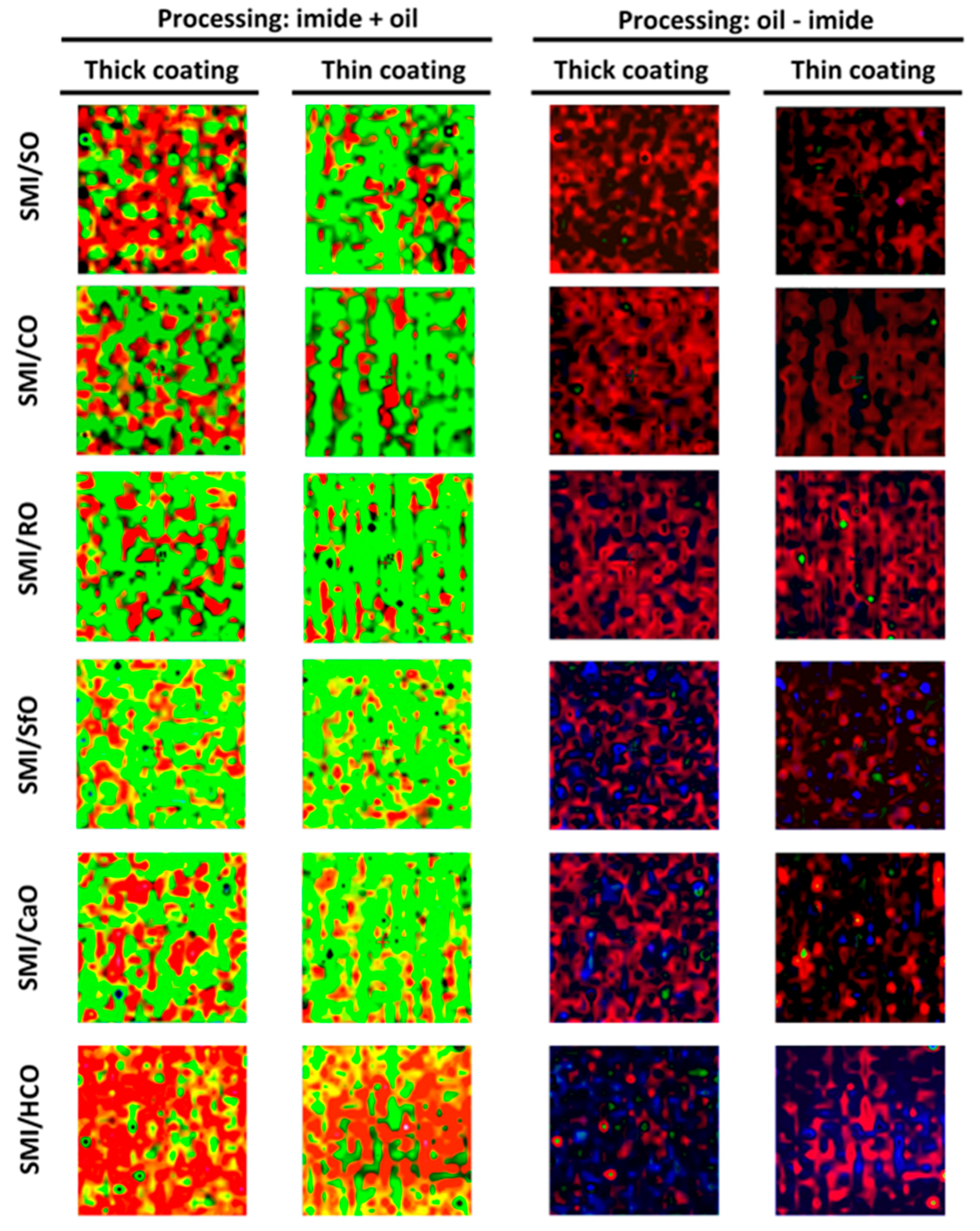
The surface maps with simultaneous coverage of the different coating components have been constructed after image processing (
Figure 10). The summation images with imide and oil coverage (“imide ADD oil”) illustrate the degree of overlap and inclusion of oil within the organic coating phase, with following color code (see online for color figures: red = oil, black = imide). The quantification of thick and thin coatings is visible from the surface areas covered with the red phase. Almost full inclusion of the red surface areas within the black surface areas is recognized for SMI/SO, SMI/MO, and SMI/RO coatings, while the amount of red surface areas outside the black surface areas increases for SMI/SfO, SMI/CaO, and SMI/HCO coatings, which is in parallel with the localization of free oil. The difference in images between oil and imide (“oil SUBTRACT imide”) directly illustrate the surface locations with free oil, following the color code (see online for color figures: blue = free oil). From the blue surface areas it can be observed that the amount of free oil increases in the formerly reported order of SMI/oil coating types, and is higher for thick coatings than for thin coatings. In conclusion, the processed images and original Raman surface maps allow us to accurately visualize the different components and local chemical composition at the coated paper surface, and systematically illustrate the effect of oil encapsulation depending on the degree of saturation of the vegetable oil.